Abstract
To elucidate the significance of mitochondrial localization of Cu/Zn-SOD (SOD1), we studied the relationship between the release of mitochondrial SOD1 and apoptosis. Kinetic analysis using HL-60 cells showed that both mitochondria-dependent and mitochondria-independent pro-apoptotic drugs, such as staurosporine and actinomycin D, increased the generation of reactive oxygen species (ROS) and decreased mitochondrial membrane potential (Δψ). ROS generation by these drugs was inhibited by Mn (III) tetrakis (5,10,15,20-benzoic acid) porphyrin (MnTBAP), a cell membrane-permeable SOD mimetic. However, MnTBAP inhibited the apoptosis induced by staurosporine but not by actinomycin D. MnTBAP failed to inhibit Δψ decrease and release of SOD1 and cytochrome c induced by actinomycin D. Moreover, 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS), an inhibitor of voltage-dependent anion channel (VDAC), inhibited the release of the two proteins and apoptosis induced by staurosporine but not actinomycin D. These results suggest that ROS plays an important role in mitochondria-dependent but not mitochondria-independent apoptosis and that the release of SOD1 increases the susceptibility of mitochondria to oxidative stress, thereby enhancing a vicious cycle leading to apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apoptosis is characterized by cell shrinkage, cell surface blebbing, DNA fragmentation, and nuclear breakdown [1, 2]. Apoptotic stimuli generally induce the opening of membrane permieablity transition (MPT) pores and swelling followed by release of pro-apoptotic proteins from the intermembranous space of mitochondria [3–5]. One such mitochondrial protein is cytochrome c, which is released into the cytosol in an early stage of apoptosis, forming an Apaf complex leading to activation of the caspase cascade [6, 7]. We previously reported that SOD1 localizes as a bound form in mitochondria of the brain and liver, and in various types of cells [8]. Neither cytochrome c nor SOD1 contains typical N-terminal presequences for mitochondrial uptake, yet both of them reside in the intermembranous space. Okado-Matsumoto et al. [9] also showed that SOD1 is localized in intermembranous space of rat liver mitochondria. Therefore, we investigated cellular distribution of SOD1 and cytochrome C during the process of apoptosis of human promyelocytic leukemia cells (HL-60).
The overproduction of superoxide and ROS induces oxidative stress, which is known to cause various forms of apoptosis [10–12]. In addition, the release of cytochrome c and consequent disruption of the electron transport chain also enhance generation of ROS [13]. Since superoxide is released into both sides of mitochondrial inner membranes [14, 15], removing superoxide in and around mitochondria would be very important in preventing apoptosis and cell death. The mitochondrial localization of the SOD1 seems to be required in protecting cells against mitochondria-derived oxidative damage and apoptosis. To elucidate the importance of subcellular localization of SOD1 in apoptosis, we investigated by using HL-60 cells the relationship between oxidative stress and release of the enzyme during apoptosis, and demonstrated that oxidative stress-triggered SOD1 release plays a critical role in mitochondria-dependent rather than mitochondria-independent apoptosis.
Materials and methods
Cell culture
HL-60 cells were grown in RPMI-1640 medium supplemented with 100 units/ml penicillin, 100 μg/ml streptomycin, and 10% heat inactivated fetal bovine serum (FBS). Cell culture was performed at 37°C and 5% CO2 atmosphere.
Chemicals
RPMI-1640, N-ethylmaleimide (NEM), and propidium iodide (PI) were purchased from Sigma-Aldrich (St. Louis, MO). Staurosporine, actinomycin D, and DIDS were obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). MnTBAP was obtained from Calbiochem (San Diego, CA). Mouse anti-cytochrome c and sheep anti-SOD1 antibodies were purchased from PharMingen and Calbiochem (San Diego, CA), respectively. Rabbit anti-DNP/HRP antibody, HRP-conjugated anti-goat IgG, anti-mouse IgG, and anti-rabbit IgG were purchased from DAKO Glostrup (Denmark). Immunoprecipitation Starter Pack and an ECL kit, and 2′,7′-dichlorofluorescein diacetate (DCFH-DA) and 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazol-carbocyanine iodide (JC-1) were obtained from Amersham Biosciences (UK) and Molecular Probes (Eugene, OR), respectively. Annexin V-APC was obtained from BD Pharmingen (San Diego, CA).
Assay for ROS generation using DCFH-DA
The intracellular generation of ROS was measured by using oxidation-sensitive fluorescent dye DCFH-DA [16]. HL-60 cells (1 × 106) were washed with ice-cold PBS and centrifuged at 1000 rpm for 5 min at 4°C. The cells were resuspended in 1 ml PBS and then 1 μl of DCFH-DA (5 mM in DMSO) was added to the cell suspension and incubated in a water-bath (37°C) for 15 min. Subsequently, the cells were kept on ice and analyzed with flow cytometry using FACS Calibur (Becton Dickenson Laboratory). Data analysis was performed using BD CELL Quest software.
Assay for mitochondrial membrane potential (Δψ) using JC-1
Mitochondrial membrane potential (Δψ) was monitored by using a lipophilic cationic probe JC-1 as described previously in detail [17]. HL-60 cells (1 × 106) were suspended in 1 ml of RPMI-1640 medium, and loaded with JC-1 (10 μM) for 10 min at 37°C. Subsequently, the cells were detected with flow cytometry using FACS Calibur (Becton Dickenson Laboratory). Both green (FL-1) and red (FL-2) fluorescence were recorded, and Δψ was determined from the ratio of red/green (FL-1/FL-2).
Cytosolic and mitochondrial protein extraction
Both cytosolic and mitochondrial fractions were isolated from HL-60 cells (1 × 106) after washing in ice-cold PBS for 5 min at 1700 rpm. The washed cells were lysed using 100 μl of CLAMI (cell lysis and mitochondria intact buffer; 250 mM Sucrose and 70 mM KCl in PBS) containing 20 μg/ml digitonin at 4°C for 5 min. The cell suspension was centrifuged at 1100 rpm and 4°C for 5 min. The supernatant was removed and stored as the cytosolic fraction at −80°C. The pellet was re-suspended in 100 μl of a “universal immunoprecipitation buffer” (50 mM Tris-HCl buffer, pH 7.4, 150 mM NaCl, 2 mM EDTA, 0.2% Triton X-100, 0.3% NP-40 and protease inhibitors) and placed at 4°C for 10 min. The suspension was centrifuged at 10,000 rpm for 10 min at 4°C and the supernatant was stored as the mitochondrial fraction at −80°C, prior to analysis by Western blotting.
Detection of protein oxidation
Mitochondrial proteins were treated with 2,4-dinitrophenylhydrazone (DNPH) to produce DNP-derivatives as described earlier [18]. The treated proteins were used for the analysis by SDS polyacrylamide gel electrophoresis (PAGE) analysis. After electrophoresis, proteins were transferred onto immobilon-P membranes at 15 V (constant voltage, 1 h) using trans-blot transfer cell (BIO-RAD). The blots were incubated with rabbit anti-DNP/HRP antibody (diluted 1:500 in TBST containing 2% BSA) at room temperature for 1 h. After washing three times with TBST, the oxidized proteins were visualized using an ECL system (Amersham Biosciences, UK).
Analysis of SOD1 and cytochrome c release
The release of SOD1 and cytochrome c from mitochondria was detected using anti-SOD1 and anti-cytochrome c antibodies, respectively. Mitochondrial or cytosolic fractions were added with a 0.2 volume of 125 mM Tris-HCl buffer (pH 6.8) containing 2% SDS, 5% β-mercaptoethanol, 25% glycerol, and 0.002% bromophenol blue. After incubation at 95°C for 5 min, these samples were subjected to SDS-PAGE followed by Western blot analysis using specific antibodies.
Western blotting
After SDS-PAGE, proteins were transferred electrophoretically from the gel onto an Immobilon-P transfer membrane (Millipore, Bedford, MA). The membrane was blocked in TBST (0.15 M NaCl, 10 mM Tris-HCl, pH 7.4, 0.05% Tween 20) containing 5% skimmed milk powder for 30 min, and then incubated with primary antibodies diluted in TBST containing 5% skimmed milk powder at room temperature for 1 h. After washing three times with TBST, the membrane was incubated at room temperature for 1 h with the HRP-conjugated secondary antibody diluted in TBST containing 5% skimmed milk powder. After washing with TBST, immunoreactive bands were visualized using an ECL system.
Measurement of cell viability and apoptosis
Apoptotic and necrotic cell deaths were distinguished by using Annexin V and PI as described previously [19]. HL-60 cells (1 × 106) were washed with ice-cold PBS and harvested by low-speed centrifugation, and resuspended in a binding buffer (10 mM HEPES buffer pH 7.4, 140 mM NaCl, 2.5 mM CaCl2) at 1 × 106 cells/ml. To 0.1 ml aliquots containing 105 cells, Annexin V-APC and PI were added at the manufacturer’s recommended concentrations. Cells were incubated for 15 min at room temperature in the dark and subsequently diluted with 0.4 ml binding buffer and analyzed by flow cytometry within 1 h. Flow cytometric analysis was performed on a Becton Dickinson FACSArray, and the data were collected and analyzed using the BD FACSArray software.
DNA fragmentation
HL-60 cells (1 × 106) were washed with ice-cold PBS and incubated in a lysis buffer (10 mM Tris-HCl buffer pH 7.4, 10 mM EDTA, 0.5% Triton X-100) at 4°C for 10 min. After centrifugation (at 15,000 rpm and 4°C for 20 min), the supernatant was collected. RNase A was added to the final concentration of 0.1 mg/ml, and the mixture was incubated at 37°C for 1 h. The cell lysate was then incubated at 37°C for 1 h with 0.1 mg/ml of proteinase K (Sigma-aldrich). DNA was extracted using 5 M NaCl and 2-propanol, followed by centrifugation at 15,000 rpm for 20 min. The DNA-extract was then dissolved in a TE buffer (10 mM Tris-HCl (pH 7.4), 10 mM EDTA) and separated on a 1.5% agarose gel. DNA fragmentation was visualized after ethidium bromide staining of the gel.
Statistical analysis
Values are expressed as mean ± S.D. The differences between control and treated groups (staurosporin or actinomycin D) were analyzed with Student’s t-test. A P value P < 0.05 was considered significant.
Results
ROS generation in HL-60 cells with staurosporine or actinomycin D
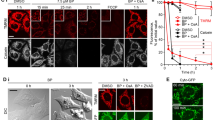
Several studies documented that both staurosporine and actinomycin D induced oxidative stress-dependent apoptosis in various types of cells [20, 21]. In our experiment, HL-60 cells were treated with either staurosporine or actinomycin D for ≈3 h, and the time-course of intracellular production of ROS was monitored by DCFH-DA using flow cytometry. Shortly after the treatment, ROS level increases transiently followed by a decrease (Fig. 1). The increase of ROS was inhibited by treating cells with MnTBAP, a membrane-permeable SOD mimic.
ROS generation assessed by flow cytometry after DCFH staining. HL-60 cells were cultured in the presence of either a 0.5 μM staurosporine or b 0.5 μg/ml actinomycin D for ≈3 h and then stained with DCFH-DA. c HL-60 cells were also cultured in the presence or absence of MnTBAP (100 μM) for 1 h, and then incubated with either staurosporine or actinomycin D for 3 h. Cellular generation of hydrogen peroxide was determined using DCFH-DA and flow cytometry. Values represent the mean ± SE for three independent experiments and the significance was set at P < 0.05
Effects of staurosporine and actinomycin D on the induction of MPT
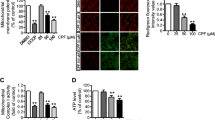
An increase of ROS level with either staurosporine or actinomycin D suggested that mitochondria would be under oxidative stress. Thus, we analyzed the effects of these drugs on the oxidation of mitochondrial proteins and induction of MPT. We detected protein oxidation using an anti-DNP antibody, which specifically binds to the carbonyl moieties of DNPH-treated mitochondrial proteins. When HL-60 cells were treated with either staurosporine or actinomycin D, a substantial increase in the carbonyl content of mitochondrial proteins was observed (Fig. 2). Protein carbonylation induced by either staurosporine or actinomycin D was inhibited by the treatment with MnTBAP. Under the same condition, we monitored the changes in Δψ using a fluorescent probe JC-1. The ratio of red/green (FL-1/FL-2) fluorescence reflecting changes in Δψ decreased in HL-60 cells treated with staurosporine or actinomycin D (Fig. 3). MnTBAP inhibited the decrease of Δψ induced by staurosporine, but not by actinomycin D.
The protein oxidation in mitochondria. HL-60 cells were cultured in the presence or absence of 100 μM MnTBAP for 1 h and then incubated with either 0.5 μM staurosporine or 0.5 μg/ml actinomycin D for 2 h. Mitochondria was isolated from HL-60 cells and treated with 2,4-dinitrophenylhydrazine (DNPH). The DNPH-modified proteins in mitochondria were detected immunochemically using HRP-conjugated rabbit anti-DNP antibody as described in the text. Mr, molecular marker; 1, untreated control; 2, +0.5 μM staurosporine, 3, +100 μM MnTBAP, +0.5 μM staurosporine; 4, +0.5 μg/ml actinomycin D; 5, +100 μM MnTBAP +0.5 μg/ml actinomycin D
Effect on mitochondrial membrane potential. HL-60 cells were treated with a 0.5 μM staurosporine or b 0.5 μg/ml actinomycin D for ≈5 h and then stained with JC-1. c HL-60 cells were pre-incubated in the presence or absence of 100 μM MnTBAP for 1 h followed by treatment with staurosporine or actinomycin D for 3 h. The green and red fluorescence correspond to JC-1 monomers and its aggregates, respectively. The values of red and green fluorescence ratio (Fl-1/Fl-2) reflect the changes in and represented by mean ± SE for three independent experiments and the significance was set at P < 0.05
Mitochondrial release of SOD1 and cytochrome c during staurosporine- and actinomycin D-induced apoptosis
We previously showed that oxidative stress induced MPT and released both SOD1 and cytochrome c from mitochondria [22]. To clarify the relationships among the time courses of MPT, SOD1, and cytochrome c releases during apoptosis, we analyzed the mitochondrial and cytosolic fractions of HL-60 cells treated with either staurosporine or actinomycin D. After the treatment, a time-dependent increase of both SOD1 and cytochrome c in the cytosolic fractions was observed (Fig. 4). At the 2 h timepoint, about 50% of SOD1 and 10% of cytochrome c were released to the cytosol. Both SOD1 and cytochrome c were completely released to cytosol after 5 h of treatment with staurosporine and actinomycin D. The release of the two proteins induced by staurosporine was inhibited by either MnTBAP or DIDS. In contrast, the release of the two proteins induced by actinomycin D was not affected by the two compounds. These results suggested that oxidative stress-induced MPT pore is required for staurosporine-stimulated but not actinomycin D-stimulated SOD1 and cytochrome c releases.
Release of SOD1 and cytochrome c from mitochondria. HL-60 cells were cultured in the presence of a 0.5 μM staurosporine or b 0.5 μg/ml actinomycin D for ≈5 h. Then, mitochondrial and cytosolic fractions were isolated as described in the text. Subcellular localizations of cytochrome c and SOD1 were determined by Western blot analysis using specific antibodies. Total proteins in mitochondrial and cytosolic fractions were calculated to 100%; proteins in cytosolic fractions were calculated and blotted in figures. c, d HL-60 cells were pre-incubated with either 100 μM MnTBAP or 500 μM DIDS for 1 h and then incubated with staurosporine or actinomycin D for 5 h. Subcellular localizations of cytochrome c (c) and SOD1 (d) were determined by Western blot analysis using specific antibodies. Values represent the mean ± SE (n = 3) and the significance was set at P < 0.05
Phosphatidylserine externalization and DNA fragmentation in HL-60 treated with staurosporine and actinomycin D
During the early stage of apoptosis, phosphatidylserine (PS) was exposed to cell surface; this exposure could be detected by annexin V that binds to PS with high affinity [23, 24]. To clarify the relationship between the mitochondrial release of intermembranous proteins and apoptosis-related events, we analyzed the exposure of annexin V and DNA fragmentation in staurosporine- or actinomycin D-treated HL-60 cells. Figure 5 shows that the proportion of annexin V-binding cells increased in a time-dependent manner. After externalization of PS, DNA fragmentation was induced by treating cells with either staurosporine or actinomycin D (Fig. 6). MnTBAP inhibited both PS externalization and DNA fragmentation induced by staurosporine but not by actinomycin D. DIDS also inhibited DNA fragmentation induced by staurosporine but not by actinomycin D.
Phosphatidylserine externalization induced by staurosporine and actinomycin D. At the indicated times, HL-60 cells were incubated with either a 0.5 μM staurosporine or b 0.5 μg/ml actinomycin D. c HL-60 cells were cultured in the presence or absence of 100 μM MnTBAP for 1 h, and then incubated with staurosporine or actinomycin D for 5 h. Cell surface expression of PS was determined by the annexin V binding assay using flow cytometry. Values represent the mean ± SE (n = 3) and the significance was set at P < 0.05
DNA fragmentation induced by staurosporine and actinomycin D. HL-60 cells were treated with a 0.5 μM staurosporine or b 0.5 μg/ml actinomycin D. c HL-60 cells were pre-incubated with or without 100 μM MnTBAP or 500 μM DIDS for 1 h and then with staurosporine or actinomycin D for 5 h. Then, DNA fragmentation of cells was analyzed by agarose gel electrophoresis. Similar results were obtained in triplicate experiments
Discussion
Recently, we reported that SOD1 is localized predominantly in mitochondria as a bound form in various types of cells [8]. We found that both SOD1 and cytochrome c were released simultaneously from mitochondria under the conditions when ROS generation and mitochondrial swelling took place [22]. Thus, we hypothesized that the release of SOD1 from mitochondria might participate in apoptosis in a similar manner to the release of cytochrome c. To validate this hypothesis, we investigated the subcellular localization and the role of SOD1 in ROS generation during mitochondria-dependent apoptosis. We demonstrated that ROS generation plays a key role in mitochondria-dependent apoptosis through increasing MPT and triggering the releases of SOD1 and cytochrome c. In addition, kinetics analysis revealed that SOD1 released from mitochondria prior to cytochrome c. Thus, the release of SOD1 might contribute to mitochondria-dependent apoptosis by promoting ROS-triggered cytochrome c release through accelerating the accumulation of intracellular ROS.
Apoptosis or programmed cell death is characterized as type 1 (extrinsic, receptor-dependent, caspase-dependent) or type 2 (intrinsic, autophagic, mitochondria-dependent) [25, 26]. In this experiment, actinomycin D, an inhibitor of DNA-dependent RNA synthesis, was used to induce the type 1 form of apoptosis [25], whereas staurosporine, a potent protein kinase c inhibitor, was used to trigger type 2 apoptosis [21]. Several lines of evidence indicate that both staurosporine and actinomycin D increase the generation of ROS, thereby inducing oxidative stress-dependent apoptosis in various types of cell lines [20, 27–30]. In fact, the observation of our time course experiments in HL-60 cells suggested that both staurosporine and actinomycin D increased ROS generation. Although the enhanced generation of ROS by both staurosporine and actinomycin D was inhibited by the treatment of MnTBAP, only staurosporine-induced cell death was blocked by MnTBAP, suggesting that ROS is indispensable for mitochondria-dependent but not independent apoptosis.
Since endogenous oxidative stress is a central inducer in staurosporine-triggered apoptosis [21, 31, 32], MnTBAP significantly inhibited MPT and release of SOD1 and cytochrome c. In contrast, Actinomycin D not only induces oxidative damage but also activates caspase 8 to cleave and translocate Bid into mitochondria [25, 33, 34]. Thus, both the loss of Δψ and the release of the two proteins induced by actinomycin D were not inhibited by MnTBAP. In actinomycin D-triggered apoptosis, tBid induced the release of cytochrome c by a mechanism that is independent of MPT pore opening. In this paper, we analyzed the effects of the VDAC blocker (DIDS) on staurosporine- and actinomycin D-induced apoptosis. It was found that DIDS inhibited the release of those two proteins and DNA fragmentation induced by staurosporine but did not inhibit the reactions induced by actinomycin D. Therefore, SOD1 and cytochrome c are released from different pathways in response to apoptosis signaling.
Oxidative stress-dependent mitochondrial degeneration and protein aggregation play principal roles in amyotrophic lateral sclerosis (ALS), a fatal, adult-onset neurodegenerative disease caused by mutations in SOD1 [35–39]. Mutations of SOD1 genes are found in 20% of patients with ALS [40]. It was also reported that a mutant SOD1 readily aggregates predominantly in the cytosol of motor neurons [41]. Preliminary experiments in our laboratory showed that the mutation of SOD1 decreased the affinity of the enzyme to mitochondria (unpublished experiment). Since oxidative stress is enhanced in and around mitochondria lacking SOD1, this would lead to oxidation of critical thiol groups in ANT, thereby triggering cytochrome c release. Guegan et al. [42] also reported that the release of cytochrome c from mitochondria has been observed in the spinal cord of transgenic mice. This may be linked with a mutSOD1-mediated ROS generation.
In conclusion, the results of our time course studies demonstrate that both SOD1 and cytochrome c are released from mitochondria concurrently with the generation of ROS and the reduction of Δψ. All these reactions are involved in oxidative stress-induced apoptosis. Since the delocalization of SOD1 from mitochondria would enhance mitochondrial oxidative injury, it would enhance apoptosis. Based upon these results, we propose that the release of SOD1 might be a critical initiator of mitochondria-dependent apoptosis. Further studies are required to fully elucidate the physiological implications of subcellular localization of SOD1 in mitochondria-dependent apoptosis.
References
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Wyllie AH (1980) Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 284:555–556
Crompton M (1999) The mitochondrial permeability transition pore and its role in cell death. Biochem J 341(Pt 2):233–249
Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281:1309–1312
Gogvadze V, Robertson JD, Zhivotovsky B et al (2001) Cytochrome c release occurs via Ca2+ -dependent and Ca2+ -independent mechanisms that are regulated by Bax. J Biol Chem 276:19066–19071
Liu X, Kim CN, Yang J et al (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86:147–157
Zou H, Henzel WJ, Liu X et al (1997) Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 90:405–413
Kira Y, Sato EF, Inoue M (2002) Association of Cu, Zn-type superoxide dismutase with mitochondria and peroxisomes. Arch Biochem Biophys 399:96–102
Okado-Matsumoto A, Fridovich I (2001) Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu, Zn-SOD in mitochondria. J Biol Chem 276:38388–38393
Simon HU, Haj-Yehia A, Levi-Schaffer F (2000) Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5:415–418
Vincent AM, Brownlee M, Russell JW (2002) Oxidative stress and programmed cell death in diabetic neuropathy. Ann N Y Acad Sci 959:368–383
Kanno T, Sato EE, Muranaka S et al (2004) Oxidative stress underlies the mechanism for Ca(2+)-induced permeability transition of mitochondria. Free Radic Res 38:27–35
Cai J, Jones DP (1998) Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J Biol Chem 273:11401–11404
Boveris A, Oshino N, Chance B (1972) The cellular production of hydrogen peroxide. Biochem J 128:617–630
Muller FL, Liu Y, Van Remmen H (2004) Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem 279:49064–49073
Himmelfarb J, Lazarus JM, Hakim R (1991) Reactive oxygen species production by monocytes and polymorphonuclear leukocytes during dialysis. Am J Kidney Dis 17:271–276
Facompre M, Wattez N, Kluza J et al (2000) Relationship between cell cycle changes and variations of the mitochondrial membrane potential induced by etoposide. Mol Cell Biol Res Commun 4:37–42
Levine RL, Williams JA, Stadtman ER et al (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233:346–357
Liu S, Kawai K, Tyurin VA et al (2001) Nitric oxide-dependent pro-oxidant and pro-apoptotic effect of metallothioneins in HL-60 cells challenged with cupric nitrilotriacetate. Biochem J 354:397–406
Flitter WD, Mason RP (1988) The enzymatic reduction of actinomycin D to a free radical species. Arch Biochem Biophys 267:632–639
Gil J, Almeida S, Oliveira CR et al (2003) Cytosolic and mitochondrial ROS in staurosporine-induced retinal cell apoptosis. Free Radic Biol Med 35:1500–1514
Li Q, Sato EF, Kira Y et al (2006) A possible cooperation of SOD1 and cytochrome c in mitochondria-dependent apoptosis. Free Radic Biol Med 40:173–181
Koopman G, Reutelingsperger CP, Kuijten GA et al (1994) Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 84:1415–1420
Vermes I, Haanen C, Steffens-Nakken H et al (1995) A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods 184:39–51
Anderson KM, Alrefai W, Bonomi P et al (2003) Caspase-dependent and -independent panc-1 cell death due to actinomycin D and MK 886 are additive but increase clonogenic survival. Exp Biol Med (Maywood) 228:915–925
Anderson KM, Bonomi P, Hu Y et al (2003) An interaction between type 1 and type 2 programmed cell death and clonogenic survival. Med Hypotheses 61:583–585
Yuste VJ, Sanchez-Lopez I, Sole C et al (2002) The prevention of the staurosporine-induced apoptosis by Bcl-X(L), but not by Bcl-2 or caspase inhibitors, allows the extensive differentiation of human neuroblastoma cells. J Neurochem 80:126–139
Kruman I, Guo Q, Mattson MP (1998) Calcium and reactive oxygen species mediate staurosporine-induced mitochondrial dysfunction and apoptosis in PC12 cells. J Neurosci Res 51:293–308
Ikeda K, Kajiwara K, Tanabe E et al (1999) Involvement of hydrogen peroxide and hydroxyl radical in chemically induced apoptosis of HL-60 cells. Biochem Pharmacol 57:1361–1365
Moore RM, Lundgren DW, Moore JJ (1999) Cyclooxygenase inhibitors decrease apoptosis initiated by actinomycin D, cycloheximide, and staurosporine in amnion-derived WISH cells. J Soc Gynecol Investig 6:245–251
Matsura T, Serinkan BF, Jiang J et al (2002) Phosphatidylserine peroxidation/externalization during staurosporine-induced apoptosis in HL-60 cells. FEBS Lett 524:25–30
Pong K, Doctrow SR, Huffman K et al (2001) Attenuation of staurosporine-induced apoptosis, oxidative stress, and mitochondrial dysfunction by synthetic superoxide dismutase and catalase mimetics, in cultured cortical neurons. Exp Neurol 171:84–97
Shim D, Kang HY, Jeon BW et al (2004) Protein kinase B inhibits apoptosis induced by actinomycin D in ECV304 cells through phosphorylation of caspase 8. Arch Biochem Biophys 425:214–220
Debatin KM, Poncet D, Kroemer G (2002) Chemotherapy: targeting the mitochondrial cell death pathway. Oncogene 21:8786–8803
Bruijn LI, Becher MW, Lee MK et al (1997) ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron 18:327–338
Higgins CM, Jung C, Xu Z (2003) ALS-associated mutant SOD1G93A causes mitochondrial vacuolation by expansion of the intermembrane space and by involvement of SOD1 aggregation and peroxisomes. BMC Neurosci 4:16
Liu R, Li B, Flanagan SW et al (2002) Increased mitochondrial antioxidative activity or decreased oxygen free radical propagation prevent mutant SOD1-mediated motor neuron cell death and increase amyotrophic lateral sclerosis-like transgenic mouse survival. J Neurochem 80:488–500
Shibata N (2001) Transgenic mouse model for familial amyotrophic lateral sclerosis with superoxide dismutase-1 mutation. Neuropathology 21:82–92
Chiu AY, Zhai P, Dal Canto MC et al (1995) Age-dependent penetrance of disease in a transgenic mouse model of familial amyotrophic lateral sclerosis. Mol Cell Neurosci 6:349–362
Rouleau GA, Clark AW, Rooke K et al (1996) SOD1 mutation is associated with accumulation of neurofilaments in amyotrophic lateral sclerosis. Ann Neurol 39:128–131
Mattiazzi M, D’Aurelio M, Gajewski CD et al (2002) Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J Biol Chem 277:29626–29633
Guegan C, Vila M, Rosoklija G et al (2001) Recruitment of the mitochondrial-dependent apoptotic pathway in amyotrophic lateral sclerosis. J Neurosci 21:6569–6576
Acknowledgments
This work was supported by Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science and Technology (16590252 and 14370062), and 21st Century COE Program “Base to Overcome Fatigue” supported by MEXT, Japan. This work was also supported by the Sasakawa Scientific Research Grant from The Japan Science Society. The authors thank Prof. S. Tsuyoshi Ohnishi for his critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Q., Sato, E.F., Zhu, X. et al. A simultaneous release of SOD1 with cytochrome c regulates mitochondria-dependent apoptosis. Mol Cell Biochem 322, 151–159 (2009). https://doi.org/10.1007/s11010-008-9952-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9952-9