Abstract
Genetic variations of the human RETN gene are associated with metabolic phenotypes, including obesity, insulin sensitivity, diabetes, and coronary heart disease (CHD). There are few studies of two gene variants, −394C>G and −420C>G, in Chinese population. This study investigated the distribution of RETN gene, single nucleotide polymorphisms (SNPs), in Chinese Han population and the association of the polymorphisms with type 2 diabetes mellitus (T2DM) and CHD. In a population-based, case–control genetic association study, a total of 961 subjects were recruited from the community, including 318 T2DM patients, 273 CHD patients, and 370 unrelated healthy control individuals. Serum lipid levels were detected. Two SNPs of RETN gene, −394C>G and −420C>G, were genotyped by PCR-RFLP. Unknown Polymorphisms were screened with the technique of denaturing high performance liquid chromatography (DHPLC). The frequencies of RETN −394G allele in T2DM group, CHD group, and control group were 0.3066, 0.3555, and 0.3481, respectively, which are met with the Hardy–Weinberg equilibrium. There is a significant difference of the comparison of sex in T2DM group of RETN gene SNP-394C>G (P < 0.05). Compared with controls, there was no significant difference in the distribution of genotypes and allele frequencies of −394C>G polymorphic site in T2DM patients and CHD patients, respectively. No direct association was found between the −394C>G polymorphism and T2DM or CHD. The frequencies of RETN −420G allele in T2DM group, CHD group, and control group were 0.4009, 0.3725, and 0.3859, respectively, which are met with the Hardy–Weinberg equilibrium. The frequencies of RETN −420G allele in T2DM groups and control groups of Chinese population are significantly different from those in European population (0.40 vs. 0.27, 0.39 vs. 0.26) (P < 0.01). Compared with controls, there was no significant difference in distribution of genotypes and allele frequencies of −420C>G polymorphic site in T2DM patients and CHD patients, respectively. No direct association was found between the −420C>G polymorphism and T2DM or CHD. In addition, we found new potential SNP +593G>C in exon 3 of RETN gene using DHPLC. The RETN gene exhibits sex and ethnic differences. +593G>C of RETN gene might be a new potential SNP in exon 3 of RETN gene. Association between SNP −394C>G and −420C>G of RETN gene with T2DM and CHD in Chinese needs more exploration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2001, Steppan et al. [1] reported that they discovered a cystein-rich polypeptide hormone, termed as RETN, which was secreted by adipocytes in response to hypoglycemic drugs like thiazolidinediones (TZDs). The human resistin gene, namely RETN gene, is located in 19p13.3 with a whole length of 1,369 bp, including four exons and three introns [2].
The RETN gene is a potential candidate for the etiology of insulin resistance and type 2 diabetes and has been implicated as the molecular link between type 2 diabetes and obesity [1]. Burnett et al. [3, 4] found that patients with premature coronary artery disease have higher plasma RETN levels compared to individuals with angiographically normal coronary arteries as well as prevalent CHD associated with higher resistin levels. There are several SNPs which are distinguished by different countries and populations located in the RETN gene. Meanwhile, the association of the two RETN genes SNPs, −394C>G and −420C>G, with T2DM and CHD in Chinese has not been known so far. We detected the distributions of the two RETN gene SNPs, −394C>G and −420C>G, in Chinese Han population and the relation of this polymorphism to T2DM and CHD by PCR-RFLP.
In addition, we took advantage of a highly sensitive and specific automated denaturing high performance liquid chromatography (DHPLC) technique to detect fragments that may harbor mutations prior to sequencing.
Subjects and methods
Subjects
Three hundred and seventy control subjects were unrelated age- and gender-matched individuals (Han people), selected via health screening at the Outpatient Department of West China Hospital of Sichuan University (224 males, 146 females, and age 58.8 ± 9.2 years old), and with exclusion of coronary vascular diseases and diabetes mellitus. None of the subjects were taking any medication. Three hundred and eighteen T2DM patients (179 males, 139 females, and age 58.2 ± 11.4 years old) diagnosed by the WHO criteria for diabetes mellitus from Department of Endocrinology of West China Hospital, Sichuan University were selected. 273 CHD patients (177 males, 96 females, age 60.4 ± 8.6 years old) with significant coronary stenosis by cardiovascular angiography (according to stenos ≥50%, at least one coronary artery) from the Department of Cardiology of West China Hospital, Sichuan University were selected. All the subjects mentioned above were unrelated Han people. The informed consent forms were obtained from all subjects studied. The study was approved by the Internal Ethical Review Board of West China Hospital.
Plasma lipid measurements
The blood sample after 12-h fasting was collected in a tube for measuring lipid. Triglycerides (TG), total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C), and low-density lipoprotein-cholesterol (LDL-C) of all subjects were measured by Automatic Biochemistry Analyzer (Olympus 5400) and GmbH diagnostic kit (Roche).
PCR amplification
Genomic DNA was extracted from peripheral blood by salting out method, stored at 4°C. PCR primers were designed according to the reference [5]. For SNP −394C>G, a 561 bp sequence of the RETN gene was amplified by PCR in a DNA Thermal Cycler (PE 9600), using the upstream primer 5′-GCACCATGCCTAGCAAGAG-3′ and the downstream primer 5′-GCTGAAAGAGGGAACCAAGAG-3′. For SNP −420C>G, a 533 bp sequence of the RETN gene was amplified by PCR in a DNA Thermal Cycler (PE 9600), using the upstream primer 5′-TGTCATTCTCACCCAGAGACA-3′ and the downstream primer 5′-TGGGCTCAGCTAACCAAATC-3′. DNA templates were pre-denatured at 94°C for 5 min and then each PCR was subjected to 35 cycles with a temperature cycle consisting of 30 s of denaturation at 94°C, 30 s of annealing at 59°C, and 30 s of extension at 72°C, the last step was 10 min of extension at 72°C.
SNP genotyping
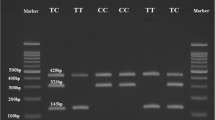
The PCR products (10 μl) of SNP −394C>G were digested with five units of Eam1104I at 37°C overnight, and the one of SNP −420C>G was digested with five units of BpiI at 37°C overnight. The fragments were separated by electrophoresis on 3% agarose gel. The DNA fragments were visualized by the gel analytic system (BIO-RAD Gel Doc 2000) and genotypes were determined. For SNP −394C>G, fragments of 561 bp for the CC wild homozygote (absence of Eam1104I cutting site) and of 150 and 411 bp for the GG mutant homozygote (presence of Eam1104I cutting site) were produced. The heterozygote CG includes three fragments of 150, 411, and 561 bp. For SNP −420C>G, fragments of 533 bp for the GG wild homozygote (absence of BpiI cutting site) and of 204 and 329 bp for the CC mutant homozygote (presence of BpiI cutting site) were produced. The heterozygote CG includes three fragments of 204, 329, and 533 bp.
Screening the new potential polymorphisms using DHPLC
Based on the sequence NT_011145, we designed seven pairs of primers of RETN gene by Primer 5.0. The amplified fragments include RETN gene’s exons and introns. The PCR fragments were applied to a preheated reversed-phase column. An elution gradient was generated by mixing buffer A (0.1 mmol/l EDTA) and buffer B (0.1 mol/l TEAA, 0.1 mmol/l EDTA, 25% acetonitrile). The predicted optimal column temperatures and elution gradient for the DHPLC analysis of each PCR amplicon were verified by confirming that the elution profile of each of the PCR amplicons generated from wild-type genomic DNA had a sharp and solitary peak. According to the different fragments’ detecting temperature and lasting time, the automated program will control the gradient concentration of mixing buffers A and B in 1.5 ml/min velocity of flow to screen the PCR fragments sequence. Inspection of the DNA in a 260-nm wavelength will be transmitted into the DHPLC chromatogram.
Statistical analysis
Statistical analysis was performed with the SPSS 11.0 statistical software package (SPSS Inc., Chicago, IL). Observed frequencies in each of the three groups (T2DM, CHD, and control) were compared to the expected frequencies (Hardy–Weinberg equilibrium) by χ 2 test of HWE software. The distribution of genotypes and allele frequencies in T2DM, CHD, and control group was analyzed by χ 2 test. The difference of lipid levels in different genotypes of T2DM, CHD, and control group was evaluated by the one-way analysis of variance (ANOVA) test and LSD test. The association of RETN gene SNPs with T2DM and CHD was analyzed by binary logistic regression. The comparison of sex in T2DM was analyzed by χ 2 test.
Results
Genotypes and allele distributions of SNP −394C>G and −420C>G polymorphic sites in study population.
Table 1 shows the genotypes and allele distributions of SNP −394C>G and −420C>G polymorphic sites in the study population. The genotype distribution in each group was met with Hardy–Weinberg equilibrium. There was no significant difference of the genotypes and allele distributions in T2DM and CHD, compared with the control group, respectively (P > 0.05).
Comparison of sex in T2DM of RETN gene SNP −394C>G
Table 2 shows the genotypes distributions of SNP −394C>G polymorphic sites in T2DM group. It exhibits significant differences of the genotype distributions in female and male groups with T2DM (P < 0.05). There are obviously more women with the CG genotype than men in T2DM group.
Association of RETN gene SNP −394C>G and −420C>G with T2DM and CHD in study population
Table 3 shows the odds ratio (OR) and 95% confidence interval (CI) of RETN gene SNP −394C>G and −420C>G to T2DM and CHD. It shows that SNP −394C>G and −420C>G have no direct association with T2DM and CHD, respectively.
Comparison of plasma lipids levels in three genotypes in study population
Table 4 displays the comparison of lipid levels of three genotypes in three groups for RETN gene SNP −394C>G and −420C>G. No significant difference of lipid levels among three genotypes was found in both T2DM and CHD patients for two SNP sites (P > 0.05).
DHPLC chromatograms
Table 5 shows the PCR primers and the amplified fragments. Table 6 shows the DHPLC analysis conditions (temperature and concentration). Homozygote genomic DNA has a sharp and solitary peak contrast to heterozygote having double or anomalous peak (Figs. 1, 2).
PCR products sequence map
Based on the initial detection by DHPLC, five mutational samples were selected to be sequenced directly. And the results showed that 100% genotypes were consistent with their previous ones (Figs. 3, 4).
The new potential SNP +585C>T in exon 3 of resistin gene. This SNP is also reported by Wang et al. [5] in Caucasians population
Discussion
This is, to our knowledge, the first study to analyze and identify an association in two loci, the −394C>G and SNP −420C>G polymorphisms, with T2DM, respectively, and find new potential SNP in exon 3 of RETN gene using DHPLC in West China. A search for genes that are downregulated by thiazolidinediones (TZDs) in mouse adipocytes led to the discovery of an adipose-specific secreted protein called resistin. Since Steppan et al. [1] reported that resistin gene, RETN, was discovered in 2001, it has attracted many scientists to research RETN gene. Resistin is a newly described circulating protein with no homology to any known hormone, cytokine, or other intercellular signaling molecule. It is secreted specifically by adipocytes and has actions that antagonize insulin action [6]. At the amino acid level, the human resistin proteins and that of the mouse exhibit 59% identity [2]. Reilly et al. [7] found that plasma resistin levels were associated with markers of inflammation, but not insulin resistance, both in SIRCA, a study of asymptomatic nondiabetic subjects, and in a type 2 diabetic sample. Furthermore, they found that resistin levels were significantly associated with coronary atherosclerosis in SIRCA even after control for multiple established risk factors and the presence of the metabolic syndrome.
The convergence of insulin resistance and inflammation in the pathogenesis of atherosclerotic CVD have been recognized over the last decade [8–11]. In Chinese Han population of Sichuan province, we analyzed the genotypes and allele frequencies of RETN gene SNP −394C>G in 318 T2DM patients, 273 CHD patients, and 370 unrelated healthy control individuals. There was no significant difference in the distribution of genotypes and allele frequencies of −394C>G polymorphic site in T2DM patients and CHD patients, respectively. In other studies, Wang et al. [5] reported in Caucasians population, the frequencies of minor allele G of RETN gene SNP −394C>G in T2DM group and control group were 0.36 and 0.29, respectively, in agreement with our findings (Table 1) in Chinese Han population. Besides, our result shows a significant difference in the comparison between male individuals and female individuals of T2DM group of RETN gene SNP −394C>G (P < 0.05). This findings suggest that the sex may play an important role in RETN gene SNP −394C>G in type 2 diabetes. As is known, T2DM is a complex metabolic disorder in which endogenous sex hormones may contribute to sex-dependent etiologies. We hypothesized that genetic variants related to T2DM might differ between men and women. We thus performed an association study to identify gene polymorphisms associated with T2DM in men and women separately. Our result revealed that the RETN gene −394 CG heterozygote was significantly associated with the prevalence of T2DM in women compared to the CC and GG homozygotes. That is to say, women with the CG genotype are more apt to develop T2DM. Further studies are needed to address the mechanism of RETN gene SNP −394C>G in humans.
We also analyzed the genotype and allele frequencies of RETN gene SNP −420C>G in 318 T2DM patients, 273 CHD patients and 370 unrelated healthy control individuals. Compared with controls, there was no significant difference in the distribution of genotype and allele frequencies of −420C>G polymorphic site in T2DM patients and CHD patients, respectively. However, our result is so close to the one of Cho [12] found in Korea. In addition, the frequencies of G allele in T2DM groups and control groups of Chinese population are significantly different from those in European population [13] (0.40 vs. 0.27, 0.39 vs. 0.26) (P < 0.01). We can draw a conclusion that the distributions of −420C>G of RETN gene exhibit ethnic difference.
Resistin was initially suggested to be a link between obesity and insulin resistance in rodents [1, 6]. Evidence to confirm this in human has been raised by several study teams recently [1, 5, 12, 14–20]. Moreover, resistin expression was found to be abundant in monocytes/macrophages [19–21], which play an important role in atherosclerosis [22]. These cells infiltrate arteries and initiate or promote atherogenesis by secreting various pro-inflammatory cytokines [10]. Since resistin is expressed mainly in inflammatory cells [20, 21], regulated by and regulating inflammatory cytokines [23, 24], and increased in serum in subclinical inflammatory conditions [7, 25–30], it may be a link between inflammation and atherosclerosis. However, we cannot exclude the possibility that resistin might also be a contributory factor to insulin resistance or metabolic syndrome. The association between resistin and atherosclerosis remains controversial. Steppan et al. [18] also found resistin circulates in the mouse, with increased levels in obesity, and has effects on glucose homeostasis that oppose those of insulin. Resistin is a potential link among TZDs, obesity and insulin resistance in the mouse. Thus, resistin is a possible major risk factor for type 2 diabetes mellitus. Recently, high concentrations of resistin were shown to induce vascular endothelial dysfunction and vascular smooth muscle cell proliferation. Resistin secreted from macrophages may contribute to atherogenesis by virtue of its effects on vascular endothelial cells and smooth muscle cells in humans [31]. Patients diagnosed with premature coronary artery disease (PCAD) were found to have higher serum levels of resistin than normal controls. Resistin protein is present in both murine and human atherosclerotic lesions, and mRNA levels progressively increase in the aortas of mice developing atherosclerosis. Resistin induced increases in monocyte chemoattractant protein (MCP)-1 and soluble vascular cell adhesion molecule (sVCAM)-1 protein expression in murine vascular endothelial cells, suggesting a possible mechanism by which resistin might contribute to atherogenesis. PCAD patients exhibited increased serum levels of resistin when compared to controls [3]. Plasma resistin levels are correlated with markers of inflammation and are predictive of coronary atherosclerosis in humans, independent of plasma C-reactive protein (CRP). Resistin may represent a novel link among metabolic signals, inflammation, and atherosclerosis [7]. Satoh et al. [32] found that chronic hyper-resistinemia leads to whole-body insulin resistance involving impaired insulin signaling in skeletal muscle, liver, and adipose tissue, resulting in glucose intolerance, hyperinsulinemia, and hypertriglyceridemia.
Mutation analysis by DHPLC involves subjecting PCR products to ion-pair reversed-phase liquid chromatography in a column containing alkylated nonporous particles. At the proper temperature, the heteroduplexes formed in PCR samples with internal sequence variations have a lower column retention time relative to their homoduplex counterparts. For analysis of autosomal recessive and X-linked recessive disorders, genomic DNA from normal individuals is added to each well so that a heteroduplex is formed if the patients are homozygous or hemizygous for the mutant allele. The major advantages of this method include rapid analysis and automated instrumentation [33]. In our study, two mutant alleles, +593G>C and +585C>T in exon 3 of RETN gene were checked out using DHPLC and confirmed by direct sequencing. Since one of the mutation has been reported by Wang et al. [5] already, We found new potential SNP +593G>C in exon 3 of RETN gene. Further experiments and large population sample investigations are needed to fully confirm this result.
In summary, our data suggest that no direct association was found in the two loci, −394C>G and −420C>G, polymorphism with T2DM or CHD in Chinese Han population. However, there is a significant gender difference in the association of gene polymorphisms with T2DM. Ethic difference was also found in the RETN gene. In addition, new potential SNP +593G>C in exon 3 of RETN gene may exist according to our detection using DHPLC. Further functional studies are needed to identify the actual biological role of RETN gene polymorphisms.
References
Steppan CM, Bailey ST, Bhat S et al (2001) The hormone RETN links obesity to diabetes. Nature 409:307–312. doi:10.1038/35053000
Ghosh S, Singh AK, Aruna B et al (2003) The genomic organization of mouse RETN reveals major differences from the human RETN: functional implications. Gene 13:27–34. doi:10.1016/S0378-1119(02)01213-1
Burnett MS, Lee CW, Kinnaird TD et al (2005) The potential role of RETN in atherogenesis. Atherosclerosis 182:241–248. doi:10.1016/j.atherosclerosis.2005.02.014
Burnett MS, Devaney JM, Adenika RJ et al (2006) Cross-sectional associations of resistin, coronary heart disease, and insulin resistance. J Clin Endocrinol Metab 91:64–68. doi:10.1210/jc.2005-1653
Wang H, Chu WS, Hemphill C et al (2002) Human RETN gene: molecular scanning and evaluation of association with insulin sensitivity and type 2 diabetes in caucasians. J Clin Endocrinol Metab 87:2520–2524. doi:10.1210/jc.87.6.2520
Steppan CM, Brown EJ, Wright CM et al (2001) A family of tissue-specific RETN-like molecules. Proc Natl Acad Sci USA 98:502–506. doi:10.1073/pnas.98.2.502
Reilly MP, Lehrke M, Wolfe ML et al (2005) RETN is an inflammatory marker of atherosclerosis in humans. Circulation 111:932–939. doi:10.1161/01.CIR.0000155620.10387.43
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (2001) Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 285:2486–2497. doi:10.1001/jama.285.19.2486
Reaven GM (1988) Banting lecture: role of insulin resistance in human disease. Diabetes 37:1595–1607. doi:10.2337/diabetes.37.12.1595
Ross R (1999) Atherosclerosis: an inflammatory disease. N Engl J Med 340:115–126. doi:10.1056/NEJM199901143400207
Festa A, D Agostino R Jr, Howard G et al (2000) Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation 102:42–47
Conneely KN, Silander K, Scott LJ et al (2004) Variation in the RETN gene is associated with obesity and insulin-related phenotypes in Finish subjects. Diabetologia 47:1782–1788. doi:10.1007/s00125-004-1537-x
Cho YM, Youn BS, Chung SS et al (2004) Common genetic polymorphisms in the promoter of RETN gene are major determinants of plasma RETN concentrations in humans. Diabetologia 47:559–565
Duman BS, Cagatay P, Hatemi H et al (2007) Association of resistin gene 3′-untranslated region EX4-44G→A polymorphism with obesity- and insulin-related phenotypes in turkish type 2 diabetes patients. Rev Diabet Stud 4:49–55. doi:10.1900/RDS.2007.4.49
Krízová J, Dolinková M, Lacinová Z et al (2007) Adiponectin and resistin gene polymorphisms in patients with anorexia nervosa and obesity and its influence on metabolic phenotype. Physiol Res 57:539–546
Bouchard L, Weisnagel SJ, Engert JC et al (2004) Human resistin gene polymorphism is associated with visceral obesity and fasting and oral glucose stimulated C-peptide in the Québec Family Study. J Endocrinol Invest 27:1003–1009
Pfützner A, Langenfeld M, Kunt T et al (2003) Evaluation of human resistin assays with serum from patients with type 2 diabetes and different degrees of insulin resistance. Clin Lab (Zaragoza) 49:571–576
Steppan CM, Lazar MA (2002) Resistin and obesity-associated insulin resistance. Trends Endocrinol Metab 13:18–23. doi:10.1016/S1043-2760(01)00522-7 Review
Savage DB, Sewter CP, Klenk ES et al (2001) RETN/Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-gamma action in humans. Diabetes 50:2199–2202. doi:10.2337/diabetes.50.10.2199
Yang RZ, Huang Q, Xu A et al (2003) Comparative studies of RETN expression and phylogenomics in human and mouse. Biochem Biophys Res Commun 310:927–935. doi:10.1016/j.bbrc.2003.09.093
Patel L, Buckels AC, Kinghorn IJ et al (2003) RETN is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun 300:472–476. doi:10.1016/S0006-291X(02)02841-3
Plutzky J (2001) Inflammatory pathways in atherosclerosis and acute coronary syndromes. Am J Cardiol 88:10K–15K. doi:10.1016/S0002-9149(01)01924-5
Lehrke M, Reilly MP, Millington SC et al (2004) An inflammatory cascade leading to hyperRETNemia in humans. PLoS Med 1:e45. doi:10.1371/journal.pmed.0010045
Bokarewa M, Nagaev I, Dahlberg L et al (2005) RETN, an adipokine with potent proinflammatory properties. J Immunol 174:5789–5795
Youn BS, Yu KY, Park HJ et al (2004) Plasma RETN concentrations measured by enzyme-linked immunosorbent assay using a newly developed monoclonal antibody are elevated in individuals with type 2 diabetes mellitus. J Clin Endocrinol Metab 89:150–156. doi:10.1210/jc.2003-031121
Fujinami A, Obayashi H, Ohta K et al (2004) Enzyme-linked immunosorbent assay for circulating human RETN: RETN concentrations in normal subjects and patients with type 2 diabetes. Clin Chim Acta 339:57–63. doi:10.1016/j.cccn.2003.09.009
Zhang JL, Qin YW, Zheng X et al (2003) Serum RETN level in essential hypertension patients with different glucose tolerance. Diabet Med 20:828–831. doi:10.1046/j.1464-5491.2003.01057.x
McTernan PG, Fisher FM, Valsamakis G et al (2003) RETN and type 2 diabetes: regulation of RETN expression by insulin and rosiglitazone and the effects of recombinant RETN on lipid and glucose metabolism in human differentiated adipocytes. J Clin Endocrinol Metab 88:6098–6106. doi:10.1210/jc.2003-030898
Al-Daghri N, Chetty R, McTernan PG et al (2005) Serum RETN is associated with C-reactive protein and LDL cholesterol in type 2 diabetes and coronary artery disease in a Saudi population. Cardiovasc Diabetol 4:10. doi:10.1186/1475-2840-4-10
Shetty GK, Economides PA, Horton ES et al (2004) Circulating adiponectin and RETN levels in relation to metabolic factors, inflammatory markers, and vascular reactivity in diabetic patients and subjects at risk for diabetes. Diabetes Care 27:2450–2457. doi:10.2337/diacare.27.10.2450
Jung HS, Park KH, Cho YM et al (2006) RETN is secreted from macrophages in atheromas and promotes atherosclerosis. Cardiovasc Res 69:76–85. doi:10.1016/j.cardiores.2005.09.015
Satoh H, Nguyen MT, Miles PD et al (2004) Adenovirus-mediated chronic “hyper-RETNemia” leads to in vivo insulin resistance in normal rats. J Clin Invest 114:224–231
Aramaki M, Udaka T, Torii C et al (2006) Screening for CHARGE syndrome mutations in the CHD7 gene using denaturing high-performance liquid chromatography. Genet Test 10:244–251. doi:10.1089/gte.2006.10.244
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 30671155) and the Chinese High Tech Programs (863) from the Ministry of Science and Technology (No. 2002BA711A08). We gratefully acknowledge the medical genetics laboratory in Sichuan University for technical support and the endocrinal department in West China Hospital for assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chi, S., Lan, C., Zhang, S. et al. Association of −394C>G and −420C>G polymorphisms in the RETN gene with T2DM and CHD and a new potential SNP might be exist in exon 3 of RETN gene in Chinese. Mol Cell Biochem 330, 31–38 (2009). https://doi.org/10.1007/s11010-009-0097-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0097-2