Abstract
The purpose of the present work was to determine differences between young men (M), and women in the follicular phase (W) and women taking oral contraceptives containing ethinylestradiol (CW) in the phagocytic process of neutrophils (chemotaxis, phagocytosis and microbicide capacity), in the serum concentrations of cytokines both pro-inflammatory (IFNγ, TNFα, IL-12, IL-6, IL-8) and anti-inflammatory ones (IL-10 and IL-13), and in neuroendocrine factors with immunomodulatory capacity (estradiol, prolactin, cortisol, catecolamines and 72 kDa heat shock proteins, Hsp72). Men showed a lower phagocytosis and microbicide capacity than women, and less serum concentrations of the pro-inflammatory cytokines IL-6 and IL-8. CW neutrophils also showed a lower phagocytic capacity than W neutrophils, together with less serum IL-8 concentration. CW showed the highest serum concentration of IL-13. However, no statistical changes were observed in the pro-inflammatory cytokines: INF-γ, TNF-α, IL-12 and in the anti-inflammatory cytokine IL-10. The greater anti-inflammatory status in CW than in W was parallel with lower concentrations of oestrogens. Cortisol, prolactin, and the extracellular Hsp72 seem to be involved in the gender- and contraceptives-induced differences in the inflammatory response. While cortisol (in general an immunosuppressive hormone) showed the highest values in CW, prolactin and Hsp72 (an immunopermissive factors) showed the lowest values in CW and M. Less clear is the participation of catecholamines in the gender-inflammatory differences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In addition to their effects on sexual differentiation and reproduction, sex hormones appear to influence the immune system, that results in a sexual dimorphism in the immune response in humans: for instance, females produce more vigorous cellular and more vigorous humoral immune reactions, they are more resistant to certain infections, and they suffer a higher incidence of autoimmune diseases. Disease expression is also affected by the reproductive status of the females. As sex steroids (oestrogens, progesterone and testosterone) differ between genders and within different reproductive stages, and a lot of research has focussed on the effects of sex hormones on immune responses [1]. Oestrogens increase humoral immunity and androgens and progesterone are natural immunosuppressors. Several physiological, pathological and therapeutic conditions, such as the menstrual cycle, chronic stress, inflammatory cytokines and the use of corticosteroids, oral contraceptives and steroid hormonal replacements may change the serum oestrogen milieu [2] and in turn, oestrogen affects immunity. However, sexual dimorphism is not only dependent of sexual hormones. The immune system is linked to the neuroendocrine system and both systems are connected bidirectionally and share signalling pathways. The neuroimmunoendocrine interactions are also dimorphic [3, 4], and many neuroimmunoendocrine interactions are mediated by stress hormones and factors, which are potent modulators of the immune system. In general, stress hormones such as cortisol [5] and catecholamines [6, 7] are immunesuppressors, while others, such as prolactin [6] and even the 72 kDa heat shock protein (Hsp72) [8] are immunestimulatory.
Neutrophils are key cells in the inflammatory responses and infectious process, and they act killing pathogens or dead tissues. In addition to sexual hormones [6, 9, 10] studies in our lab have shown that cortisol [11], catecholamines and Hsp72 at physiological concentrations modulate the phagocytic process of neutrophils [8]. However, whereas inflammation plays an important role in host defence, uncontrolled inflammatory reactions are responsible of the initiation and progression of autoimmune and inflammatory diseases. Local response to infection also implies cytokine production, which are released in the inflammation site. Cytokines are generally divided into pro-inflammatory and anti-inflammatory. The balance between pro- and anti-inflammatory cytokines is crucial in the inflammatory response and moreover in the development of autoimmune diseases [12]. Pharmacological manipulation of gonadal hormones levels also influences susceptibility to several autoimmune diseases [13] and affects the clinical course of these pathologies [14].
The present work analyzes and compares the inflammatory basal response on men, women in the follicular phase of their menstrual cycle and women who take oral contraceptives. For this, all stages of neutrophils phagocytic process were studied and serum concentration of several pro- and anti-inflammatory cytokines as well as neuroendocrine factors with immunomodulatory capacity (Hsp72, catecholamines, estradiol, cortisol and prolactin) were also evaluated.
Materials and methods
Subjects
Men (n = 10) and women (n = 20) volunteers aged between 20 and 24 years participated in the study. They had to be healthy, non-smokers, and not heavy consumer of alcohol. The volunteers were divided in three experimental groups: women in the follicular phase (W), women taking oral contraceptives (ethinylestradiol) (SCHERING) (CW) and men (M).
Isolation of neutrophils
Peripheral venous blood samples were drawn by antecubital vein puncture. The blood was centrifuged in a density gradient (Histopaque, Sigma), and the neutrophils were harvested, washed twice in Hank´s medium (Sigma), counted, and adjusted to 106 cells ml−1 of medium. Cell viability was checked by the Trypan blue exclusion test, which gave 98% viable cells.
Chemotaxis assay
Chemotaxis was evaluated using a modification of the original technique described by Boyden. Briefly, 300 μl aliquots of neutrophils suspension (1 × 106 cells ml−1 medium) were deposited in the upper compartment of a Boyden chamber. The filter used (isopore, polycarbonate) had a pore diametre of 3 μm (Millipore). fMLP peptide (10−8 M; Sigma) was put into the lower compartment to induce chemotaxis. After 90 min of incubation at 37°C and 5% CO2, the filter was fixed and stained. A chemotactic index, representing the total number of neutrophils counted at random (under microscope, ×100) in 16 fields of the lower face of the filters, was calculated [15].
Serum
Serum was obtained from venous blood from volunteers by centrifugation at 700g for 10 min. For phagocytosis and microbicide capacity tests, serum was used as opsonin source and serum was obtained from venous blood taken from the volunteers at their basal state as described. Hormones and cytokines determinations were performed on serum obtained from the volunteers at basal state, immediately after exercise and 24 h. later as described. These samples were stored at −20°C until assay.
Phagocytosis assay
Phagocytosis of C. albicans by neutrophils was evaluated ex vivo using a technique previously described for isolated neutrophils [11]. Briefly, 0.5 ml C. albicans suspension (106 cells ml−1) and 50 μl of serum were added to 0.5 ml of the neutrophil suspension (106 neutrophils ml−1), followed by incubation in a thermostatic bath at 37°C for 60 min with shaking. The samples were then centrifuged at 300g for 10 min, discarding two-thirds of the supernatant. The remainder of the supernatant was shaken, and an aliquot was taken for counting the number of C. albicans ingested by 100 neutrophils (phagocytic index) in a Neubauer haemocytometer under a phase contrast microscope.
Phagocytosis of latex beads was studied incubating 200 μl of neutrophil suspension (106 cells/ml) on MIF plates at 37°C and 5% CO2 for 30 min and then 20 μl of latex beads (1.09 μl diameter diluted to 1% in PBS, SIGMA) were added. After 30 min of incubation, the plates were washed, fixed, and stained, and the number of particles ingested by 100 macrophages was determined under microscope ×100.
Microbicide capacity of neutrophils
Microbicidal capacity of neutrophils has been studied by using both direct (killing of phagocytosed Candida albicans) and indirect (superoxide anion production after phagocytosis of inert particles) techniques.
Killing of phagocytosed C. albicans (living cells) was evaluated ex vivo using a technique previously described for isolated neutrophils [11]. Briefly, the technique is similar to phagocytosis but 1.5 ml of methylene blue (0.01%), (that stained the dead C. albicans), was added at 50 min of incubation. The samples were then centrifuged at 300 g for 10 min, and the number of phagocytosed and dead C. albicans counted. Results are expressed as the percentage of dead C. albicans of the total phagocytosed by 100 neutrophils (Candidicide index).
The oxygen-dependent microbicide capacity of neutrophils was evaluated by means of the superoxide anion production. The assay was performed by the nitroblue tetrazolium (NBT; SIGMA) reduction test. 250 μl of neutrophil suspension (1 × 106 cells ml−1) were incubated with 250 μl of NBT (1 mg ml−1 in PBS) and 25 μl of latex beads (1% in PBS) (stimulated samples), or 25 μl of PBS (non-stimulated samples). After 30 min of incubation, the reaction was stopped with HCl (O.5 N) and the samples centrifuged (30 min, 700g). The intracellular reduced NBT (intracellular concentration of superoxide anion) was extracted with dioxan (SIGMA). The absorbances were deterinated using the same amounts of Hank’s solution, NBT, and HCl or dioxan, respectively as blank.
Cytokine determinations
Serum concentration of pro- (INFγ, TNFα, IL-6, IL-8 and IL-12) and anti-inflammatory cytokines (IL-10 and IL-13) were evaluated by ELISA (IZASA and Sankin).
Determination of Hsp72, cortisol, prolactin, estradiol, epinephrine and norepinephrine concentrations
For the Hsp72, cortisol, prolactin and estradiol, serum was obtained by centrifuging (700g for 10 min) 1 ml of blood from each volunteer. Cortisol, prolactin and 17-β estradiol were measured by electrochemiluminiscence immunoassay (ECLIA) by using a automatic analyzer (ROCHE ELECSYS). Hsp72 concentration was measured by ELISA (Stressgen). For the catecholamine assay, 40 ml stabilizing solution (900 mg of EGTA and 700 mg of glutathione in 10 ml of 0.1 M NaOH) was added to 2 ml of each blood sample before separation of the plasma. The plasma was then isolated by centrifugation as before. Catecholamines were measured by HPLC (Electrochemical detection, Coulochem) through a commercial kit (Cromosystems Instruments and Chemicals GMBH). All plasma samples were stored at −20°C until assay.
Statistical analysis
The variables were normally distributed. The Student t-test among the pair of groups (non-paired samples) was used for comparisons, taking P < 0.05 as the minimum significance level. Values are given as means (SEM).
Results
Chemotaxis, phagocytosis of latex beads and the production of O −2 did not seem to be influenced by sex or by oral contraceptives intake, because no statistical differences were found between the three experimental groups (W, CW, M) (Table 1).
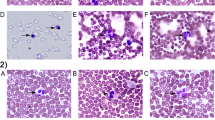
Men showed a lower phagocytosis (P < 0.001) and microbicide (P < 0.01) capacity against C. albicans than women (Fig. 1), and less serum concentrations (P < 0.05) of the pro-inflammatory cytokines IL-6 and IL-8. CW neutrophils also showed lower phagocytic (P < 0.01) and microbicide (P < 0.05) capacity than W neutrophils (Fig. 1), together with less serum IL-8 concentration (a cytokine that activates neutrophils) (Table 2). Nevertheless, phagocytic and microbicide capacity of neutrophils were still higher (P < 0.05) in CW than in M. Serum IL-13 concentration (an anti-inflammatory cytokine) showed the highest values in CW (P < 0.05) (Table 2).
Serum concentrations of all evaluated cytokines were in the average range of healthy people. Nevertheless, although without statistical differences, W showed slightly lower values than men in the serum concentrations of pro-inflammatory cytokines INFγ, TNFα, IL-12 as well as in the serum concentrations of the IL-10.
Estradiol, cortisol, prolactin, catecholamines and the extracellular Hsp72 seems to be involved in the gender and contraceptives-induced differences in the inflammatory response (Table 3). M and CW showed the lowest levels of estradiol whereas cortisol (in general an immunosuppressive hormone) showed the highest values in CW with significant differences with respect to W (P < 0.05) and M (P < 0.05). Furthermore, prolactin in CW and M showed lower (P < 0.01) values than in W. Hsp72 also presented the lowest (P < 0.05 with respect to W) serum concentration in both M and CW (Table 3). With respect to catecholamines, men showed higher values of epinephrine (P < 0.001 with respect to both W and P < 0.001 CW) and lower values of norepinephrine (P < 0.05 only with respect to CW).
Discussion
Women are more susceptible to suffer autoimmune diseases and moreover these diseases have been linked to abnormalities of the systemic anti-inflammatory factors [16]. Although nowadays there are evidences which show that autoimmune diseases are more prevalent in women than in men [17–21], there are not many studies which have analyse the influence of gender on the inflammatory response, which is frequently in relation with these pathologies. In addition to sex hormones, immune system, especially the innate or inflammatory response, is also modulated by stress hormones. The aim of this research was to study if there were differences in the inflammatory response between men (M), women who did not take oral contraceptives (W) and women who take oral contraceptives (CW).
In order to study the inflammatory response it was evaluated neutrophil function (chemotaxis, phagocytic and microbicidal capacity against C. albicans, phagocytic capacity of latex beads and O −2 production) as well as the systemic release of pro- and anti-inflammatory cytokines. Moreover possible differences in estradiol, prolactin, cortisol, catecholamine and Hsp72 were also evaluated.
Chemotaxis, the first stage of the phagocytic process of neutrophils, is not different between men and women. Neither, it was not found statistical differences between men and women in the phagocytosis of inert particles and in the oxygen-dependent microbicide capacity (O −2 production after phagocytosing latex beads) of neutrophils. However, neutrophils from M showed lower phagocytic and microbicide capacity against C. albicans than both groups of women (W, CW), but neutrophils from CW showed lower capacity than W, which could be a subjacent mechanism involved in the higher incidence of candidiasis reported in general in women taking contraceptives [22]. The lower neutrophil function in the neutrophils of M and CW was in parallel with a lower serum concentration of IL-8. IL-8 is a pro-inflammatory cytokine generated by monocytes and endothelial cells that activate neutrophils. For this reason, a systemic higher concentration of IL-8, may be responsible of the higher neutrophils function found in W with respect to M and CW. It has been recently reported that IL-8 concentration does not change during the menstrual cycle [23] but however, it seems that the use of oral contraceptives containing ethynilestradiol may reduce IL-8 concentration and, in turn, this fact could decrease phagocytic and microbicide function of neutrophils. Although no previous studies have evaluated the effect of this synthetic estrogenic hormone on phagocytosis and microbicide capacity of neutrophils, there are studies that have reported ethynilestradiol as an anti-inflammatory hormone, and it has been used in the treatment of autoimmune/inflammatory diseases [24, 25]. These anti-inflammatory effects results in the inhibition of TNFα and IL-1β [25] and in the recruitment of inflammatory cells [24]. However, we have not detected significant differences induced by oral contraceptives containing ethynilestradiol between W and CW neither in chemotaxis nor in the serum concentration of INFγ, TNFα, IL-12, IL-6.
The equilibrium between pro- and anti-inflammatory cytokines is crucial in several infections, allergic and inflammatory/autoimmune diseases [12]. Our results did not show significant differences in the concentrations of the pro-inflammatory cytokines INF-γ, TNF- α and IL-12, and in the anti-inflammatory cytokine IL-10 between the three groups. On the contrary, CW showed a lower concentration of IL-8 and a higher concentration of IL-13. Moreover, M also showed lower concentration of IL-6 than W. Sexual differences in the concentration of IL-6 can be involved in the higher incidence of autoimmune diseases in women, such as systemic lupus erythematosus (SLE) [26], in fact, women with SLE have higher concentration of IL-6 and also showed greater IL-6 response during stress than men [27], and stress is closely associated with autoimmune/inflammatory diseases.
Stress hormones, such as glucocorticoids and catecholamines can decrease the systemic concentrations of pro-inflammatory cytokines but stimulate the anti-inflammatory ones [12]. In our study, CW showed the highest cortisol and norepinephrine concentrations that correlated with a less systemic IL-8 concentration, and with the highest values of IL-13. This indicates a lower inflammatory systemic status in women taking contraceptives. In addition, high levels of oestrogens and prolactin (pro-inflammatory hormones) and low levels of glucocorticoids (an anti-inflammatory hormone), have been described in the active phase of autoimmune/inflammatory diseases [28]. Nevertheless, although oestrogens have been clearly involved in the gender differences and severity of inflammatory diseases, anti-inflammatory as well as pro-inflammatory responses to oestrogens have been reported [29]. In our study, the greater anti-inflammatory status in CW than in W was parallel with lower concentrations of oestrogens and prolactin and, greater concentration of cortisol.
Stress hormones are also strong modulators of the neutrophils function. The lower neutrophils function in CW seems to be in agreement with a higher cortisol concentration and a lower prolactin concentration. This suggests that CW women could be better protected from an inflammatory overstimulation. In fact, cortisol has showed beneficial effects in patients with inflammatory diseases. The higher concentration of epinephrine in men than in women correlated with a lower phagocytic and microbicide capacity of neutrophils. In this way, previous studies in our lab found that physiological basal concentrations of epinephrine inhibit phagocytosis and killing of C. albicans by neutrophils in men [30]. On the contrary, epinephrine can stimulate the phagocytic activity of neutrophils in women [31], which suggests a role for epinephrine in the sexually dimorphic phagocytic function of neutrophils. In addition, men showed the lowest concentration in norepinephrine concentration, supporting a role for norepinephrine in the lower phagocytic function of neutrophils. Recent studies in our lab support this hypothesis since physiological concentrations of norepinephrine stimulates phagocytosis of C. albicans by neutrophils with the participation of β-adrenoreceptors [8, 31, 32]. It seems clear that catecholamines (perhaps with different functions for epinephrine and norepinephrine) participate in the gender immune differences. In fact, it has been reported that β2-adrenergic receptors regulation of human neutrophils function is also sexually dimorphic [21].
Heat shock protein of 72 kDa (Hsp72) is upregulated intracellularly in response to different stressors, enhancing cellular survival following stress [33–35]. These proteins may also be released into the blood during several stressors and they may be involved in the modulation of innate/inflammatory response during stress [36, 37]. Recently, it has been also reported a sexual dimorphism in the intracellular Hsp72 concentration in several tissues in response to stress. Thus, although no differences were found in the Hsp72 concentration at basal state, female rats showed an attenuated Hsp72 response after stress than male [35].
Up to our knowledge this is the first study to evaluate differences between men and women (taking or not oral contraceptives) in the extracellular concentration of Hsp72. W group presented higher concentration of Hsp72 than CW and M. The higher Hsp72 concentration in W may be involved in the greater activation of neutrophils for phagocytosing and killing C. albicans. In fact, Hsp72 stimulates the microbicide capacity of neutrophils against C. albicans [8]. Several studies have linked oestrogen and Hsp72 [38] involving oestrogen also in protecting cells from damage [35, 39]. Nickerson et al. [35] have suggested that when oestrogen levels are the lowest, stress-induced intracellular Hsp72 would be at its highest. However, we found that W group presented higher serum concentration of extracellular Hsp72 in parallel with the highest concentration of estradiol. A possible explanation for our results may be that high levels of oestrogens allow the release of Hsp72 into the blood from cells from different tissues, explaining the high serum concentration of the extracellular protein. In fact, M and CW, who have the lowest serum concentration of oestrogens, also present the lowest extracellular Hsp72 concentration in blood. The lower oestrogen concentration in the bloodstream in CW and in M could avoid the release of Hsp72 from cells to blood, avoiding its stimulatory effects on the phagocytic and microbicide capacity of human neutrophils, which explain the lower neutrophil function in these groups.
In conclusion, there is a clear gender dimorphism in phagocytic and microbicide capacity of neutrophils against C. albicans. Neutrophils function is higher in women than in men and women taking contraceptives. In these differences in neutrophil function seems to be involved the concentration of IL-8 and the difference in “stress factors”, both immunostimulatories and immunoinhibitories.
The higher phagocytic and microbicide capacity of neutrophils could increase resistance to infection in women compared to men. Nevertheless, the higher “inflammatory status” (greater neutrophil activation and IL-8 and IL-6 concentrations) could also contribute to the higher incidence of inflammatory diseases in women. The intake of oral contraceptives containing etynilestradiol can reduce the resistance to infection of C. albicans. Nevertheless, these contraceptives improve the “inflammatory status” (lower neutrophils activation, and IL-8 concentration, together with higher IL-13 concentration) and they could decrease the prevalence of inflammatory diseases in women.
References
Bouman A, Heineman MJ, Faas MM (2005) Sex hormones and the immune response in humans. Hum Reprod Update 11:411–423
Cutolo M, Capellino S, Sulli A, Serioli B, Secchi ME, Villaggio B, Straub RH (2006) Estrogens and autoimmune diseases. Ann N Y Acad Sci 1089:538–547
Besedovsky HO, Del Rey A (1996) Immune-neuro-endocrine interactions: facts and hypotheses. Endocr Rev 17:64–102
De Leon-Nava MA, Morales-Montor J (2006) Immune sexual dimorphism: can sex steroids affect the Th1/Th2 cytokine profile? Rev Invest Clin 58:161–169
Munk A, Guyre PM (1991) Glucocorticoids and immune function. In: Ader R, Felten DL, Cohen N (eds) Psychoneuroimmunology. Academic Press, New York, pp 447–464
Ortega E (2003) Neuroendocrine mediators in the modulation of phagocytosis by exercise: physiological implications. Exerc Immunol Rev 9:70–94
Nagatomi R, Kaifu T, Okutsu M, Zhang X, Kanemi O, Ohmori H (2000) Modulation of the immune system by the autonomic nervous system and its implication in immunological changes after training. Exerc Immunol Rev 6:54–74
Ortega E, Giraldo E, Hinchado MD, Martinez M, Ibañez S, Cidoncha A, Collazos ME, Garcia JJ (2006) Role of Hsp72 and norepinephrine in the moderate exercise-induced stimulation of neutrophils’ microbicide capacity. Eur J Appl Physiol 98:250–255
Molloy EJ, O’Neill AJ, Grantham JJ, Sheridan-Pereira M, Fitzpatrick JM, Webb DW, Watson RW (2003) Sex-specific alterations in neutrophil apoptosis: the role of estradiol and progesterone. Blood 102:2653–2659
Yavuz S, Ozilhan G, Elbir Y, Tolunay A, Eksioglu-Demiralp E, Direskeneli H (2007) Activation of neutrophils by testosterone in Behçet’s disease. Clin Exp Rheumatol 25:46–51
Ortega E, Collazos ME, Maynar M, Barriga C, De la Fuente M (1993) Stimulation of the phagocytic fuction of neutrophils in sedentary men after acute moderate exercise. Eur J Appl Physiol Occup Physiol 66:60–64
Elenkov IJ, Chrousos GP (2002) Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann N Y Acad Sci 966:290–303
Da Silva JA (1999) Sex hormones and glucocorticoids: interactions with the immune system. Ann N Y Acad Sci 876:102–117
Roubinian J, Talal N, Siiteri PK, Sadakian JA (1979) Sex hormone modulation of autoimmunity in NZB/NZW mice. Arthritis Rheum 22:1162–1169
Ortega E, Barriga C, de la Fuente M (1993) Study of the phagocytic process in neutrophils from elite sports women. Eur J Appl Physiol 66:37–42
Elenkov IJ, Chrousos GP (2006) Stress system-organization, physiology and immunoregulation. Neuroimmunomodulation 13:257–267
Green MS (1992) The male predominance in the incidence of infectious diseases in children: a postulated explanation for disparities in the literature. Int J Epidemiol 21:381–386
Da Silva JA (1995) Sex hormones, glucocorticoids and autoimmunity: facts and hypothesis. Ann Rheum Dis 54:6–16
Gaillard RC, Spinedi E (1998) Sex- and stress-steroids interactions and the immune system: evidence for a neuroendocrine-immunological sexual dimorphism. Domest Anim Endocrinol 15:345–352
Castagnetta L, Granata OM, Traina A, Cocciadiferro L, Saetta A, Stefano R, Cutolo M, Carruba G (2002) A role for sex steroids in autoimmune diseases: a working hypothesis and supporting data. Ann N Y Acad Sci 966:193–203
de Coupade C, Gear RW, Dazin PF, Sroussi HY, Green PG, Levine JD (2004) Beta 2-adrenergic receptor regulation of human neutrophil function is sexually dimorphic. Br J Pharmacol 43:1033–1041
Lebedeva OP, Kalutskiĭ PV (2007) Anti-infectious defense of vagina during use of low-dose monophasic contraceptives. Zh Mikrobiol Epidemiol Immunobiol 1:67–70
O’Brien SM, Fitzgerald P, Scully P, Landers A, Scott LV, Dinan TG (2007) Impact of gender and menstrual cycle phase on plasma cytokine concentrations. Neuroimmunomodulation 14:84–90
Subramanian S, Matejuk A, Zamora A, Vandenbark AA, Offner H (2003) Oral feeding with ethinyl estradiol suppresses and treats experimental autoimmune encephalomyelitis in SJL mice and inhibits the recruitment of inflammatory cells into the central nervous system. J Immunol 170:1548–1555
Subramanian S, Tovey M, Afentoulis M, Krogstad A, Vandenbark AA, Offner H (2003) Ethinyl estradiol treats collagen-induced arthritis in DBA/1LacJ mice by inhibiting the production of TNF-alpha and IL-1beta. Clin Immunol 115:162–172
Hu S, Xu Q, Xiao W, Huang M (2006) The expression of molecular chaperone HSP90 and IL-6 in patients with systemic lupus erythematosus. J Huazhong Univ Sci Technol Med Sci 26:664–666
Edwards KM, Burns VE, Ring C, Carroll D (2006) Sex differences in the interleukin-6 response to acute psychological stress. Biol Psychol 71:236–239
Jara LJ, Navarro C, Medina G, Vera-Lastra O, Blanco F (2006) Immune-neuroendocrine interactions and autoimmune diseases. Clin Dev Immunol 13:109–123
Nilso BO (2007) Modulation of the inflammatory responses by estrogens with focus on the endothelium and its interactions with leukocytes. Inflam Res 56:269–273
Malpica MI, Rodríguez AB, Sáez MC, García JJ, Barriga C, Ortega E (2002) In vitro study of the effect of adrenaline on the functional capacity of human neutrophils: role during exercise. J Neuroendocrinol 14:824–828
Ortega E, Giraldo E, Hinchado MD, Martin L, Garcia JJ, dela Fuente M (2007) Neuroimmunomodulation during exercise: role of catecholamines as ‘stress mediator’ and/or ‘Danger signal’ for the innate immune response. Neuroimmunomodulation 14:206–212
Ortega E, Marchena JM, García JJ, Barriga C, Rodríguez AB (2005) Norepinephrine as mediator in the stimulation of phagocytosis induced by moderate exercise. Eur J Appl Physiol 93:714–718
Bertrand N, Sirén AL, Tworek D, McCarron RM, Spatz M (2000) Differential expression of HSC73 and HSP72 mRNA and proteins between young and adult gerbils after transient cerebral ischemia: relation to neuronal vulnerability. J Cereb Blood Flow Metab 20:1056–1065
Bidmon B, Endemann M, Arbeiter K, Ruffingshofer D, Regele H, Herkner K, Eickelberg O, Aufricht C (2004) Overexpression of HSP-72 confers cytoprotection in experimental peritoneal dialysis. Kidney Int 66:2300–2307
Nickerson M, Kennedy SL, Johnson JD, Fleshner M (2006) Sexual dimorphism of the intracellular heat shock protein 72 response. J Appl Physiol 101:566–575
Flescher M, Campisi J, Jonhson JD (2003) Can exercise stress facilitate innate immunity? A functional role for stress-induced extracellular Hsp72. Exerc Immunol Rev 9:6–24
Asea A (2005) Stress proteins and initiation of immune response: chaperokine activity of hsp72. Exerc Immunol Rev 11:34–45
Heneka MT, Galea E, Gavriluyk V, Dumitrescu-Ozimek L, Daeschner J, O’Banion MK, Weinberg G, Klockgether T, Feinstein DL (2002) Noradrenergic depletion potentiates beta-amyloid-induced cortical inflammation: implications for Alzheimer’s disease. J Neurosci 22:2434–2442
Heneka MT, Gavrilyuk V, Landreth GE, O’Banion MK, Weinberg G, Feinstein DL (2003) Noradrenergic depletion increases inflammatory responses in brain: effects on IkappaB and HSP70 expression. J Neurochem 85:387–398
Acknowledgements
This investigation was supported in part by grants of II (2PR04A076) and III (PRI06A172) and a fellowship from the “Consejería de Infraestructura y Desarrollo Tecnológico , Junta de Extremadura”, and the “Fondo Social Europeo” and a fellowship from UEX (“Banco Santander Program”).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giraldo, E., Hinchado, M.D., Garcia, J.J. et al. Influence of gender and oral contraceptives intake on innate and inflammatory response. Role of neuroendocrine factors. Mol Cell Biochem 313, 147–153 (2008). https://doi.org/10.1007/s11010-008-9752-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9752-2