Abstract
Acacetin (5,7-dihydroxy-4′-methoxyflavone), a flavonoid compound, has anti-peroxidative and anti-inflammatory effects. The effect of acacetin on antimetastasis in human prostate cancer DU-145 cells was investigated. First, the result demonstrated acacetin could exhibit an inhibitory effect on the abilities of the adhesion, invasion, and migration by cell–matrix adhesion assay, wound-healing assay, and Boyden chamber assay. Data also showed acacetin could inhibit the phosphorylation of p38 mitogen-activated protein kinase (p38 MAPK) involved in the downregulation of the expressions of matrix metalloproteinase-2 (MMP-2), matrix metalloproteinase-9 (MMP-9), and urokinase-type plasminogen activator (u-PA) at both the protein and mRNA levels. Next, acacetin significantly decreased the nuclear levels of nuclear factor kappa B (NF-κB), c-Fos, and c-Jun. Also, the treatment with acacetin to DU145 cells also leads to a dose-dependent inhibition on the binding ability of NF-κB and activator protein-1 (AP-1). Furthermore, the treatment of inhibitors specific for p38 MAPK (SB203580) to DU145 cells could cause reduced expressions of MMP-2, MMP-9, and u-PA. These results showed acacetin could inhibit the invasion and migration abilities of DU145 cells by reducing MMP-2, MMP-9, and u-PA expressions through suppressing p38 MAPK signaling pathway and inhibiting NF-κB- or AP-1-binding activity. These findings proved acacetin might be offered further application as an antimetastatic agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the most common cancer as well as the second leading cause of cancer-related deaths in men in Western countries [1]. One out of nine men over 65 years of age is frequently diagnosed with prostate cancer in the United States [2]. Nearly 50% of prostate cancer patients present with pathologic or clinical evidence of brain metastasis [3]. Thus, metastasis has been a major challenge for a successful cancer treatment to improve patient outcome.
Flavonoids are widespread in fruits, vegetables, seeds, and medicinal herbs. All might be one type of polyphenolic flavonoid, which have the diphenylpropane (C6C3C6) skeleton, including monomeric flavanols, flavones, flavanols, and flavanones, and have been intensively studied for their role in human health, including cancer prevention. Acacetin (5,7-dihydroxy-4′-methoxyflavone) (Fig. 1a), a flavonoid compound, has been reported to possess anti-peroxidative, anti-inflammatory, and anti-plasmodial [4–6]. Previous studies showed acacetin have also exerted an antiproliferative effect on the liver, prostate, lung, stomach, and breast cancer cells [7–11]. Although it was quite clear, acacetin may inhibit the growth of various cancers by inducing cancer cells toward apoptosis and anti-proliferation, whereas the precise impact and related molecular mechanism of acacetin on metastasis of cancer cells need further research.
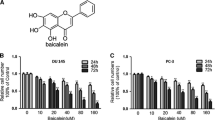
Effect of acacetin on the viability in DU145 cells. a Chemical structures of acacetin. b Cells (4 × 104 cells/ml) were treated with various concentrations (0, 1, 5, 10, 20, 40, and 60 μM) of acacetin for 24 and 48 h. Cell viability was determined by MTT assay. The survival cell number was directly proportional to formazan, which was measured spectrophotometrically at 563 nm. Values are expressed as mean ± SD of three independent experiments. * P < 0.05, ** P < 0.01, *** P < 0.001 compared with the untreated control (dose 0)
Cancer metastasis, the spread of cancer cells from the primary neoplasm to distant sites and their growth there, is the major cause of poor clinical outcome or death in various cancer patients [12]. Tumor invasion and migration are complex cascades of events involving a finely tuned interplay between malignant cells and multiple host factors. As cancer cells become metastatic and as endothelial cells become angiogenic, they develop altered affinity and avidity for their extracellular matrix (ECM), including the basement membrane. Excess ECM degradation is one of the hallmarks of tumor invasion and migration [13]. The metastasis is a multistep process involving an over-expression of proteolytic enzymes, such as matrix metalloproteinases (MMPs) and u-PA. MMP-2 and MMP-9 (also known as type IV collagenases or gelatinases) can degrade most ECM components forming the basal membrane [14]. In addition, u-PA may initiate the activation of an enzymatics cascade and convert the zymogen plasminogen to plasmin. Activating these enzymes enables the degradation of ECM by tumor cells, allowing their access to the vasculature, invasion, and migration into the target organ and development of tumor metastasis [15, 16].
As well as MMPs, the mitogen-activated protein kinases surperfamily members (MAPK) are associated with increased scattering/motility, invasion, proliferation, survival, and morphogenesis [17, 18]. Three major mammalian MAP kinases include extracellular signal-regulated kinase1 and 2 (ERK1/2), c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK), and p38 MAPK. The diverse MAP kinase members are activated in response to different extracellular stimuli and have distinct downstream targets, thus serving different roles in cellular responses. ERK1/2, p38 MAPK, and JNK/SAPK play a central role in regulating the expressions of MMPs and u-PA [19–21]. The promoter of MMP-2, MMP-9, and u-PA is highly conserved and shown to contain multiple functional elements, including NF-κB and AP-1 elements [22–24].
NF-κB is an inducible transcription factor, which is involved in inflammation, immune response, and malignant transformation. NF-κB is a dimeric transcription factor that consists of p50, p52, p65 (RelA), RelB, and c-Rel. NF-κB is maintained in the cytoplasm through interactions with an inhibitor of NF-κB (IκB), but upon dissociation, it moves into the nucleus and promotes cancer cells proliferation, angiogenesis, and metastasis. The AP-1 transcription factor plays a critical role in regulating tumor cell proliferation and has been implicated in controlling a program of gene expression that mediates cell motility and invasion in vitro [25, 26]. AP-1 consists of homodimers and heterodimers of members from the Fos (c-Fos, Fos B, Fra-1, and Fra-2) and Jun (c-Jun, Jun B, and Jun D) families. MAPKs are intricately involved in the expression of the components involved in MMPs or u-PA promoter induction by NF-kB, AP-1, and its association with c-Fos and c-Jun. Hence, MMPs, u-PA, and their regulatory pathways have been considered as promising targets for anti-cancer drugs and chemopreventive agents [27, 28]. Further, to establish the antimetastastic mechanism of acacetin, our study is aimed to examine the inhibitory effects and the related signaling pathways of acacetin on the invasion and migration of human DU145 prostate cancer cells in vitro.
Materials and methods
Reagents and antibodies
Acacetin (purity >97%), DMSO, Tris–HCl, EDTA, SDS, phenylmethylsulfonyl fluoride, bovine serum albumin (BSA), gelatin, casein, plasminogen, leupeptin, Nonidet P-40, deoxycholic acid, sodium orthovanadate, and SB203580 were purchased from Sigma-Aldrich (St. Louis, MO); the protein assay kit was obtained from Bio-Rad Labs. (Hercules, CA). Dulbecco’s phosphate buffer solution (PBS), trypsin-EDTA, and powdered MEM Eagle’s medium were purchased from Gibco/BRL (Gaithersburg, MD). Matrigel was from BD Biosciences (Bedford, MA). Antibody against Akt, ERK1/2, JNK/SAPK, and p38 MAPK, proteins, and phosphorylated proteins were purchased from Cell Signaling Tech. (Beverly, MA). PI3K, MMP-2, MMP-9, u-PA, NF-κB (p65), c-Fos, c-Jun, β-actin, and C23 antibodies were from BD Transduction Laboratories (San Diego, CA). The enhanced chemiluminescence (ECL) kit was purchased from Amersham Life Science (Amersham, UK).
Cell culture and acacetin treatment
DU145, a human prostate carcinoma cell line, was obtained from BCRC (Food Industry Research and Development Institute in Hsin-Chu, Taiwan). Cells were cultured in MEM supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 U/ml of penicillin, 100 mg/ml streptomycin mixed antibiotics, and 1 mM sodium pyruvate. All cell cultures were maintained at 37°C in a humidified atmosphere of 5% CO2–95% air. The culture medium was renewed every 2–3 days. Adherent cells were detached by incubation with trypsin. For acacetin treatment, the stock solution of acacetin was dissolved in dimethyl sulfoxide (DMSO) and sterilized by filtration through 0.2 μm disc filters. Appropriate amounts of stock solution (1 mg/ml in DMSO) of acacetin were added to the cultured medium to achieve the indicated concentrations (Final DMSO concentration was <0.2%) and then incubated with cells for the indicated time periods.
Analysis of cell viability (MTT assay)
In order to evaluate the cytotoxicity of acacetin, an MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide] assay was performed to determine the cell viability. In brief, cells were seeded at a density of 4 × 104 cells/ml in a 24-well plate for 24 h. Then, the cells were treated with acacetin at various concentrations (0, 1, 5, 10, 20, 40, and 60 μM) for various periods of time (24 and 48 h). Each concentration was repeated three times. After the exposure period, the medium was removed and followed by washing the cells with PBS. Then, the medium was changed and incubated with MTT solution of 5 mg/ml/well for 4 h. The medium was removed, and formazan was solubilized in isopropanol and measured spectrophotometrically at 563 nm. The percentage of viable cells was estimated by comparing with the untreated control cells.
Cell–matrix adhesion assay
After a pre-treatment with acacetin (0, 1, 5, and 10 μM) for 24 h or 1 μM acacetin for 6, 12, 24, and 48 h, cells were seeded at a density of 5 × 104 cells/ml in a 24-well plate and coated with 150 μl type I collagen (10 μg/ml); then they were cultured for 30 min. Then, non-adherent cells were removed by PBS washes and adherent cells were fixed in ethanol. After a staining with 0.1% crystal violet, fixed cells were lysed in 0.2% Triton-100 and measured spectrophotometrically at 550 nm.
Wound-healing assay
For cell motility determination, DU145 cells (1 × 105 cells/ml) were seeded in 6-well tissue culture plate and grown to 80–90% confluence. After aspirating the medium, the center of the cell monolayers was scraped with a sterile micropipette tip to create a denuded zone (gap) of constant width. Subsequently, cellular debris was washed with PBS, and the DU145 cells were exposed to various concentrations of acacetin (0, 1, 5, and 10 μM). The wound closure was monitored and photographed at 0, 12, 24, 36, and 48 h with an Olympus CKX-41 inverted microscope and an Olympus E-410 camera. In order to quantify the migrated cells, pictures of the initial wounded monolayers were compared with the corresponding pictures of cells at the end of the incubation. Artificial lines fitting the cutting edges were drawn on pictures of the original wounds and overlaid on the pictures of cultures after incubation. Migrated cells across the white lines were counted in six random fields from each triplicate treatment, and data are presented as mean ± SD.
Boyden chamber invasion and migration assay
The ability of DU145 cells for passing through Matrigel-coated filters was measured by the Boyden chamber invasion assay. Matrigel was diluted to 200 μg/ml with distilled water and applied to the top side of the 8 μm pore polycarbonate filter. In brief, DU145 cells were treated with various concentrations of acacetin. After 48 h, cells were detached by trypsin and resuspended in serum-free medium. Medium containing 10% FBS was applied to the lower chamber as chemoattractant, and then the cells were seeded on the upper chamber at a density of 1 × 105 cells/well in 50 μl of serum-free medium. The chamber was incubated for 8 h at 37°C. At the end of incubation, the cells in the upper surface of the membrane were carefully removed with a cotton swab and cells invaded across the Matrigel to the lower surface of the membrane were fixed with methanol and stained with 5% Giemsa solution. The invasive cells on the lower surface of the membrane filter were counted with a light microscope. The data are presented as the average number of cells attached to the bottom surface from randomly chosen fields. Each experiment was carried out in triplicate.
In order to measure the ability of DU145 cells on migration, cells were seeded into a Boyden chamber with 8 μm pore polycarbonate filters which were not coated with Matrigel. The migrative cells were treated with various concentrations of acacetin for 48 h or 1 μM acacetin for 6, 12, 24, and 48 h. The migration assay was measured as described in the invasion assay.
Analysis of MMP-2, MMP-9, and u-PA activities (Zymography assay)
The activities of MMP-2 and MMP-9 were assayed by gelatin zymography as described previously. In brief, conditioned media from cells cultured in the absence of serum for 24 h were collected. Samples were mixed with loading buffer and electrophoresed on 8% SDS-polyacrylamide gel containing 0.1% gelatin. Electrophoresis was performed at 140 and 110 V for 3 h. Gels were then washed twice in Zymography washing buffer (2.5% Triton X-100 in double-distilled H2O) at room temperature to remove SDS, followed by incubation at 37°C for 12–16 h in Zymography reaction buffer (40 mM Tris–HCl (pH 8.0), 10 mM CaCl2, 0.02% NaN3), stained with Coomassie blue R-250 (0.125% Comassie blue R-250, 0.1% amino black, 50% methanol, 10% acetic acid) for 1 h and destained with destaining solution (20% methanol, 10% acetic acid, 70% double-distilled H2O). Nonstaining bands representing the levels of the latent form of MMP-2 and MMP-9 were quantified by densitometer measurement using a digital imaging analysis system.
Visualization of u-PA activity was performed by casein-plasminogen zymography. In brief, 2% casein and 20 μg/ml plasminogen were added to 8% SDS-PAGE gel. Samples with a total protein of about 20 μg were then loaded onto the gels. The u-PA activity of cells treated or untreated with acacetin was measured as described in the gelatin zymography section.
Isolation of total RNA, reverse transcriptase polymerase chain reaction (RT-PCR), and DNA electrophoresis
Total RNA was isolated from human prostate cells using the total RNA Extraction Midiprep System (Viogene BioTek Corporation, Taiwan). Total RNA (2 μg) was transcribed to 20 μl cDNA with 1 μl dNTPs (2.5 mM), 1 μl Oligo dT (10 pmole/μl), and 1 μl RTase (200 U), 1 μl RNase inhibitor and 5× reaction buffer. The appropriate primers (sense of MMP-2, 5′-GGCCCTGTCACTCCTGAGAT-3′, nt 1337–1356; antisense of MMP-2, 5′-GGCATCCAGGTTATCGGGGA-3′, nt 2026–2007; sense of MMP-9, 5′-AGGCCTCTACAGAGTCTTTG-3′, nt 1201–1220; antisense of MMP-9, 5′-CAGTCCAACAAGAAAGGACG-3′, nt 1700–1683; sense of u-PA, 5′-TTGCGGCCATCTACAGGAG-3′, nt 654–672; antisense of u-PA 5′-ACTGGGGATCGTTATACATC-3′, nt 1068–986; sense of GADPH, 5′-CGGAGTCAACGGATTGGTGTT-3′, nt 94–126; antisense of 5′-AGCCTTCTCCATGGTTGGTGAAGAC-3′, nt 399–375) were used for PCR amplifications. PCR was performed with Platinum Taq polymerase (Invitrogen) under the following conditions: 30 cycles of 94°C for 1 min, 59°C (MMP-2) or 60°C (MMP-9 and GAPDH) for 1 min, 72°C for 1 min followed by 10 min at 72°C.
Preparation of whole-cell lysates and nuclear extracts
The cells were lysed with ice-cold RIPA buffer (1% NP-40, 50 mM Tris-base, 0.1% SDS, 0.5% deoxycholic acid, 150 mM NaCl, pH 7.5), and then the following were added phenylmethylsulfonyl fluoride (10 mg/ml), leupeptin (17 mg/ml), and sodium orthovanadate (10 mg/ml). After vortexing for 30 min on ice, the samples were centrifuged at 12000g for 10 min, and then the supernatants were collected, denatured, and subjected to SDS-PAGE and Western blotting. Nuclear extracts were prepared as previously described [29]. Each nuclear pellet was resuspended in nuclear extract buffer (1.5 mM MgCl2, 10 mM HEPES, pH 7.9, 0.1 mM EDTA, 0.5 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 25% glycerol, and 420 mM NaCl). The nuclear suspension was incubated on ice for 20 min and then centrifuged at 14,000g for 5 min. The supernatant (corresponding to the soluble nuclear fraction) was saved and then used for NF-κB, c-Fos, c-Jun, and AP-1 detection. The protein content was determined with Bio-Rad protein assay reagent using BSA as a standard.
Western blotting analysis
In order to analyze the migration-related proteins, Western blotting was performed as follows. The denatured samples (50 μg purified protein) were resolved on 10–12% SDS-PAGE gels. The proteins were then transferred onto nitrocellulose membranes. Non-specific binding of the membranes was blocked with Tris-buffered saline (TBS) containing 1% (w/v) nonfat dry milk and 0.1% (v/v) Tween-20 (TBST) for more than 2 h. Membranes were washed with TBST three times for 10 min and incubated with an appropriate dilution of specific primary antibodies in TBST overnight at 4°C. Subsequently, membranes were washed with TBST and incubated with appropriate secondary antibody (horseradish peroxidase-conjugated goat antimouse or antirabbit IgG) for 1 h. After washing the membrane three times for 10 min in TBST, the band detections were revealed by enhanced chemiluminescence using ECL Western blotting detection reagents and exposed ECL hyperfilm in FUJFILM Las-3000 mini (Tokyo, Japan). Then proteins were quantitatively determined by densitometry using FUJFILM-Multi Gauge V3.0 software.
Analysis of NF-κB and AP-1 binding assay (electrophoretic mobility shift assay)
Cell nuclear proteins were extracted with a nuclear extract buffer and measured by an electrophoretic mobility shift assay (EMSA). Cells (1 × 105/ml) were collected in PBS buffer (pH 7.4) and centrifuged at 2000g for 5 min at 4°C. Cells were lysed with buffer A (10 mM HEPES, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, and 0.5 mM PMSF (pH 7.9) containing 5% NP-40) for 10 min on ice, and this was followed by vortexing to shear the cytoplasmic membranes. The lysates were centrifuged at 2000g for 10 min at 4°C. The pellet containing the nuclei was extracted with high salt buffer B (20 mM HEPES, 420 mM NaCl, 1.5 mM MgCl2, 0.5 mM DTT, 0.5 mM PMSF, 0.2 mM EDTA, and 25% glycerol) for 15 min on ice. The lysates were centrifugated at 13000g for 10 min at 4°C. The supernatant containing the nuclear proteins was collected and frozen at −80°C until use. The protein content of nuclear fractions was determined with Bio-Rad protein assay. A 5-μg aliquot of nuclear proteins was mixed with either biotin-labeled NF-κB or AP-1 oligonucleotide probes for 15 min at room temperature or with oligonucleotides containing (sense of NF-κB, 5′-AGTTGAGGGGACTTTCCCAGGC-3′, antisense of NF-κB, 3′-TCAACTCCCCTGAAAGGGTCCG-5′; sense of AP-1, 5′-CGCTTGATGACTCAGCCGGAA-3′, antisense of AP-1, 3′-GCGAACTACTGAGTCGGCCTT). DNA probes were added to 10 μl binding reactions containing double-distilled H2O, 5 μg nuclear proteins, 1 μl poly (dI-dC), 1 μl biotin-labeled double-stranded NF-κB or AP-1 oliginucleotides, and 2 μl of 10-fold binding buffer into a microcentrifuge tube and were incubated for 15 min at room temperature. Specific competition binding assays were performed by adding 200-fold excess of unlabeled probe as a specific competitor. Following protein–DNA complexes formation, samples were loaded on a 6% nondenaturing polyacrylamide gel in 0.5× TBE buffer and then transferred to positively charged nitrocellulose membranes (Milipore, Bedford, MA) by a transfer blotting apparatus and cross-linked in a Stratagene crosslinker. Gel shifts were visualized with streptavidin–horseradish peroxidase followed by chemiluminescent detection.
Statistical analysis
Data were expressed as means ± standard deviation of three independent experiments and analyzed by Student’s t test (Sigmaplot 2001). Significant differences were established at P ≤ 0.05.
Results
The cytotoxicity of acacetin in DU145 cells
We first assayed the cytotoxicity of acacetin by treating DU145 cells with acacetin at various concentrations (0, 1, 5, 10, 20, 40, and 60 μM) for 24 and 48 h followed by MTT assay. As shown in Fig. 1b, acacetin showed a dose- and time-dependent inhibitory effect on the growth of DU145 cells. Compared to 0 μM (DMSO was treated alone, data not shown), after 24 and 48 h treatment with acacetin at a concentration between 0 and 10 μM was not significantly altered, indicating acacetin was not toxic to DU145 cells at these dosages. When cells were treated with 20–60 μM acacetin for 24 and 48 h, cell viability was significantly decreased. These results demonstrated treating with acacetin with doses higher than 10 μM for 24 and 48 h resulted in dose- and time-dependent loss of cell viability in DU145 cells, but doses lower than 10 μM for 24 and 48 h did not cause cytotoxicity. In the following experiments, these doses below 10 μM of acacetin were applied in all subsequent experiments.
Acacetin inhibited adhesion, invasion, and migration in DU145 cells
In order to investigate the antimetastatic low-cytotoxic acacetin in DU145 cells, a cell–matrix adhesion assay, a wound-healing assay, and a Boyden chamber assay were used. In the cell–matrix adhesion assay, acacetin treated DU145 cells at 10 μM for 24 h, showed significantly decreasing on the cell adhesion ability of DU145 cells in a dose-dependent manner. Also, time course experiments with acacetin at a low concentration of 1 μM also indicated acacetin could significantly inhibit the adhesion of DU145 cells in a time-dependent manner (Fig. 2a, b). For the wound-healing assay, according to a quantitative assessment, treatment with 5 and 10 μM of acacetin inhibited 60 and 88% of cell migration after 24 h, respectively; and such doses of acacetin inhibited 49 and 63% of cell migration at 48 h, respectively. Also, the cells were treated with various concentrations of acacetin for 0, 12, 24, 36, and 48 h. The results showed 10 μM of acacetin had greatest inhibitory effect on cell motility after 48 h incubation (Fig. 3a, b). Also, compared with the untreated cells, the level of DU145 cell number decreased almost 2.7-fold when treating with 10 μM acacetin for 48 h. These results revealed acacetin significantly inhibited the motility of DU145 cells.
Concentration- and time-dependent effects of acacetin on cell–matrix adhesion in DU145 cells. In concentration-dependent assay (a), DU145 cells were treated with various concentrations (0, 1, 5, and 10 μM) of acacetin for 24 h. In time-dependent assay (b), DU145 cells were treated with acacetin of 1 μM for 6, 12, 24, and 48 h, treated cells were then subjected to analyses for cell–matrix adhesion as described in Materials and methods section. Values are expressed as mean ± SD of three independent experiments. * P < 0.01, ** P < 0.001 compared with the untreated control (dose 0)
Effect of acacetin on the motility in DU145 cells. a DU145 cell monolayers were scraped by a sterile micropipette tip, and the cells were treated with various concentrations (0, 1, 5, and 10 μM) of acacetin for 0, 12, 24, 36, and 48 h. The number of cells in the denuded zone was quantitated after indicated times (0, 12, 24, 36, and 48 h) by inverted microscopy. White lines indicate the wound edge. Pictures only were presented 24 and 48 h. b Migrated cells across the white lines were counted in six random fields from each treatment. Quantitative assessment of the mean number cells in the denuded zone is expressed as mean ± SD of three independent experiments. * P < 0.05, ** P < 0.01, *** P < 0.001 compared with the untreated control (dose 0); # P < 0.01, ## P < 0.001 compared with the 0 h-treated time
One important characteristic of metastasis is the invasive and migratory ability of tumor cells. Using a cell invasion and migration assay with the Boyden chamber, the reductions in invasion and migration of DU145 cells were noted when the acacetin concentrations were beyond 1 μM. The results showed acacetin induced a dose- and time-dependent decrease in invasion and migration with an increasing concentration of acacetin. In the 10-μM acacetin-treated group, the invasion and migration of DU145 cells showed 37 and 34%, respectively, compared with those of the non-acacetin treated group (Fig. 4a, b). Subsequently, time course experiments with acacetin at a low concentration of 1 μM also indicated acacetin could significantly inhibit the invasion and migration of DU145 cells in a time-dependent manner (Fig. 4c, d). The results demonstrated acacetin significantly inhibited the invasion and migration of highly metastatic DU145 cells.
Concentration- and time-dependent effects of acacetin on invasion and migration in DU145 cells. In concentration-dependent assays (a, b), DU145 cells were treated with various concentrations (0, 1, 5, and 10 μM) of acacetin for 48 h. In time-dependent assay (c, d), cells were treated with acacetin of 1 μM for 6, 12, 24, and 48 h. Cell invasion were measured by Boyden chamber for 8 h; polycarbonate filters (pore size, 8 μm) were precoated with matrigel. Cell migration was measured by Boyden chamber for 6 h with polycarbonate filters (pore size, 8 μm); Invasion and migration abilities of DU145 cells were quantified by counting the number of cells that invaded to the underside of the porous polycarbonate membrane under microscopy and represent the average of three experiments ± SD. * P < 0.05, ** P < 0.01, *** P < 0.001 compared with the untreated control (dose 0)
Acacetin inhibited the expressions of MMP-2, MMP-9, and u-PA in DU145 cells
In order to explore the possible antimetastatic mechanism of acacetin, the expressions of MMP-2, MMP-9, and u-PA in DU145 cells exposed to various concentrations of acacetin were examined. As shown in Fig. 5a, acacetin led to a significant reduction of MMP-2, MMP-9, and u-PA at the protein level in a dose-dependent manner. Gelatin and casein zymography was also carried out to assess the activities of MMP-2, MMP-9, and u-PA in cells treated with acacetin. As shown in Fig. 5b, acacetin inhibited the activities of MMP-2, MMP-9, and u-PA in a dose-dependent manner. Quantification analysis indicated that the MMP-9 activity reduced by 8, 34, and 79% and MMP-2 activity reduced by 15, 40, and 70% while u-PA by 13, 38, and 52% when cells were treated with 1, 5, and 10 μM of acacetin, respectively. Moreover, acacetin also exerted inhibitive effects on the MMP-2, MMP-9, and u-PA at the mRNA level in a dose-dependent manner compared with control group (Fig. 5c). These results suggested that the antimetastatic effect of acacetin was related to the inhibition of enzymatically degradative processes of tumor metastasis.
Effect of acacetin on MMP-2, MMP-9, and u-PA expressions in DU145 cells. a Cells were treated with various concentrations (0, 1, 5, and 10 μM) of acacetin for 24 h. The protein levels of MMP-2 and MMP-9 from whole-cell lysates were analyzed by Western blot. β-Actin was used as loading control. It was determined that the protein expressions of MMP-2, MMP-9, and u-PA were subsequently quantified by densitometric analysis with that of control being onefold. b The conditioned media were collected and MMP-2, MMP-9, and u-PA activities were determined by gelatin and casein zymography. MMP-2, MMP-9, and u-PA activities were quantified by densitomeric analysis. c Cells were treated with various concentrations of acacetin for 24 h. And then, RNA samples were extracted and subjected to a semi-quantitative RT-PCR for MMP-2, MMP-9, and u-PA with GADPH being an internal control. The PCR products were quantitated by densitometric analysis with that of the untreated group being 100%. Values are expressed as mean ± SD of three independent experiments. * P < 0.05, ** P < 0.01, *** P < 0.001 compared with the untreated control (dose 0)
Acacetin inhibited the phosphorylation of p38 MAPK in DU145 cells
Since we have shown treatment of DU145 cells with acacetin inhibited the cell metastasis and activities of MMP-2, MMP-9, and u-PA, the underlying mechanisms were further investigated. Several studies have indicated the transcription factors (for example, NF-kB, c-Fos, c-Jun), JNK1/2, ERK1/2, p38 MAPK, and Akt that are involved in the activities of MMP-2, MMP-9, and u-PA on different cell types [19, 30]. In order to assess whether acacetin mediates and/or inhibits phosphorylation of JNK1/2, ERK1/2, p38 MAPK, Akt, and the protein level of PI3K, we explored the effect of acacetin on the phosphorylated status of MAPK family members (JNK1/2, ERK1/2, p38 MAPK) and Akt in DU145 cells which were treated with various concentrations of acacetin for 6 h. Figure 6 showed acacetin significantly inhibited the activation of p38 MAPK as shown by decreasing the phosphorylation of p38 MAPK. In contrast, acacetin did not significantly affect phospho-JNK1/2, phospho-ERK, and phospho-Akt activity. Moreover, no significant change in the total amount of JNK1/2, ERK1/2, p38 MAPK, and Akt proteins was noted (data not shown).
An inhibitory effect of acacetin on the phosphorylation of p38 MAPK. Cells were treated with various concentrations (0, 1, 5, and 10 μM) of acacetin for 6 h, and then cell lysates were subjected to SDS-PAGE followed by Western blotting with anti-phospho-JNK1/2, anti-phospho-ERK1/2, anti-phospho-p38, and anti-phospho-Akt antibodies. β-Actin was used as a loading control. Results from three repeated and separated experiments were similar
In order to further investigate whether the inhibition of acacetin was mainly through inhibition of the p38 MAPK signaling pathway, DU145 cells were pretreated with a p38 inhibitor (SB203580; 40 or 50 μM) for 1 h and then incubated in the with or without acacetin (1 μM) for 24 h. Results of gelatin zymography assay showed sole treatment of SB203580 (40 or 50 μM) or acacetin (1 μM), respectively, reduced the activity of MMP-9 or MMP-2 or by 30, 72, and 18% or 29, 60, and 12%, respectively, and the combination treatment could reduce the activities of MMP-9 or MMP-2 by 65 or 53% (40 μM SB203580 + 1 μM acacetin) (Fig. 7a). Similarly, in a casein zymography assay, a sole treatment of SB203580 (40 or 50 μM) or acacetin (1 μM) reduced the activity of u-PA by 8, 47, and 10%, respectively, and the combination treatment could further reduce the activity of u-PA by 64% (40 μM SB203580 + 1 μM acacetin) (Fig. 7b). Data finding revealed that the inhibition of the activities of MMP-2, MMP-9, and u-PA by acacetin on DU145 cells could occur through p38 inactivation.
Effect of p38 MAPK inhibitor (SB203580) and acacetin on the activities of MMP-2, MMP-9, and u-PA. Cells were plated in 6-well and pre-treated with SB203580 (40 or 50 μM) for 1 h and then incubated in the presence or absence of acacetin (1 μM) for 24 h. Afterward, the culture medium was subjected to gelatin and casein zymography to analyze the activities of a MMP-2 and MMP-9, and b u-PA. Determined activities of these proteins were subsequently quantified by densitometric analysis with that of control being 100% as shown just below the gel data. Data represented the mean ± SD of three independent experiments (* P < 0.05, ** P < 0.01, *** P < 0.001)
Acacetin inhibited the DNA binding activities of NF-κB, c-Fos, and c-Jun in DU145 cells
NF-κB and AP-1 family of transcriptional factors have been known to translocate to the nucleus and regulate the expression of multiple genes involved in MMPs or u-PA secretion. In order to clarify the involvement of NF-κB and AP-1 proteins in the mechanism of acacetin’s action, the effect of acacetin on the DNA-binding activities of NF-κB and AP-1 in DU145 cells was investigated by EMSA. As shown in Fig. 8a, DU145 cells were treated with 0–10 μM of acacetin for 12 h, acacetin inhibited NF-κB, and AP-1-binding activities in a dose-dependent manner. Especially, the binding activities of NF-κB and AP-1 were inhibited by treating with 10 μM acacetin. Further, the expression of NF-κB, c-Fos, and c-Jun in the cytoplasmic and nuclear extracts were analyzed by Western blotting to assess the possible inhibitory effect of acacetin. As shown in Fig. 8b and c, acacetin treatment resulted in a concentration-dependent decrease in NF-kB, c-Fos, and c-Jun protein levels in the cytoplasmic as well as nuclear fraction. Result showed that the cytoplasmic and nuclear levels of NF-κB, c-Fos, and c-Jun were gradually diminished at doses of 1, 5, and 10 μM acacetin when compared to the 0 μM after treatment for 12 h.
Effects of acacetin on the DNA-binding activities of NF-κB and AP-1 in DU145 cells. Cells were treated with various concentrations (0, 1, 5, and 10 μM) of acacetin for 12 h, and then nuclear extracts were prepared and analyzed for a NF-κB and AP-1 DNA-binding activity using biotin-labeled consensus NF-κB and AP-1 specific oligonucleotide. Then EMSA assay was performed as described in Materials and methods section. Lane 1 biotin-NF-κB or biotin-AP-1 oligonucleotide (probe), no nuclear extracts for probe to bind. Lane 2 nuclear extracts incubated with 100-fold excess unlabeled consensus oligonucleotide (comp.) to confirm the specificity of binding. Lane 3–6 biotin-NF-κB or biotin-AP-1 oligonucleotide plus various concentrations of acacetin. Excess free probe is indicated at the bottom. Results from three repeated and separated experiments were similar. b Cytoplamic extract and c nuclear extracts were also analyzed by Western blotting with anti-NF-κB (p65), c-Fos, and c-Jun antibodies. β-Actin and C23 were used as loading controls. Determined the protein expressions of NF-κB, c-Fos, and c-Jun were subsequently quantified by densitometric analysis with that of control being onefold. The densitometric results are expressed as mean ± SD of three independent experiments. * P < 0.05, ** P < 0.01, *** P < 0.001 compared with the untreated control (dose 0)
Acacetin inhibited the cell invasion and migration via an inactivation of the p38 MAPK signaling pathway
In order to further delineate whether the inhibition of cell invasion and migration by acacetin mainly occurred through an inhibition of the p38 MAPK signaling, DU145 cells were pretreated with a p38 inhibitor (SB203580; 40 μM) and then incubated in the with or without acacetin (1 μM) for 48 h. Compared with the control, the invasion and migration assays revealed sole treatment with acacetin or SB203580 decreased the cell invasion and migration by 53, 65% and 44, 63%, respectively, and the combination treatment (40 μM SB203580 + 1 μM myricetin) could further reduce the cell invasion and migration by 42% and 31%, respectively (Fig. 9a, b).
Effect of p38 MAPK inhibitor (SB203580) and acacetin on invasion (a) and migration (b) in DU145 cells. Cells were plated in 6-well and pre-treated with SB203580 (40 μM) for 1 h and then incubated in the presence or absence of acacetin (1 μM) for 48 h. Afterward, cells were subjected to analyse for invasion and migration as described in Materials and methods section. Data represented the mean ± SD of three independent experiments (* P < 0.01, ** P < 0.001)
Discussion
Prostate cancer continues to be a major problem in the developed world. Owing to the high mortality and unsatisfactory treatment options available, prostate cancer remains the most common malignancy and is a leading cause of cancer-related deaths among men. Prostate cancer usually progresses from androgen-dependent to -independent stage, making antiandrogen therapy ineffective leading to an increase in metastatic potential and incurable malignancy [31]. However, prostate cancer is highly resistant to chemotherapy and there is still no effective cure for patients with advanced stages of the disease. Thus, effective chemopreventive treatment for metastasis would have an important impact on prostate cancer mortality rates.
The lethality of most malignant tumors is the result of local invasion and metastasis from the primary tumors to other tissues. The prostate cancer cells spread by vascular metastasis, typically in the brain [3]. Metastasis has been found to be accompanied by various physiological alterations involved in degradation of ECM, such as the over-expression of proteolytic enzyme activity, such as u-PA and MMPs, as well as the invasion and migration of tumor cells into blood stream or lymphatic system to spread to another tissue or organ. Also, invasion and migration of cells are the important factors for cancer cell metastasis, it was reported that enhanced production of MMPs and u-PA is correlated with invasion, metastasis, and angiogenesis of the tumors [32–34]. Therefore, the impacts of acacetin on several proteases involved in ECM degradation were investigated in this study. We first explored the antimetastatic mechanism of acacetin on the invasion and migration of human DU145 prostate cancer cells, and found acacetin can inhibit the adhesion, invasion, and migration of the DU145 cells in vitro model. Also, to further explore the exact mechanism of acacetin-induced inhibition on the invasion and migration, we performed a set of experiments, including zymography, Western blot, and RT-PCR, to detect MMP-2, MMP-9, and u-PA at both the protein and mRNA levels. We demonstrated here that the expressions of MMP-2, MMP-9, and u-PA were reduced by acacetin treatment. Also, several studies have identified signal transduction pathways that are involved in regulation of MMP-2, MMP-9, and u-PA expressions in malignant cells. It is well known that the activation of p38 MAPK will stimulate two cis-acting regulatory elements including the binding sites of NF-κB and AP-1 which play an important role in controlling MMP-2, MMP-9, and u-PA gene expressions. [21, 35, 36]. Our present data have revealed acacetin treatment inhibited phosphorylation of p38 MAPK and concurrent reduction in the expression levels of MMP-2, MM-9, and u-PA, indication a possible mechanism of inhibition of MMP-2, MM-9, and u-PA synthesis by acacetin. The involvement of p38 MAPK signaling pathway was further supported by the use of p38 MAPK inhibitor in our experimental model. A treatment with an inhibitor specific for p38 MAPK could inhibit the MMP-2, MM-9, and u-PA secretions.
The transcription of MMPs and u-PA gene is regulated by upstream regulatory sequences, including NF-κB, AP-1, and Ets-1 binding sites [23, 37]. Therefore, our work provides insight into how acacetin suppressed the p38 MAPK signaling pathway and reduces NF-kB- and AP-1-binding activities in DU145 prostate cancer cells. Indeed, one or more of these binding sites have been implicated in mediating the effects of a diverse set of agents. Here, we have also found the treatment of acacetin to DU145 cells results in an inhibition of NF-kB- and AP-1-DNA binding activities and, in turn, reduced the MMP-2, MMP-9, and u-PA expressions.
Finally, the involvement of p38 MAPK signaling pathway in cell metastasis was further supported by experiment with p38 MAPK inhibitor, showing treatment with inhibitor of p38 MAPK to DU145 cells inhibited the cell invasion and migration. In summary, we demonstrated acacetin could inhibit the invasion and migration of DU145 cells in vitro via inactivation of the p38 MAPK signaling pathway. As shown from the above results, acacetin may be a powerful candidate in developing preventive agents for cancer metastasis.
Abbreviations
- MMPs:
-
Matrix metalloproteinases
- u-PA:
-
Urokinase-type plasminogen activator
- ECM:
-
Extracellular matrix
- ERK:
-
Extracellular signal-regulated kinase
- JNK/SAPK:
-
c-Jun N-terminal kinase/stress-activated protein kinase
- p38 MAPK:
-
p38 Mitogen-activated protein kinase
- PI3K:
-
Phosphoinositide 3-kinase
- NF-κB:
-
Nuclear factor kappa B
- AP-1:
-
Activator protein-1
- IκB:
-
Inhibitor of NF-κB
References
Greenlee RT, Murray T, Bolden S, Wingo PA (2000) Cancer statistics. CA Cancer J Clin 50:7–33
Yim D, Singh RP, Agarwal C, Lee S, Chi H, Agarwal R (2005) A novel anticancer agent, decursin, induces G1 arrest and apoptosis in human prostate carcinoma cells. Cancer Res 65:1035–1044
Sandberg L, Papareddy P, Silver J, Bergh A, Mei YF (2009) Replication-competent Ad11p vector (RCAd11p) efficiently transduces and replicates in hormone-refractory metastatic prostate cancer cells. Hum Gene Ther 20:361–373
Kraft C, Jenett-Siems K, Siems K, Jakupovic J, Mavi S, Bienzle U, Eich E (2003) In vitro antiplasmodial evaluation of medicinal plants from Zimbabwe. Phytother Res 17:123–128
Pan MH, Lai CS, Wang YJ, Ho CT (2006) Acacetin suppressed LPS-induced up-expression of iNOS and COX-2 in murine macrophages and TPA-induced tumor promotion in mice. Biochem Pharmacol 72:1293–1303
Yin Y, Gong FY, Wu XX, Sun Y, Li YH, Chen T, Xu Q (2008) Anti-inflammatory and immunosuppressive effect of flavones isolated from Artemisia vestita. J Ethnopharmacol 120:1–6
Hsu YL, Kuo PL, Lin CC (2004) Acacetin inhibits the proliferation of HepG2 by blocking cell cycle progression and inducing apoptosis. Biochem Pharmacol 67:823–829
Hsu YL, Kuo PL, Liu CF, Lin CC (2004) Acacetin-induced cell cycle arrest and apoptosis in human non-small cell lung cancer A549 cells. Cancer Lett 212:53–60
Pan MH, Lai CS, Hsu PC, Wang YJ (2005) Acacetin induces apoptosis in human gastric carcinoma cells accompanied by activation of caspase cascades and production of reactive oxygen species. J Agric Food Chem 53:620–630
Shim HY, Park JH, Paik HD, Nah SY, Kim DS, Han YS (2007) Acacetin-induced apoptosis of human breast cancer MCF-7 cells involves caspase cascade, mitochondria- mediated death signaling and SAPK/JNK1/2-c-Jun activation. Mol Cells 24:95–104
Singh RP, Agrawal P, Yim D, Agarwal C, Agarwal R (2005) Acacetin inhibits cell growth and cell cycle progression, and induces apoptosis in human prostate cancer cells: structure-activity relationship with linarin and linarin acetate. Carcinogenesis 26:845–854
Weiss L (1990) Metastatic inefficiency. Adv Cancer Res 54:159–211
Huang SC, Ho CT, Lin-Shiau SY, Lin JK (2005) Carnosol inhibits the invasion of B16/F10 mouse melanoma cells by suppressing metalloproteinase-9 through down-regulating nuclear factor-kappa B and c-Jun. Biochem Pharmacol 69:221–232
Bernhard EJ, Gruber SB, Muschel RJ (1994) Direct evidence linking expression of matrix metalloproteinase 9 (92-kDa gelatinase/collagenase) to the metastatic phenotype in transformed rat embryo cells. Proc Natl Acad Sci USA 91:4293–4297
Duffy MJ, Duggan C (2004) The urokinase plasminogen activator system: a rich source of tumour markers for the individualized management of patients with cancer. Clin Biochem 37:541–548
Itoh Y, Nagase H (2002) Matrix metalloproteinases in cancer. Essays Biochem 38:21–36
Chan-Hui PY, Weaver R (1998) Human mitogen-activated protein kinase kinase kinase mediates the stress-induced activation of mitogen-activated protein kinase cascades. Biochem J 336:599–609
Trusolino L, Comoglio PM (2002) Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nat Rev Cancer 2:289–300
Chen PN, Hsieh YS, Chiou HL, Chu SC (2005) Silibinin inhibits cell invasion through inactivation of both PI3K-Akt and MAPK signaling pathways. Chem Biol Interact 156:141–150
Kwon GT, Cho HJ, Chung WY, Park KK, Moon A, Park JH (2008) Isoliquiritigenin inhibits migration and invasion of prostate cancer cells: possible mediation by decreased JNK/AP-1 signaling. J Nutr Biochem (in press)
Lee SJ, Park SS, Lee US, Kim WJ, Moon SK (2008) Signaling pathway for TNF-alpha -induced MMP-9 expression: mediation through p38 MAP kinase, and inhibition by anti-cancer molecule magnolol in human urinary bladder cancer 5637 cells. Int Immunopharmacol 8:1821–1826
Nagase H, Woessner JF Jr (1999) Matrix metalloproteinases. J Biol Chem 274:21491–21494
Sliva D (2004) Signaling pathways responsible for cancer cell invasion as targets for cancer therapy. Curr Cancer Drug Targets 4:327–336
Westermarck J, Kahari VM (1999) Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J 13:781–792
Kunnumakkara AB, Anand P, Aggarwal BB (2008) Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett 269:199–225
Jiang J, Grieb B, Thyagarajan A, Sliva D (2008) Ganoderic acids suppress growth and invasive behavior of breast cancer cells by modulating AP-1 and NF-kappaB signaling. Int J Mol Med 21:577–584
Lee SO, Jeong YJ, Im HG, Kim CH, Chang YC, Lee IS (2007) Silibinin suppresses PMA-induced MMP-9 expression by blocking the AP-1 activation via MAPK signaling pathways in MCF-7 human breast carcinoma cells. Biochem Biophys Res Commun 354:165–171
Hoppe-Seyler F, Butz K, Rittmuller C, von Knebel Doeberitz M (1991) A rapid microscale procedure for the simultaneous preparation of cytoplasmic RNA, nuclear DNA binding proteins and enzymatically active luciferase extracts. Nucleic Acids Res 19:5080
Rao JS (2003) Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer 3:489–501
Turner NA, Aley PK, Hall KT, Warburton P, Galloway S, Midgley L, O’regan DJ, Wood IC, Ball SG, Porter KE (2007) Simvastatin inhibits TNFalpha-induced invasion of human cardiac myofibroblasts via both MMP-9-dependent and -independent mechanisms. J Mol Cell Cardio 43:168–176
Aquilina JW, Lipsky JJ, Bostwick DG (1999) Androgen deprivation as a strategy for prostate cancer chemoprevention. J Natl Cancer Inst 89:689–696
Kim D, Kim S, Koh H, Yoon SO, Chung AS, Cho KS, Chung J (2001) Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J 15:1953–1962
Kleiner DE, Stetler-Stevenson WG (1999) Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol 43:S42–S51
Stetler-Stevenson WG, Aznavoorian S, Liotta LA (1993) Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol 9:541–573
Gum R, Lengyel E, Juarez J, Chen JH, Sato H, Seiki M, Boyd D (1996) Stimulation of 92-kDa gelatinase B promoter activity by ras is mitogen-activated protein kinase kinase 1-independent and requires multiple transcription factor binding sites including closely spaced PEA3/ets and AP-1 sequences. J Biol Chem 271:10672–10680
Hung SH, Shen KH, Wu CH, Liu CL, Shih YW (2009) α-Mangostin suppresses PC-3 human prostate carcinoma cell metastasis by inhibiting matrix metalloproteinase-2/9 and urokinase-plasminogen activator expression through the JNK signaling pathway. J Agric Food Chem 57:1291–1298
Rothhammer T, Hahne JC, Florin A, Poser I, Soncin F, Wernert N, Bosserhoff AK (2004) The Ets-1 transcription factor is involved in the development and invasion of malignant melanoma. Cell Mol Life Sci 61:118–128
Acknowledgment
This work was supported by the grant from the Subsidized Project of the Chung Hwa University, Tainan, Taiwan (97-HT-08008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, KH., Hung, SH., Yin, LT. et al. Acacetin, a flavonoid, inhibits the invasion and migration of human prostate cancer DU145 cells via inactivation of the p38 MAPK signaling pathway. Mol Cell Biochem 333, 279–291 (2010). https://doi.org/10.1007/s11010-009-0229-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0229-8