Abstract
The chronic effects of type 2 diabetes mellitus on myofilament sensitivity to Ca2+ in ventricular myocytes from the Goto–Kakizaki (GK) rat have been investigated. Experiments were performed in ventricular myocytes isolated from 17-month GK rats and age-matched Wistar controls. Myocytes were loaded with fura-2 (an indicator for intracellular Ca2+ concentration) and the fura-2 ratio (340/380 nm), and shortening were measured simultaneously in electrically stimulated myocytes. Myofilament sensitivity to Ca2+ was assessed from phase-plane diagrams of fura-2 versus cell length by measuring the gradient of the fura-2–cell length trajectory during late relaxation of the twitch contraction. Non-fasting and fasting blood glucose were elevated in GK rats compared to controls. Fasting blood glucose was 151.5 ± 15.3 mg/dl (n = 8) in GK rats compared to 72.1 ± 3.6 mg/dl (n = 9) in controls. At 120 min after intraperitoneal injection of glucose (2 g/kg body weight), blood glucose was 570.8 ± 36.8 mg/dl in GK rats compared to 148 ± 8.6 mg/dl in controls. Amplitude of shortening was significantly increased in myocytes from GK rats (6.56 ± 0.54%, n = 31) compared to controls (5.05 ± 0.43%, n = 36), and the amplitude of the Ca2+ transient was decreased in myocytes from GK rats (0.23 ± 0.02 RU, n = 31) compared to controls (0.30 ± 0.02 RU, n = 36). The fura-2–cell length trajectory during the late stages of relaxation of the twitch contraction was steeper in myocytes from GK rats (89.2 ± 16.6 μm/RU, n = 27) compared to controls (31.9 ± 5.9 μm/RU, n = 35). Increased amplitude of shortening, accompanied by a decrease in amplitude of the Ca2+ transient, might be explained by an increased myofilament sensitivity to Ca2+.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The effects of type 2 diabetes mellitus (DM) on heart function are poorly understood, and in general, manifestations of cardiac dysfunction in the experimental setting are only evident several months following the onset of DM. The Goto–Kakizaki (GK) rat is a genetic animal model of type 2 DM which was created by selective breeding of an outbred colony of Wistar rats, with selection for high glucose levels in an oral glucose tolerance test [1]. Several studies have investigated the effects of DM on heart function in the GK rat. In isolated perfused hearts, El Omar et al. reported unaltered left ventricular diastolic pressure and left ventricular pressure rise (LV dP/dt max) and fall (LV dP/dt min) and in ventricular myocytes, unaltered amplitude of twitch shortening and amplitude of the Ca2+ transient [2]. A magnetic resonance imaging study reported reduced myocardial blood flow, reduced ejection fraction mainly as a result of loss in left ventricular longitudinal contraction [3]. A recent study in ventricular myocytes reported unaltered amplitude of shortening and an increase in amplitude of the Ca2+ transient which might be attributed to an altered relationship between intracellular Ca2+ and the myofilaments [4]. The aim of this study was to investigate the chronic effects of DM on myofilament sensitivity to Ca2+ in ventricular myocytes from GK rats aged 17 months.
Methods
Experimental model
Groups of young male GK and age-matched control Wistar rats were maintained on the same diet and water ad libitum. Experiments were conducted at 17 months of age. Glucose tolerance tests were applied after an overnight fast [5]. Blood glucose was measured (One Touch II Blood Glucose Meter, LifeScan Inc., USA) at time 0 and 30, 60, 120 and 180 min after an intraperitoneal injection of glucose (2 g/kg body weight). Principles of laboratory animal care were followed throughout. Approval for this project was obtained from the Faculty of Medicine & Health Sciences Ethics Committee, United Arab Emirates University.
Ventricular myocyte isolation
Single ventricular myocytes were isolated according to previously described techniques [6] with minor modifications. Animals were killed humanely with a guillotine, and hearts were then removed rapidly and mounted in Langendorff mode. Hearts were perfused at a constant flow of 8 ml g heart−1 min−1 and at physiological temperature (36–37°C) with HEPES-based salt solution (isolation solution, see below) containing 0.75 mmol/l Ca2+. Perfusion flow rate was adjusted to allow for differences in heart weight between animals. When the heart had stabilized, perfusion was continued for 4 min with Ca2+-free isolation solution containing 0.1 mM EGTA and then for 6 min with isolation solution containing 0.05 mmol/l Ca2+, 0.75 mg/ml collagenase (type 1; Worthington Biochemical Corp., USA) and 0.075 mg/ml protease (type X1V; Sigma, Germany). After this time, the ventricles were excised from the heart, minced and gently shaken in collagenase-containing isolation solution supplemented with 1% BSA. Cells were filtered from this solution at 4-min intervals and resuspended in isolation solution containing 0.75 mmol/l Ca2+. Ventricular myocytes were isolated from five control and five GK rats.
Simultaneous measurement of ventricular myocyte shortening and intracellular Ca2+ concentration
Myocytes were loaded with the fluorescent indicator fura-2 AM (F-1221, Molecular Probes, USA) as described previously [6]. In brief, 6.25 μl of a 1.0 mmol/l stock solution of fura-2 AM (dissolved in dimethylsulphoxide) was added to 2.5 ml of cells to give a final fura-2 concentration of 2.5 μmol/l. Myocytes were shaken gently for 10 min at 24°C (room temperature). After loading, myocytes were centrifuged, washed with normal Tyrode solution (see below) to remove extracellular fura-2 and then left for 30 min to ensure complete hydrolysis of the intracellular ester. Ventricular myocytes were allowed to settle on the glass bottom of a Perspex chamber mounted on the stage of an inverted microscope (Axiovert 35, Zeiss, Germany). Myocytes stimulated at 1 Hz were superfused (3–5 ml/min) with a HEPES-based normal Tyrode solution containing 1.8 mmol/l Ca2+ at room temperature 25–27°C. The animals used in these experiments were 17 months of age. At this elderly age, ventricular myocytes are less robust compared to cells isolated from younger animals. In order to facilitate the simultaneous measurement of myocyte shortening and intracellular Ca2+ experiments were performed at room temperature.
Unloaded shortening was used as an index of contractility [7]. Shortening was followed using a video edge detection system (VED-114, Crystal Biotech, USA). The degree of shortening [expressed as a % of resting cell length (RCL)], the time to peak (TPK) shortening and time from peak shortening to half (THALF) relaxation were recorded. To measure intracellular Ca2+ concentration, myocytes were alternately illuminated by 340 and 380 nm light using a monochromator (Cairn Research, UK) which changed the excitation light every 2 ms. The resultant fluorescent emission at 510 nm was recorded by a photomultiplier tube, and the ratio of the emitted fluorescence at the two excitation wavelengths (340/380 ratio) was calculated to provide an index of intracellular Ca2+ concentration. The amplitude of the Ca2+ transient, the TPK Ca2+ transient and the THALF relaxation of the Ca2+ transient were recorded. Data were analysed using Signal Averager (v 6.16, Cambridge Electronic Design, UK).
Assessment of myofilament sensitivity to Ca2+
Myofilament sensitivity to Ca2+ was assessed from phase-plane diagrams of fura-2 ratio versus cell length by measuring the gradient of the fura-2–cell length trajectory during late relaxation of the twitch contraction. The position of the trajectory reflects the relative myofilament response to Ca2+ and, hence, can be used as a measure of myofilament sensitivity to Ca2+ [8].
Solutions
The Ca2+-free isolation solution contained (in mmol/l): 130 NaCl, 5.4 KCl, 1.4 MgCl2, 0.4 NaH2PO4, 5 HEPES, 10 glucose, 20 taurine and 10 creatine set to pH 7.3 with NaOH. The normal Tyrode solution contained (in mmol/l): 140 NaCl, 5 KCl, 1 MgCl2, 10 glucose, 5 HEPES, 1.8 CaCl2 (pH 7.4).
Statistics
Results were expressed as the mean ± SEM of ‘n’ observations. ‘n’ Refers to the number of animals or cells. Statistical comparisons were performed using the independent sample t-test. P < 0.05 was considered to indicate a significant difference.
Results
General characteristics
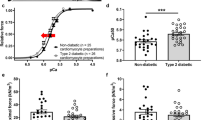
Experiments were performed in GK animals and age-matched controls at 17 months of age. Neither body weight or heart weight was significantly (P > 0.05) different in GK rats compared to controls. Mean body weight was 399 ± 22.1 g (n = 5) in GK rats compared to 436.0 ± 43.2 g (n = 5) in controls. Heart weight was 1.50 ± 0.05 g (n = 5) in GK rats compared to 1.52 ± 0.06 g (n = 5) in controls. Non-fasting and fasting blood glucose were significantly (P < 0.05) elevated in GK rats compared to controls. Non-fasting blood glucose was 188.8 ± 10.1 mg/dl (n = 5) in GK rats compared to 101.4 ± 6.1 mg/dl (n = 5) in controls. Fasting blood glucose was 151.5 ± 15.3 mg/dl (n = 8) in GK rats compared to 72.1 ± 3.6 mg/dl (n = 9) in controls. The results of the glucose tolerance test are shown in Fig. 1. Animals were fasted overnight and after a glucose challenge (2 g/kg bodyweight, i.p.), blood glucose was measured at 30, 60, 120 and 180 min. At 17 months blood glucose was significantly elevated at 30, 60, 120 and 180 min in GK rats compared to controls. At 120 min blood glucose was 570.8 ± 36.8 mg/dl (n = 8) compared to 148.1 ± 8.6 mg/dl (n = 9) in controls.
Uptake of glucose from blood following a glucose challenge at 17 months of age. After an overnight fast animals received an intraperitoneal injection of glucose (2 g glucose/kg body weight). Blood glucose was measured at time 0 and at 30, 60, 120 and 180 min after administration of glucose. Data are mean ± SEM, n = 8–9 animals. * P < 0.05, ** P < 0.01
Ventricular myocyte shortening and intracellular Ca2+
Typical records of shortening and Ca2+ transients in electrically stimulated (1 Hz) myocytes from GK and control rats are shown in Fig. 2a. Resting cell length was not significantly altered in ventricular myocytes from GK rats (109.3 ± 2.6 μm, n = 31) compared to controls (109.7 ± 2.4 μm, n = 36). TPK shortening was significantly prolonged in ventricular myocytes from GK rats (337.5 ± 8.0 ms, n = 31) compared to controls (302.7 ± 7.8 ms, n = 36) (Fig. 2b). THALF relaxation was also significantly prolonged in ventricular myocytes from GK rats (275.4 ± 10.7 ms, n = 31) compared to controls (231.3 ± 11.1 ms, n = 36) (Fig. 2c). Amplitude of shortening was significantly increased in myocytes from GK rats (6.56 ± 0.54%, n = 31) compared to controls (5.05 ± 0.43%, n = 36) (Fig. 2d).
Ventricular myocyte shortening and intracellular Ca2+. (a) Typical records of shortening and Ca2+ transient in myocytes from control (left panel) and GK (right panel) rat hearts. (b) TPK shortening, (c) THALF relaxation, (d) amplitude of shortening, (e) TPK Ca2+ transient, (f) THALF relaxation of the Ca2+ transient and (g) amplitude of the Ca2+ transient in ventricular myocytes from GK and control rat hearts. Data are mean ± SEM, n = 31–36 cells from five hearts
Resting fura-2 ratio (340/380) was not significantly altered in ventricular myocytes from GK rats (1.23 ± 0.04 RU, n = 31) compared to controls (1.32 ± 0.04 RU, n = 36). Neither the TPK nor THALF relaxation of the Ca2+ transient was significantly altered in ventricular myocytes from GK rats compared to controls. TPK Ca2+ transient and THALF relaxation of the Ca2+ transient were, respectively, 104.3 ± 5.9 and 199.0 ± 6.9 ms (n = 31) in ventricular myocytes from GK rats compared to 91.7 ± 4.0 and 199.1 ± 6.3 ms (n = 36) in controls. The amplitude of the Ca2+ transient was significantly reduced in myocytes from GK rats (0.23 ± 0.02 RU, n = 31) compared to controls (0.30 ± 0.02 RU, n = 36).
Myofilament sensitivity to Ca2+
Typical phase-plane diagrams of length versus fura-2 ratio in myocytes from GK rats and controls are shown in Fig. 3a. Myofilament sensitivity to Ca2+ was assessed by measuring the gradient of the fura-2–cell length trajectory during late relaxation of the twitch contraction. Measurements were carried out at 600–700 ms (Fig. 3b), 700–800 ms (Fig. 3c), 800–900 ms (Fig. 3d) and 600–900 ms (Fig. 3e). The position of the trajectory reflects the relative myofilament response to Ca2+ and, hence, can be used as a measure of myofilament sensitivity to Ca2+ [8]. The gradients of the trajectory for GK myocytes were significantly greater at 600–700 and at 600–900 ms compared to controls. Gradients for GK myocytes compared to controls were, respectively: 49.3 ± 11.4 (n = 27) and 23.4 ± 6.1 (n = 35) μm/RU at 600–700 ms; 22.8 ± 6.8 (n = 27) and 12.5 ± 2.4 (n = 35) μm/RU at 700–800 ms; 12.2 ± 4.4 (n = 27) and 5.0 ± 1.3 (n = 35) μm/RU at 800–900 ms; 89.2 ± 16.6 (n = 27) and 31.9 ± 5.9 (n = 35) μm/RU at 600–900 ms.
Myofilament sensitivity to Ca2+. (a) Typical phase-plane diagrams of length versus fluorescence for twitch contractions in myocytes from control (left panel) and GK (right panel) rat hearts. Dotted lines indicate the time (600–900 ms) during which trajectory measurements were calculated. Mean trajectories at (b) 600–700, (c) 700–800, (d) 800–900, (e) 600–900 ms during the relaxation phase of contraction. Data are mean ± SEM, n = 25–35 cells from five hearts. * P < 0.05, ** P < 0.01
Discussion
Experiments were performed in GK rats and Wistar controls aged 17 months. The major findings of this study were: (i) TPK and THALF relaxation of shortening were prolonged and amplitude of shortening was increased in GK myocytes compared to controls; (ii) amplitude of the Ca2+ transient was reduced in GK myocytes compared to controls; (iii) the gradient of the trajectory during the relaxation phase of the twitch contraction was significantly greater for GK myocytes compared to controls.
The GK rats displayed increased non-fasting and fasting blood glucose, characteristics which are typical of type 2 DM. Previous studies have demonstrated elevations in blood plasma glucose in GK rats from as young as 1 month after weaning [9]. The GK rats also displayed glucose intolerance which is consistent with impaired secretion of insulin and peripheral insulin resistance [1, 9], which may contribute to cardiac hypertrophy [10].
The time course of myocyte shortening, including the TPK and THALF relaxation, was prolonged, and the amplitude of shortening was increased in electrically stimulated (1 Hz) ventricular myocytes from GK rats compared to age-matched controls. Previous studies in isolated perfused heart preparations from younger animals have reported unaltered left ventricular diastolic pressure and left ventricular pressure rise (LV dP/dt max) and fall (LV dP/dt min) and unaltered amplitude of myocyte twitch shortening [2]. Another study which employed magnetic resonance imaging in female GK rats demonstrated reduced myocardial blood flow, reduced ejection fraction mainly as a result of loss in left ventricular longitudinal contraction [3]. Interestingly, there is exaggerated diastolic dysfunction during hypoxia [2], and ageing female GK rat heart has an increased susceptibility to ischaemic injury compared to male GK rat heart [11]. A more recent study also demonstrated unaltered amplitude of shortening in ventricular myocytes from GK rats compared to controls [4].
Although the time course of the Ca2+ transient was not significantly altered, the amplitude of the Ca2+ transient was reduced in myocytes from GK rats compared to controls. Previous studies have reported unaltered or increased intracellular Ca2+ levels in GK animals [2, 4, 10]. Increased, or unaltered, amplitude of myocyte shortening accompanied by decreased amplitude of the Ca2+ transient may be explained by altered myofilament sensitivity to Ca2+. Previous studies in the STZ-induced diabetic rat have demonstrated diminished sensitivity of myofilaments to Ca2+ and alterations in myosin isoenzyme pattern, for example from V1 to V3 isoenzyme, which in turn may partly underlie the contractile defects which have been frequently reported in the diabetic heart [12–14]. An increase in systolic Ca2+ might provide a mechanism to compensate for defective myofilament sensitivity to Ca2+.
Myofilament sensitivity to Ca2+ was assessed from phase-plane diagrams of fura-2 ratio versus cell length by measuring the gradient of the fura-2–cell length trajectory during late relaxation of the twitch contraction. The position of the trajectory during the relaxation phase reflects the relative myofilament response to Ca2+ and, hence, can be used as a measure of myofilament sensitivity to Ca2+ [8]. The gradient of the trajectory during the relaxation phase of the twitch contraction was significantly greater for GK myocytes compared to controls suggesting that myofilaments sensitivity to Ca2+ is increased in GK myocytes compared to controls. Previous studies in STZ-induced diabetic rats, which is a widely used experimental model of type 1 DM, have also demonstrated an increase in myofilament sensitivity to Ca2+ [15–17]. In the STZ-induced diabetic rat heart decreased expression of SR Ca2+-ATPase and ryandodine receptor protein partly underlies the decreased uptake and release of Ca2+ which in turn contributes to the prolonged time course and decreased amplitude of the Ca2+ transient [18]. Interestingly, there was a decrease in the amplitude of the Ca2+ transient, whilst there was a small increase in the amplitude of myocyte shortening. Alterations in myofilament sensitivity to Ca2+ might provide a compensatory mechanism to preserve mechanical function of the diabetic heart.
It is concluded that altered myofilament sensitivity to Ca2+ partly underlies contractile dysfunction in ventricular myocytes from the GK diabetic rat.
References
Goto Y, Kakizaki M, Masaki N (1975) Spontaneous diabetes produced by selective breeding of normal Wistar rats. Proc Jpn Acad 51:80–85
El Omar MM, Yang ZK, Phillips AO, Shah AM (2004) Cardiac dysfunction in the Goto-Kakizaki rat. A model of type II diabetes mellitus. Basic Res Cardiol 99:133–141. doi:10.1007/s00395-004-0440-4
Iltis I, Kober F, Desrois M, Dalmasso C, Lan C, Portha B et al (2005) Defective myocardial blood flow and altered function of the left ventricle in type 2 diabetic rats: a noninvasive in vivo study using perfusion and cine magnetic resonance imaging. Invest Radiol 40:19–26
Howarth FC, Shafiullah M, Qureshi MA (2007) Chronic effects of type 2 diabetes mellitus on cardiac muscle contraction in the Goto-Kakizaki rat. Exp Physiol 92:1029–1036. doi:10.1113/expphysiol.2007.038703
Howarth FC, Qureshi MA (2001) Characterisation of ventricular myocyte shortening after administration of streptozotocin (STZ) to neonatal rats. Arch Physiol Biochem 109:200–205. doi:10.1076/apab.109.3.200.11598
Howarth FC, Qureshi MA, White E (2002) Effects of hyperosmotic shrinking on ventricular myocyte shortening and intracellular Ca(2+) in streptozotocin-induced diabetic rats. Pflügers Arch 444:446–451. doi:10.1007/s00424-002-0830-0
White E, Boyett MR, Orchard CH (1995) The effects of mechanical loading and changes of length on single guinea-pig ventricular myocytes. J Physiol 482:93–107
Spurgeon HA, DuBell WH, Stern MD, Sollott SJ, Ziman BD, Silverman HS et al (1992) Cytosolic calcium and myofilaments in single rat cardiac myocytes achieve a dynamic equilibrium during twitch relaxation. J Physiol 447:83–102
Portha B, Serradas P, Bailbe D, Suzuki K, Goto Y, Giroix MH (1991) Beta-cell insensitivity to glucose in the GK rat, a spontaneous nonobese model for type II diabetes. Diabetes 40:486–491. doi:10.2337/diabetes.40.4.486
Darmellah A, Baetz D, Prunier F, Tamareille S, Rucker-Martin C, Feuvray D (2007) Enhanced activity of the myocardial Na(+)/H (+) exchanger contributes to left ventricular hypertrophy in the Goto-Kakizaki rat model of type 2 diabetes: critical role of Akt. Diabetologia 50:1335–1344. doi:10.1007/s00125-007-0628-x
Desrois M, Sidell RJ, Gauguier D, Davey CL, Radda GK, Clarke K (2004) Gender differences in hypertrophy, insulin resistance and ischemic injury in the aging type 2 diabetic rat heart. J Mol Cell Cardiol 37:547–555. doi:10.1016/j.yjmcc.2004.05.014
Malhotra A, Sanghi V (1997) Regulation of contractile proteins in diabetic heart. Cardiovasc Res 34:34–40. doi:10.1016/S0008-6363(97)00059-X
Takeda N, Nakamura I, Hatanaka T, Ohkubo T, Nagano M (1988) Myocardial mechanical and myosin isoenzyme alterations in streptozotocin-diabetic rats. Jpn Heart J 29:455–463
Takeda N, Hatanaka T, Nakamura I, Ohkubo T, Iwai T, Tanamura A et al (1989) Ventricular myosin isoenzyme pattern and myocardial contractility. Prog Clin Biol Res 315:597–599
Howarth FC, Qureshi MA (2001) Myofilament Ca2+ sensitivity in ventricular myocytes from streptozotocin-induced diabetic rat. Int J Diabetes Metab 9:67–74
Woodall A, Bracken N, Qureshi A, Howarth FC, Singh J (2004) Halothane alters contractility and Ca2+ transport in ventricular myocytes from streptozotocin-induced diabetic rats. Mol Cell Biochem 261:251–261. doi:10.1023/B:MCBI.0000028763.15680.07
Khandoudi N, Guo AC, Chesnais M, Feuvray D (1993) Skinned cardiac fibres of diabetic rats: contractile activation and effects of 2,3-butanedione monoxime (BDM) and caffeine. Cardiovasc Res 27:447–452. doi:10.1093/cvr/27.3.447
Choi KM, Zhong Y, Hoit BD, Grupp IL, Hahn H, Dilly KW et al (2002) Defective intracellular Ca(2+) signaling contributes to cardiomyopathy in type 1 diabetic rats. Am J Physiol 283:H1398–H1408
Acknowledgements
The project was supported by a grant from the Faculty of Medicine & Health Sciences, United Arab Emirates University, Al Ain, United Arab Emirates.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Howarth, F.C., Qureshi, M.A. Myofilament sensitivity to Ca2+ in ventricular myocytes from the Goto–Kakizaki diabetic rat. Mol Cell Biochem 315, 69–74 (2008). https://doi.org/10.1007/s11010-008-9790-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9790-9