Abstract
Breast cancer is the principle cause of death among women worldwide. In this study, we investigated the anti-tumor potential of lycopene (Lyco) alone or combined with melatonin (Lyco + Mel) for 120 days against a single oral dose of (50 mg/kg B.W.) 7,12-dimethylbenz(a)anthracene (DMBA)-induced oxidative stress and mammary carcinogenesis in female rats. The treatment protocol started from the day immediately after DMBA administration. Results obtained indicated that there was an elevation in the levels of malondialdhyde and nitric oxide in serum and breast tissues of DMBA injected rats. The combined treatment (Lyco + Mel) group showed a potential reduction of these parameters more than lyco individually. The activities of SOD, CAT, and GPx were found to be significantly high than lyco alone treated rats. In DMBA group a negative significant correlation between weight and serum nitric oxide (r = −0.59), and a positive significant correlation between NO and MDA (r = 0.81) was observed. Histopathological examination revealed the formation of tumor and angiogenesis in DMBA-induced rats and these abnormal changes were ameliorated by combined treatment with Lyco + Mel. In conclusion, these results suggested that supplementation of diet with lycopene with melatonin provided antioxidant defense with strong chemo preventive activity against DMBA-induced mammary tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is one of the most serious problems in oncology. It is a leading cause of death among women in many countries [1]. Reactive oxygen species (ROS) are involved in a variety of important pathophysiological conditions including mutagenesis and carcinogenesis [2]. Free radicals play an important role in tumor promotion by direct chemical reaction or alteration of cellular metabolic processes [3].
Human body is equipped with various antioxidants such as superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), glutathione (GSH), ascorbic acid (Vitamin C), and α-tocopherol (Vitamin E), which can counteract the deleterious action of ROS and protect from cellular and molecular damage [4].
Various molecules can inhibit the formation of free radicals associated with carcinogenesis. Bioactive compounds from plant origin have the potential to subside the biochemical imbalances induced by various toxins associated with free radicals. They provide protection without causing any side effects, and therefore, development of drugs from plant products is desired. For that account, natural antioxidants from plant sources have been viewed as promising therapeutic drugs [5].
Lycopene, the pigment principally responsible for the characteristic deep-red color of ripe tomato fruits and tomato products, has received much attention in recent years because of its beneficial effect in the treatment of diseases [6]. It was demonstrated that lycopene provided the best protection against singlet oxygen-induced cell damage [7].
Epidemiologic studies revealed that lycopene may increasingly be identified as sharing inverse relationships to cancer with other common carotenoids or as being the only carotenoid to show such an association [8].
Lycopene in human plasma has a half life of about 2 to 3 days [9]. Only a few metabolites, such as 5,6-dihydroxy-5,6-dihydro lycopene, have been detected in human plasma [6]. Owing to its lipophilic nature, lycopene was found to concentrate in LDL and VLDL fractions and not in HDL fraction of the serum [10].
Recent studies showed that some substances such as melatonin (Mel), retinoic acid, and nigalla sativa have a potential protective effect in cancer. Mel, a secretory product of the pineal gland, is a powerful antioxidant that not only scavenges the hydroxyl radical [11], but also inhibits the production of NO synthase. Mel can enter the nucleus, where it protects DNA from oxidation damage thereby decreasing the incidence of cancer [12]. Also, it modulates the immune response and inhibits the development of hormone dependent cancer [13].
There is mixed epidemiologic evidence that lycopene consumption is associated with a lower risk of prostate cancer. Lycopene inhibits the growth of benign and malignant prostatic epithelial cells in vitro [14].
No previous studies were done to investigate the protective effect of lycopene against the genesis of breast cancer. The goal of this study was to investigate the effect of lycopene alone or combined with melatonin in inhibiting the oxidative stress and carcinogenic effect of DMBA-induced mammary cancer in rats.
Materials and methods
Animals
A total of 48 Female Sprague Dawley rats (colony of laboratory animals from the King Fahd Medical Research center, Jeddah, KSA) were maintained and handled according to the recommendations of the Institutional Ethic Committee. The animals weighing 60–80 g were housed in a room maintained at 22°C with a 12-h light-dark cycle with free access to foods. Rats were randomized into four groups (12 rats per group) Group I rats served as control. Rats in groups II, III, and IV were intragastrically administrated with a single dose of 50 mg/kg B.W. of 7,12-dimethylbenz(a)anthracene (DMBA, Sigma Chemicals). DMBA was dissolved in corn oil and given in a volume of 1 ml [15]. On the day after DMBA administration, rats in group III fed on diet supplemented with lycopene (50 mg/kg of diet) [16], and rats in group IV supplemented with lycopene and injected subcutaneously in a dose of 2.5 mg melatonin/kg B.W. Melatonin was prepared by dissolving 50 mg in 2 ml ethanol and diluted to 100 ml with distilled water [17].
Rats were palpated weekly for tumor appearance. The first was detected after 75 days from DMBA administration.
After 120 days, rats were starved overnight and sacrificed by decapitation. Blood was collected. Serum was obtained after blood coagulation and centrifugation at 12,000g for 10 min, and stored at −80°C for further analyses. Breast tissues were excised and quickly removed for estimation of antioxidant enzymes. Tissues were washed thoroughly with ice-cold normal phosphate buffer saline, pH 7.2 (PBS, 0.9%), and cut into small pieces. Tissues were homogenized by a glass homogenizer tube in cold PBS, centrifuged at 20,000 rpm for 10 min, and the supernatant was stored at −80°C for further analysis.
Biochemical analysis
Nitric oxide measurement
Serum and tissue nitrite and nitrate were analyzed by the modified micro assay as described [18]. Briefly, nitrate was reduced to nitrite by cadmium and assayed by Griess reagent.
Malondialdhyde measurement
Serum and tissue MDA was assayed by thiobarbituric acid method as described by [19].
Assay of SOD activity
The SOD activity was assessed by the Nitroblue tetrazolium (NBT) reduction method [20]. A total of 0.1 ml of tissue supernatant was added to a reaction mixture containing 0.1 mM EDTA (200 μl), 0.12 mM riboflavin (50 μl) and 0.6 M phosphate buffer (pH 7.8) in a final volume of 3 ml. The optical density was measured at 560 nm. The activity was expressed as U/min/mg protein.
Assay of CAT activity
The reaction mixture (2 ml) contained 1.95 ml of 10 mM H2O2 in 60 mM phosphate buffer (pH 7.0). The reaction was initiated by adding 0.5 ml tissue supernatant to it, and the absorbance was taken for 3 min at 240 nm. Phosphate buffer (60 mM, pH 7.0) was used as a reference. The data is expressed as μmol H2O2 consumed/(min (mg protein) [21].
Assay of GPx activity
The activity of glutathione peroxidase (GPx) was assayed by the method of Rotruck et al. [22]. The reaction mixture containing 0.2 ml of EDTA (0.8 mM, pH 7.0), 0.4 ml of phosphate buffer (10 mM), and 0.2 ml of tissue homogenate was incubated with 0.1 M of H2O2 and 0.2 ml of glutathione for 10 min. Oxidation of glutathione by the enzyme was measured spectrophotometrically at 420 nm. The activity of GPx was expressed as μmol glutathione oxidized/min/mg protein.
Histopathological examination
Mammary tissues were fixed in 10% buffered formalin, embedded in paraffin using a conventional automated system. The blocks were cut to obtain 5-μm-thick sections and stained with hemotoxylin–eosin [23]. Serial paraffin sections of each tissue image were captured by light microscopy (Olympus BX51).
Statistical analysis
Data are presented as mean ± standard deviation (S.D.). One way analysis of variance (ANOVA) followed by Tukey multiple comparison method was carried out to compare the mean value of different groups by using SPSS 7.5 student version. Comparisons were made between Group I versus Groups II, III, and IV, and between Group II versus Groups III and IV. P < 0.05 was considered statistically significant in all cases.
Results
Table 1 illustrates the body weight and the protection percentage in rats received either lycopene (Lyco) individually or combined with melatonin (Mel). The body weight was significantly decreased in tumor-induced animals versus control (P < 0.01). Conversely, supplementation of (Lyco + Mel) increased the body weight more than Lyco alone treated rats till near the control group. It was found that protection against DMBA-induced carcinogenesis was 66.5% in animals that received lycopene alone, while it was 80% in animals that received lycopene with melatonin as compared with non treated rats. In DMBA group, 4 out of 12 died (2 after 65 days and 2 after 90 days). In Lyco treated group, 3 out of 12 died and in combined (Lyco + Mel) treatment, 2 out of 12 died. In DMBA group, there was severe inflammatory reaction in the skin of the submandibular region and development of multiple masses involving the mammary gland tissues.
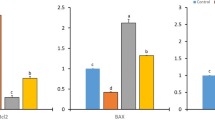
Malondialdhyde and nitric oxide levels in serum and breast tissues were statistically significant elevated in the breast cancer bearing animals, whereas little reduction was found in the rats treated with Lyco, and much reduction in combined treatment (P < 0.01) as compared with non treated rats to near control value (Table 2).
Results in Table 3 showed a significant elevation in the activities of SOD in the combination of (Lyco + Mel) treated group versus tumor bearing rats than the activity in the lyco individually treated.
CAT activity was significantly (P < 0.05) lower in animals with breast cancer versus the control group. Moreover, higher levels of CAT activity were recorded in Lyco + Mel treated groups than Lyco alone versus non treated rats. GPx activities were significantly (P < 0.01) high in the combined (Lyco + Mel) treatment group than Lyco individually treated group of rats. The activity of GPx was reduced in cancer bearing rats versus the normal group.
Histological examination
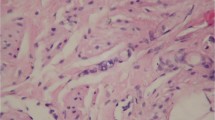
Histological examination of DMBA-induced invasive breast lesions versus treated with Lyco and Mel and normal control was done. Figure 1a shows the mammary gland tissues in control group formed of an admixture of fibro fatty tissues and ductal structures. Figure 1b shows the development of carcinoma in DMBA group as was evident by neovascularization, presence of uniformly malignant ductal epithelial cells growing in vague cribriform pattern, and necrosis formation along the tumor and cell destructions. DMBA group treated with Lyco showed absence of the malignant changes with normal appearance of ductal tissues (Fig. 1c). Treatment with Lyco + Mel resulted in complete disappearance of abnormal changes that caused by DMBA (Fig. 1d).
Discussion
A new strategy possessing anti-neoplastic and free radical scavenging properties. Therefore, it is appropriate to investigate various phytotherapeutic origins to detect anti-tumor and free radical scavenging activities [24]. The available semi synthetic anticancer drugs have more side effects and are cytotoxic to human beings. Since modern medicine has no effective cure for malignant cancers and tumors, scientists are interested in finding a potent phototherapeutic agent with non-cytotoxic properties
DMBA-induced mammary gland tumor in rodents has been widely used as an animal model for the development of chemo preventive drugs for breast cancer in humans [25]. Data of the present study indicated that daily intake of Lyco alone or combined with Mel could prevent or delay the development of breast cancer in the rat. The effects are highly significant, because fewer of the lyco supplemented animals (6/9) developed tumor versus DMBA group and are much lower (8/10) in combined treatment. Also, (Lyco + Mel), decreases the number and size of tumors induced by the carcinogen.
Toxic manifestation of DMBA is associated with its oxidative metabolism leading to the formation of reactive metabolites (epoxides and quinines) capable of generating free radicals. Metabolism of DMBA by the mixed function oxidases system often results in the formation of oxyradicals “O2 •−, H2O2, and •OH,” which bind covalently to nucleophilic sites on cellular macromolecules thereby eliciting cancerous responses [26]. The generation of ROS and the peroxidation of membrane lipids are well associated with the initiation of carcinogenesis affecting the normal biochemical process, which further leads to the reduction of body weight [27]. This is in accordance with our results, which revealed a significant reduction in body weight as result of DMBA administration. Serum and tissue malondialdhyde and nitric oxide levels were increased significantly in the breast cancer-bearing animals; their levels showed little reduction in the groups treated with Lyco alone and much reduction when combined with Mel near to the normal control group. The reduction of NO level in combined treatment may be due to the inhibition of NO synthase enzyme as described by Hussein et al. [11]. In the DMBA group, a negative significant correlation between weight and serum nitric oxide (r = −0.59) and a positive significant correlation between NO and MDA (r = 0.81) was observed.
Oxidative stress induced due to the generation of free radicals and/or decreased antioxidant level in the target cells and tissues has been suggested to play an important role in carcinogenesis [28]. During cell membrane damage, various enzymes leak down to the circulatory fluid and their assessment in serum serves as markers in clinical studies. SOD is the first antioxidant enzyme to deal with oxyradicals by accelerating the dismutation of superoxide to hydrogen peroxide. CAT is a peroxisomal haem protein that catalyses the removal of hydrogen peroxide formed during the reaction catalysed by SOD. Thus, SOD and CAT act as mutually supportive antioxidative enzymes, which provide protective defence against reactive oxygen species [29].
The present study revealed that SOD activity was decreased in the cancer-bearing animal, which may be due to altered antioxidant status caused by carcinogenesis. Decrease of CAT activity was measured in patients with breast cancer and benign breast disease conditions [30]. This is in accordance with our results, which indicate that decreased CAT in cancer-bearing animals may be due to the utilization of antioxidant enzymes in the removal of H2O2 released. GPx is an important defense enzyme against oxidative damage and this in turn requires glutathione as a cofactor. GPx catalyses the oxidation of GSH to GSSG at the expense of H2O2 [31]. Decreased GPx activity was also observed in cancerous conditions [32]. Our findings agree well with this observation, and the activity of GPx significantly decreased in cancer-bearing animals. CAT activity was significantly higher in the combination-treated group versus drug and Lyco individually treated animals.
This study is in line of cohen et al. [33], who reported that melatonin and retinoic acid have potential anticancer effect due to their ability to act as antioxidants or inhibit the production of NO by reducing NO synthase. Also, Tamarkin et al. [34] stated that subcutaneous injection of Mel can inhibit the development of DMBA-induced mammary tumors in rats.
The combination treatment showed a significant reduction of tumors at the molecular level versus the cancer controls. However, biochemical analysis revealed that combination treatment significantly inhibited lipidperoxidation, thus suggesting the antioxidant mechanism of Lyco when combined with Mel. The combination treatment may be useful for the prevention of breast cancer.
References
Bray F, McCarron P, Parkin DM (2004) The changing global patterns of female breast cancer incidence and mortality. Breast Cancer Res 6:229–239. doi:10.1186/bcr932
Aruoma OI (1994) Nutrition and health aspects of free radicals and antioxidants. Food Chem Toxicol 32:671–685. doi:10.1016/0278-6915(94)90011-6
Cerutti PA (1985) Prooxidant states and tumor promotion. Science 227:375–381. doi:10.1126/science.2981433
Jagetia GC, Rao SK (2006) Evaluation of the antineoplastic activity of guduchi (Tinospora cordifolia) in Ehrlich ascites carcinoma bearing mice. Biol Pharm Bull 29:460–466. doi:10.1248/bpb.29.460
Vijayavel K, Anbuselvam C, Balasubramanian MP (2006) Free radical scavenging activity of the marine mangrove Rhizophora apiculata bark extract with reference to naphthalene induced mitochondrial dysfunction. Chem Biol Int 163:170–175. doi:10.1016/j.cbi.2006.06.003
Rao V, Agarwal S (1999) Role of lycopene as antioxidant carotenoids in the prevention of chronic diseases: a review. Nutr Res 19(2):305–323. doi:10.1016/S0271-5317(98)00193-6
Joseph BG, Michael C, Wieslawa K, Seth T, Zhonglin Z, Leonard AC (2001) Effects of a lycopene-rich diet on spontaneous and benzo[a]pyrene-induced mutagenesis in prostate, colon and lungs of the lacZ mouse. Cancer Lett 164:1–6. doi:10.1016/S0304-3835(00)00705-9
Gerster H (1997) The potential role of lycopene for human health. J Am Coll Nutr 16:109–126
Clinton SK (1998) Lycopene: chemistry, biology, and implications for human health and disease. Nutr Rev 56:35–51
Fuhramn B, Elis A, Aviram M (1997) Hypocholesterolemic effect of lycopene and ß-carotene is related to suppression of cholesterol synthesis and augmentation of LDL receptor activity in macrophage. Biochem Biophys Res Commun 233:658–662. doi:10.1006/bbrc.1997.6520
Hussein MR, Abu-Dief EE, Abdel Reheem MH, Abdel Rahman A (2005) Ultra structural evaluation of the radio protective effects of melatonin against X-ray induced skin damage in Albino rats. Int J Exp Pathol 86:45–55. doi:10.1111/j.0959-9673.2005.00412.x
Reiter MH, Tan DX, Cabrera J et al (1999) The oxidant/antioxidant network; role of melatonin. Biol Signals Recept 8:56–63. doi:10.1159/000014569
Maestroni GJ, Conti A (1989) Beta-endorphin and dynorphin mimic the circadian immunoenhancing and anti-stress effects of melatonin. Int J Immunopharmacol 11:333–340. doi:10.1016/0192-0561(89)90078-7
Astrog P, Gradelet S, Berges R, Suschetet M (1997) Dietary lycopene decreases initiation of liver preneoplastic foci by diethylnitrosamine in rat. Nutr Cancer 29:60–65
Henry L, Narendra PS (2006) Oral artemisinin prevents and delays the development of 7,12-dimethylbenz[a]anthracene (DMBA)-induced breast cancer in the rat. Cancer Lett 231:43–44. doi:10.1016/j.canlet.2005.01.019
Delphine G, Bernard L, Jérémie T, Andrzej M, Stéphane G, Catherine C, Edmond R (2007) Serum from rats fed red or yellow tomatoes induces Connexin43 expression independently from lycopene in a prostate cancer cell line. Biochem Biophys Res Commun 364:578–582. doi:10.1016/j.bbrc.2007.10.030
Mohamed AM, Hosny AH, Mahmoud HM, Abdelreheim AA, Sary KH, Mhmoud RH (2005) The biochemical and morphological alterations following administration of melatonin, retinoic acid and nigella sativa in mammary carcinoma: an animal model. Int J Exp Pathol 86:383–396. doi:10.1111/j.0959-9673.2005.00448.x
Vodovotz Y (1996) Modified microassay for serum nitrite and nitrate. Biotechniques 20(3):390–394
Zima T, Stipek S, Crkovska J, Platenik J (1995) Measurement of lipid peroxidation products in biological samples by spectrophotometric and HPLC assay. Klin Biochem Metab 3(24):98–102
Flohe L, Otting F (1984) Superoxide dismutase assays. Methods Enzymol 105:93. doi:10.1016/S0076-6879(84)05013-8
Claiborne A (1985) Catalase activity. In: Greenwald RA (ed) CRC handbook of methods for oxygen radical research. CRC Press, Boca Raton, pp 283–284
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. doi:10.1016/0003-9861(59)90090-6
Costa I, Solanas M, Escrich E (2002) Histopathologic characterization of mammary neoplastic lesions induced with 7,12-dimethylbenz(a)anthracene in the rat. A comparative analysis with human breast tumor. Arch Pathol Lab Med 126:915–927
Ramar PS, Ponnampalam G, Savarimuthu I (2006) Anti-tumor promoting potential of luteolin against 7,12-dimethylbenz(a)anthracene-induced mammary tumors in rats. Chem Biol Interact 164:1–14. doi:10.1016/j.cbi.2006.08.018
Mehta RG (2000) Experimental basis for the prevention of breast cancer. Eur J Cancer 36:1275–1282. doi:10.1016/S0959-8049(00)00100-3
Giri U, Sharma SD, Abdulla M, Athar M (1995) Evidence that in situ generated reactive oxygen species act as a potent stage I tumor promoter in mouse skin. Biochem Biophys Res Commun 209(2):698–705. doi:10.1006/bbrc.1995.1555
Davis L, Kuttan G (2001) Effect of Withania somnifera on DMBA induced carcinogenesis. J Ethnopharmacol 75:165–168. doi:10.1016/S0378-8741(00)00404-9
Huang YL, Sheu JY, Lin TH (1999) Association between oxidative stress and changes of trace elements in patients with breast cancer. Clin Biochem 32:31–36. doi:10.1016/S0009-9120(98)00096-4
Weydert CJ, Waugh TA, Ritchie JM, Iyer KS, Smith JL, Li L, Spitz DR, Oberley LW (2006) Overexpression of manganese or copper-zinc superoxide dismutase inhibits breast cancer growth. Free Radic Biol Med 41:226–237
Gonenc A, Erten D, Aslan S, Akyncy M, Simşek B, Torun M (2006) Lipid peroxidation and antioxidant status in blood and tissue of malignant breast tumour and benign breast disease. Cell Biol Int 30:376–380. doi:10.1016/j.cellbi.2006.02.005
Cerutti P, Ghosh R, Oya Y, Amstad P (1994) The role of cellular antioxidant defence in oxidant carcinogenesis. Environ Health Perspect 102(10):123–129. doi:10.2307/3432228
Bewick M, Coutie W, Tudhope GR (1987) Superoxide dismutase, glutathione peroxidase and catalase in the red cells of patients with malignant lymphoma. Br J Haematol 65:347–350. doi:10.1111/j.1365-2141.1987.tb06866.x
Cohen M, Lippman M, Chabner B (1978) Role of pineal gland in aetiology and treatment of breast cancer. Lancet 2:814–816. doi:10.1016/S0140-6736(78)92591-6
Tamarkin L, Cohen M, Roselle D, Reichert C, Lippman M, Chabner B (1981) Melatonin inhibition and pinealectomy enhancement of 7,12-dimethylbenz(a)anthracene-induced mammary tumors in the rat. Cancer Res 41(11):4432–4436
Acknowledgment
The authors would like to thank prof. Dr. Taha Kumosani, Head of Biochemistry Dep., Faculty of Science, KAU, Jeddah, KSA for his kind facilitation of supplies for this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moselhy, S.S., Al mslmani, M.A.B. Chemopreventive effect of lycopene alone or with melatonin against the genesis of oxidative stress and mammary tumors induced by 7,12 dimethyl(a)benzanthracene in sprague dawely female rats. Mol Cell Biochem 319, 175–180 (2008). https://doi.org/10.1007/s11010-008-9890-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9890-6