Abstract
Derivatives of 4,5,6,7-tetrabromobenzotriazole (TBBt) and 4,5,6,7-tetrabromobenzimidazole (TBBi) with IC50 in the low micromolar range and with high selectivity belong to the most promising inhibitors of protein kinase CK2 (casein kinase 2). Treatment of various cell lines with TBBt, TBBi or 2-dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole (DMAT) affected cell viability with simultaneous induction of apoptosis. The inhibitory activity of newly synthesized hydroxyalkyl derivatives of TBBi and TBBt depends on the length of the alkyl chain. The hydroxypropyl substituted derivatives show higher or similar inhibitory activity than the parent compounds when tested with human protein kinase CK2. To test the distribution of this class of compounds in mammals, [14C] TBBi was synthesized.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protein kinase CK2, an ubiquitous serine/threonine kinase, plays a significant role in many cellular functions, including the regulation of cell cycle progression [1], malignant transformation, cell survival, angiogenesis [2] and apoptosis. Nowadays, more than 300 substrates of CK2 are known, and identification of others may be expected since approximately 20% of the isolated phosphopeptides conform to the consensus for phosphorylation by CK2 [3]. Dysfunctions of this enzyme are frequently associated with various pathological states [4], and major efforts are currently devoted to development of specific inhibitors.
A number of CK2 inhibitors effective in the low micromolar ranges have been synthesized. Those are derivatives of benzotriazole, benzimidazole [5, 6], flavonoids, antraquinones, quinazolones, quinolines, polypeptides and polysaccharides. The most specific inhibitor of CK2, 4,5,6,7-tetrabromo-1H-benzotriazole (TBBt) [7] is used very widely to elucidate the role of phosphorylation by CK2 of a variety of proteins of important metabolic functions. Much structural information has been accumulated on Zea mays CK2 alfa subunit in complex with various inhibitors [8], indicating that TBBt and derivatives of 4,5,6,7-tetrabromo-1H-benzimidazole (TBBi) may have an effect on the conformation of the Gly-rich loop at the active site of CK2. The ATP-binding site holds conserved water molecules [9], so it seemed interesting to explore the possibility of replacing them with substituents with a hydroxyl group. To test this idea for finding more potent inhibitors, new derivatives of TBBt and TBBi with hydroxyl group were designed and synthesized, and their influence on the activity of human CK2 alfa subunit and holoenzyme was determined.
Bearing in mind the role of CK2 in regulation of cell proliferation and knowing that TBBt, TBBi and DMAT can induce apoptosis [9, 10], we undertook the study of the distribution of this class of compounds in mammals. The [14C]TBBi was synthesized and this compound was used to study its organ distribution in rats.
Materials and methods
The starting compounds- 4,5,6,7-tetrabromo-1H-benzimidazole (TBBi) and 4,5,6,7-tetrabromo-1H-benzotriazole (TBBt) were synthesized by bromination of 1H-benzimidazole or 1H-benzotriazole according to published method [5].
Expression of subunits of CK2 and determination of CK2 activity
The pT7-7 vector carrying human CK2α subunit (hCK2α) cDNA and the pT7-7 vector carrying human CK2β subunit (hCK2β) cDNA were a kind gift of Dr. Stefania Sarno, University of Padova, Italy. Expression and purification of rhCK2α and rhCK2β subunits was according to published methods [11, 12].
The reaction mixture (final volume of 50 μl) for the determination of CK2 activity (CK2α or holoenzyme CK2α2β2) contained: protein substrate casein (75 μg) or peptide substrate (20 μM RRRDDDSDDD, Biosyntan, Germany), Tris–HCl pH 7.5 (20 mM), MgCl2 (20 mM), γ[32P]ATP (10 μM, 100–200 cpm pmol−1) and appropriate concentrations of inhibitor in 1 μl DMSO. After 20 min incubation at 30°C, 40 μl of the assay mixture was spotted onto a square (2 cm × 2 cm) of Whatman 3MM (for casein) or P81 paper (for peptide substrate), which was immediately immersed in cold 5% (w/v) trichloroacetic acid containing 0.3% o-phosphoric acid (10 ml per square), and washed with H2O five times for 10 min. Then the squares were washed in 96% ethanol and allowed to dry. The radioactivity was quantified using a LKB Flexi-Vial liquid scintillation spectrometer.
Results
N-hydroxyalkyl derivatives were prepared by alkylation of TBBt or TBBi with the use of NaH or DBU as bases and appropriate hydroxyalkyl halide as previously reported [13, 14]. The ratio of N 1 to N 2 substituted anomers of TBBt depends on the reaction conditions and on the length of the alkyl chain.
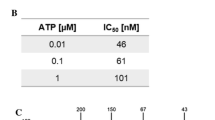
The influence of the synthesized compounds on human CK2 alfa subunit and on the holoenzyme activity was determined as described in the section “Materials and methods”. The results are shown in Table 1. Some of the new analogues showed IC50 similar to that of the parent compounds, and their inhibitory activity depended on the length of the alkyl substituents. The 3-(4,5,6,7-tetrabromo-1H-benzimidazol-1-yl)propan-1-ol (MB001) exerted a higher potency (IC50 0.5 μM) than the parent compound TBBi (IC50 1.5 μM). The 3-(4,5,6,7-tetrabromo-1H-benzotriazol-1-yl)propan-1-ol (MB002) exerted only a slightly higher potency against the catalytic subunit of CK2 α, with IC50 0.3 μM compared to 0.5 μM for TBBt. Derivatives with shorter or longer chains exerted lower inhibitory activity.
[14C]TBBi was synthesized starting from 2,4-dibromo-6-nitroaniline, through reduction to 2,4-dibromophenylene-1,6-diamine, condensation with 14C formic acid and subsequent bromination. This compound was used to study its organ distribution in rats during 0–72 h after a single per os administration of a single dose of 25 mg/kg body weight; these results will be published separately.
Discussion
To improve the inhibitory activity versus CK2, TBBt and TBBi have been subjected to chemical modifications (to be published elsewhere). Most modifications either partially or completely abrogated the ability of the new derivatives to inhibit CK2. However, selected modifications with the hydroxypropyl substituent have led to compounds, with comparable or better inhibitory activity than the parent compounds. Two derivatives in particular, 3-(4,5,6,7-tetrabromo-1H-benzimidazol-1-yl)propan-1-ol (MB 001, Fig. 1) and 3-(4,5,6,7-tetrabromo-1H-benzotriazol-1-yl)propan-1-ol (MB 002, Fig. 1), showed a small improvement in CK2 inhibitory activity.
The newly synthesized compound [14C]TBBi proved to be very useful for study of the distribution of TBBt/TBBi derivatives in mammalian tissues. Although the solubility of this type of compounds in water is low, the [14C] radioactivity was observed in all the examined organs. It is clear that these compounds can cross the blood/brain barrier, which makes them promising candidates for further modifications as potential therapeutic agents.
Abbreviations
- TBBi:
-
4,5,6,7-tetrabromo-1H-benzimidazole
- TBBt:
-
4,5,6,7-tetrabromo-1H-benzotriazole
- DMAT:
-
4,5,6,7-tetrabromo-1H-benzimidazol-2-N,N-dimethylamine
- N 1HETBBt:
-
2-(4,5,6,7-tetrabromo-1H-benzotriazol-1-yl)ethan-1-ol
- MB001:
-
3-(4,5,6,7-tetrabromo-1H-benzimidazol-1-yl)propan-1-ol
- MB002:
-
3-(4,5,6,7-tetrabromo-1H-benzotriazol-1-yl)propan-1-ol
- MB003:
-
3-(4,5,6,7-tetrabromo-2H-benzotriazol-2-yl)propan-1-ol
- BK005:
-
4-(4,5,6,7-tetrabromo-2H-benzotriazol-2-yl)butan-1-ol
References
Wang H, Yu S, Davis AT, Ahmed K (2003) Cell cycle dependent regulation of protein kinase CK2 signaling to the nuclear matrix. J Cell Biochem 88:812–822. doi:10.1002/jcb.10438
Ljubimov AV, Caballero S, Aoki AM, Pinna LA, Grant MB, Castellon R (2004) Involvement of protein kinase CK2 in angiogenesis and retinal neovascularization. Invest Ophthalmol Vis Sci 45:4583–4591. doi:10.1167/iovs.04-0686
Ficarro SB, McCleland ML, Stukenberg PT, Burke DJ, Ross MM, Shabanowitz J et al (2002) Phosphoproteome analysis by mass spectrometry and its application to Saccharomyces cerevisiae. Nat Biotechnol 20:301–305. doi:10.1038/nbt0302-301
Landesman-Bollag E, Song DH, Romieu-Mourez R, Sussman DJ, Cardiff RD, Sonenshein GE et al (2001) Protein kinase CK2: signaling and tumorigenesis in the mammary gland. Mol Cell Biochem 227(1–2):153–165. doi:10.1023/A:1013108822847
Zień P, Bretner M, Zastąpiło K, Szyszka R, Shugar D (2003) Selectivity of 4, 5, 6, 7-tetrabromobenzimidazole as an ATP-competitive potent inhibitor of protein kinase CK2 from various sources. Biochem Biophys Res Commun 306:129–133. doi:10.1016/S0006-291X(03)00928-8
Pagano MA, Andrzejewska M, Ruzzene M, Sarno S, Cesaro L, Bain J et al (2004) Optimization of protein kinase CK2 inhibitors derived from 4, 5, 6, 7-tetrabromobenzimidazole. J Med Chem 47:6239–6247. doi:10.1021/jm049854a
Sarno S, Reddy H, Meggio F, Ruzzene M, Davies SP, Donella-Deana A et al (2001) Selectivity of 4,5,6,7-tetrabromobenzotriazole, an ATP site-directed inhibitor of protein kinase CK2 (`casein kinase-2). FEBS Lett 496:44–48. doi:10.1016/S0014-5793(01)02404-8
Battistutta R, Mazzorana M, Cendron L, Bortolato A, Sarno S, Kazimierczuk Z et al (2007) The ATP-binding site of protein kinase CK2 holds a positive electrostatic area and conserved water molecules. ChemBioChem 8(15):1804–1809. doi:10.1002/cbic.200700307
Zień P, Duncan JS, Skierski J, Bretner M, Litchfield DW, Shugar D (2005) Tetrabromobenzotriazole (TBBt) and tetrabromobenzimidazole (TBBz) as selective inhibitors of protein kinase CK2: evaluation of their effects on cells and different molecular forms of human CK2. Biochim Biophys Acta 1754:271–280
Mishra S, Pertz V, Zhang B, Kaur P, Shimada H, Groffen J et al (2007) Treatment of P190 Bcr/Abl lymphoblastic leukemia cells with inhibitors of the serine/threonine kinase CK2. Leukemia 21(1):178–180. doi:10.1038/sj.leu.2404460
Grankowski N, Boldyreff B, Issinger OG (1991) Isolation and characterization of recombinant human casein kinase II subunits alpha and beta from bacteria. Eur J Biochem 198:25–30. doi:10.1111/j.1432-1033.1991.tb15982.x
Sarno S, Vaglio P, Meggio F, Issinger OG, Pinna LA (1996) Protein kinase CK2 mutants defective in substrate recognition. Purification and kinetic analysis. J Biol Chem 271:10595–10601. doi:10.1074/jbc.271.18.10595
Bretner M, Baier A, Kopańska K, Najda A, Schoof A, Reinholz M et al (2005) Synthesis and biological activity of 1H-benzotriazole and 1H-benzimidazole analogues—inhibitors of the NTpase/helicase of HCV and of some related Flaviviridae. Antivir Chem Chemother 16(5):315–326
Bretner M, Zień P, Najda A, Kulikowski T, Shugar D (2006) Patent Application P 380112
Acknowledgements
These studies were supported by the PBZ-MIN 014/P05/2004 grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bretner, M., Najda-Bernatowicz, A., Łebska, M. et al. New inhibitors of protein kinase CK2, analogues of benzimidazole and benzotriazole. Mol Cell Biochem 316, 87–89 (2008). https://doi.org/10.1007/s11010-008-9827-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9827-0