Abstract
Objective To determine the incidence of methylene tetrahydrofolate reductase (MTHFR) gene 677C→T polymorphism and plasma homocysteine (Hcy) levels in a group of subjects who underwent coronary angiography, in an attempt to establish a correlation between these parameters and the severity of coronary artery disease (CAD) and to investigate the correlation between hyperhomocysteinemia (HHcy) and the presence of 677C→T polymorphism. Background Elevated plasma Hcy level is an independent risk factor for CAD. A common mutation (677C→T) in the gene coding for MTHFR has been reported to reduce the enzymatic activity and is associated with elevated levels of Hcy, especially in subjects with low folate intake. Methods The study group comprised of 84 patients with CAD and 100 age-and-sex matched controls who had no history or clinical evidence of CAD and/or MI. DNA was extracted from peripheral blood and genotypes were determined by polymerase chain reaction, restriction mapping with Hinf1, and gel electrophoresis. Conventional risk factors for CAD were prospectively documented. Results Allele and genotype frequencies in cases and control subjects were compatible with Hardy–Weinberg equilibrium. The frequencies of TT, CT, and CC genotypes among CAD patients were 4.8, 27.4, and 67.8% and in controls were 1.0, 19.0, and 80%. Hcy levels were higher in patients with triple-vessel disease compared to single and double vessel disease (P = 0.002). Multivariate analyses identified HHcy, diabetes mellitus, and hypertension as the independent predictors of CAD. Conclusions HHcy appears to have a graded effect on the risk of CAD as well as the severity and extent of coronary atherosclerosis. Our findings support that homozygous genotype of MTHFR is a genetic risk factor for CAD. A further study with larger sample size including assessment of vitamin status is needed to better clarify the relationship between MTHFR genotypes and CAD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperhomocysteinemia (HHcy) is one of the newly recognized risk factors of CAD, MI, stroke, and peripheral arterial and venous thrombosis. Basic and clinical research has established that an increase in plasma Hcy is an independent and graded risk factor for CVD [1]. The magnitude of disease risk is not entirely explained by traditional risk factors, which account ≈50% of all cause mortality [2–4]. HHcy is thought to be responsible for about 10% of the total risk.
The Hcy metabolism represents an interesting model of gene–environment interaction. Elevations of Hcy may be caused by genetic defects in enzymes involved in its metabolism or by deficiencies in cofactor levels. The 5, 10-methylene tetrahydrofolate reductase (MTHFR) catalyzes the reduction of 5, 10-methylene tetrahydrofolate to 5-methyltetrahydrofolate, which is required as a methyl donor for conversion of Hcy to methionine via a reaction that is regulated by the vitamin B12-dependent methionine synthase. A common 677C→T transition in the MTHFR gene results in a thermolabile variant with specific decreased enzymic activity and is well established as a genetic determinant of HHcy. The molecular basis of this thermolability is a missense mutation in exon 4 of the MTHFR gene, a cytosine to thymine substitution at nucleotide 677, which converts an alanine to a valine codon [5]. The protective effect of folate may be mediated by stabilization of FAD binding. This association of the MTHFR genotype with Hcy is well known to be contingent on folate status [6]. Several studies have reported thermolabile MTHFR as a risk factor in vascular disease [7–10] with few contrary studies [11, 12]. Nevertheless, due to the high incidence ∼40% in the general population and its physiological role, the 677C→T mutation may represent an important genetic risk factor of Hcy associated CAD [13–15].
The prevalence of the 677C→T polymorphism varies greatly in different ethnic groups. This polymorphism among Asians in relation to CAD was studied mainly in Japanese and Chinese [16]. Limited data are available for other Asian populations, especially for Indians. Though, Indians have high prevalence of early CAD [17–19]. During the next decade (2020), the prevalence is likely to increase to 90% for females and 103% for males [20]. We therefore undertook the present study to determine the incidence and correlation of MTHFR polymorphism with plasma Hcy levels and CAD.
Materials and methods
Study subjects
The study population comprised 100 healthy controls and 84 patients with CAD. All the subjects were of North Indian origin. For inclusion, cases had to have ≥70% stenosis of any of the major coronary arteries confirmed by cardiac catheterization. Of 84 patients, 41 were single vessel, 31 were double vessel, and 12 were triple vessel disease. Age-and gender-matched clinically healthy controls with background as similar to cases as possible were recruited from hospital-based staff and friends. Controls had to be free of overt vascular disease and a negative family history of CAD. A written informed consent was obtained from every participant after explaining the aims and objectives of the study, which was approved by the local ethics committee.
Blood samples and biochemical investigations
An overnight fasting venous blood sample was collected in EDTA tubes by puncture of cubital vein and a part of sample was kept on ice. It was immediately centrifuged at 2500 rpm for 10 min at 4°C. The plasma fraction was aliquoted and stored at −80°C until Hcy estimation. Lipid profile was done on fresh sample on Beckmann (Beckmann, US) auto-analyzer using enzymatic kits (Randox, UK). Cells were further assessed for genetic polymorphism.
Homocysteine estimation
Homocysteine plasma concentration was assayed by enzyme-linked immunoassay (ELISA) using a standard commercial kit (Diazyme, CA, USA). Briefly, protein-bound Hcy in the serum sample is reduced to free Hcy and converted enzymatically to S-adenosyl Hcy (SAH) before the immunoassay. This is followed by a solid-phase enzyme immunoassay based on competition between SAH in the sample and immobilized SAH bound to the wall of the microtiter plate for binding sites on a monoclonal anti-SAH antibody. After removal of the unbound anti-SAH antibody, a secondary rabbit anti-mouse antibody labeled with the enzyme horseradish peroxidase is added. The absorbance is inversely related to the concentration of total Hcy in the sample. A calibration curve was constructed using a standard (2.0–50.0 μmol/l) provided with the kit.
Genetic analysis
DNA was extracted from peripheral blood leukocytes using standard phenol-chloroform extraction method. The DNA quality was confirmed by electrophoresis using a 0.7% agarose gel and quantity determined using absorbance spectrophotometry. Genetic polymorphism was studied by PCR-RFLP analysis. In order to detect the 677C→T mutation in exon 4, genomic DNA was amplified by PCR using primers as previously reported [5] in a PTC-100 thermal cycler (MJ Research Inc.) followed by restriction enzyme digestion. The PCR mixture (50 μl) contained 100 ng of DNA template, 20 pmol of each primer, 200 mmol each dNTP, 0.5U of Taq polymerase (New England Biolab, UK), and 10× PCR buffer having 25 mmol MgCl2. The cycle parameters were as follows: 1 cycle of initial denaturation for 3 min at 94°C followed by 35 cycles of denaturation for 50 s at 94°C, annealing for 60 s at 65°C, extension for 50 s at 72°C, and a final extension for 7 min at 72°C. The 198-bp PCR product (10 μl) was digested with the restriction enzyme Hinf1 (Roche Diagnostics, USA), at 37°C for 3–4 h in the buffer recommended by the manufacturer. Hinf1 can recognize the C-to-T substitution in the fragments. The 198-bp fragment derived from the C allele is not digested by Hinf1, whereas the fragments of the same length from the T allele are digested into two fragments of 175, and 23-bp, hence heterozygous subjects showed three fragments of 198, 175 and 23-bp. The HinfI-treated PCR fragments were analyzed by 10% polyacrylamide gel electrophoresis at 90 V for 2.0 h, stained with ethidium bromide and visualized under ultraviolet light.
Statistical analyses
Continuous variables were expressed as mean ± SD and were compared by student’s t-test or ANOVA for more than two groups. Categorical variables were compared between groups by use of χ 2 test and crude odds ratio. The χ 2 test for homogeneity of proportions was used to test the equality of the prevalence of different genotypes for the healthy control. Multivariate analyses were done with a logistic regression model adjusted for clinically significant variables. Statistical analyses were performed with SPSS 11.0. A probability value of <0.05 was considered statistically significant.
Results
Clinical and biochemical analysis
The clinical and biochemical characteristics of the patients and controls are summarized in Table 1. As expected the patients with CAD had significantly high value of Hcy, apoB, systolic and diastolic blood pressure, fasting sugar, and BMI, whereas low levels of HDL C compared to controls. A significantly higher prevalence of HHcy, diabetes, and hypertension was observed in patients. Mean age of the patients was 51.90 ± 11.36 years while for controls it was 49.83 ± 10.67. Among patients 81.5% were male as compared to 75% male in control group.
Genotype association with phenotype
The prevalence of the MTHFR genotypes among subjects from the general population was in the range of Hardy–Weinberg equilibrium. The T and C allele frequency was 0.11 and 0.89 (χ2 = 0.012, df = 1, P = NS) in the control group. However, T allele frequency was significantly higher in patients and was found to be associated with the increased risk of CAD (OR = 1.93; 95% CI: 1.02–3.66, P = 0.042). The frequency distribution of three genotypes was as follows in patients (CC genotype, 67.8%; CT genotype, 27.4%, and TT genotype, 4.8%) and in controls (CC genotype, 80%; CT genotype, 19%, and TT genotype, 1%) (P = 0.096). A trend for high prevalence of TT mutant genotype was observed in patients. The MTHFR CT genotypes were found to be associated with increased risk of CAD (OR = 1.61; 95% CI: 0.76–3.40) and TT genotypes (OR = 4.95; 95% CI: 0.51–48.65). CT and TT genotypes combined showed a significant association with CAD risk (OR = 1.89; 95% CI: 0.96–3.91, P = 0.059). The χ2 P-value for CT genotypes was found to be associated with increased risk of CAD (OR = 1.70; 95% CI: 0.80–3.61, P = 0.133) when compared to CC genotype. No significant difference was observed between male and female subjects for either any genotype or allele frequency (Table 1).
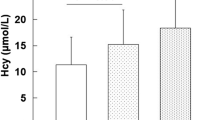
The frequencies of the TT genotype were 0% for single, 50% each for double and triple vessel disease patients, respectively (P = 0.003). Hcy levels were significantly higher in patients with multi-vessel disease compared with single and double vessel disease (P = 0.002). HHcy was more prevalent among TVD than DVD or SVD patients (Table 2).
The association of the biochemical parameters and other risk factors for CAD was studied in different MTHFR genotypes. None of the parameters showed any significant association with MTHFR genotypes except Hcy (TT = 33.41 ± 14.89, CT = 23.92 ± 11.09 and CC = 19.93 ± 7.59, P = 0.0001). All the four patients with TT genotype had Hcy > 17.5 μmol/l. No significant association was observed between any of the MTHFR genotype with diabetes or hypertension (Table 3). Among controls, no significant difference was found for the studied parameters for any of the genotypes.
Multivariate regression analysis
Multiple logistic regression analysis was performed in order to evaluate the risk of HHcy for CAD in the context of other known CAD risk factors such as diabetes mellitus, hypertension, genotypes, age, sex, lipids, and cigarette smoking. Results showed that HHcy was independently associated with the risk of CAD (OR = 5.31, 95% CI: 2.42–11.68; P = 0.0001) than others adjusted in the model (Table 4).
Discussion
The MTHFR, a gene involved in the metabolism of Hcy, is of particular medical interest as being a younger genetic risk factor for CAD. The relation between elevated plasma Hcy and vascular disease risk is independent of traditional risk factors [21]. Although the association is stronger in case-control studies with established vascular disease [21] than in prospective studies [22, 23], a meta-analysis of prospective studies indicates a robust relation [24]. Further studies suggest that the Hcy level is a strong predictor of cardiovascular events in the early follow-up period and that this risk relation may become attenuated over time [25]. Kluijtmans et al. [8] suggested that risk due to the TT mutant genotype might be confined to those with CAD.
From the analysis of the present study and studies from India and in Indians abroad, the prevalence of T allele is found to be lesser than Caucasians and other Asians [16]. The ‘T’ allele frequency observed by this study (0.11) is comparable to that of the recent studies conducted in South and North Indian subjects but is higher than Sri Lankans (0.049) [18, 26, 27]. Mexican population has the highest ‘T’ allele frequency of 0.59. The Japanese and Taiwanese population showed a relatively high ‘T’ allele frequency of 0.37 and 0.29 [8]. In this study, frequencies of MTHFR 677TT genotype in CAD cases and controls were 4.8% and 1% respectively, which is comparable to our previous reports [28] and were slightly higher than recently conducted case–control studies in Asian Indians [18, 26, 29]. The Korean study [30] found 18% TT genotype while Morita et al. [7] reported a 16% TT genotype in Japanese CAD patients, which is 11–13% higher than our results. This polymorphism is reported to have a relatively high frequency throughout the world and can be regarded as a balanced polymorphism that escaped natural selection. Such a polymorphism does not usually imply a serious, lethal disorder. However, it can contribute to the pathogenesis of multifactorial diseases, such as CAD.
The result of present study does support the 677C→T mutation as a risk factor for CAD. The TT MTHFR genotype is associated with increased plasma Hcy levels, which could be associated with increased risk of vascular disease. Meta-analyses conducted on individual data from case–control studies [13, 31, 32] have concluded a modest but statistically significant increased risk of CAD in subjects with the 677TT genotype. Accordingly, a recent large meta-analysis of 32 studies (n = 14,870) showed a graded increase in ischemic stroke risk with increasing 677T allele dose, supporting the causal relationship between the C677T MTHFR genotype, elevated Hcy and vascular disease [33]. Mager et al. [34] reported a significantly higher frequency of homozygosity (TT) in patients with early-onset CAD than in patients with later-onset CAD or in control subjects (OR = 2.4; 95% CI: 1.2–4.7). In contrast, a meta-analysis failed to support such hypothesis [35]. One possible reason for these inconsistencies may be associated with differences in the ethnicity of the study subjects.
In current study, TT genotype showed highest concentration of Hcy as reported by others. It shows insignificantly high OR = 4.95, which could be due to small sample size. Further study with larger sample size is required to find out better correlation of TT genotype with CAD.
MTHFR mutation exerts its influence on coronary atherosclerosis through the action of Hcy, which interacts with vascular smooth muscle cells, endothelium function, plasma lipoprotein, coagulation factors, and platelets [36, 37]. However, Hcy concentration is not only influenced by the gene mutation but also by non-genetic factors, such as folate status and/or vitamin B12. Guenther et al. [6] showed that the diminished enzyme activity was attributable to reduce FAD binding. Furthermore, they observed that folate derivatives protected both the wild type and mutant E. coli against FAD loss. The effect of this genetic defect may be largely compensated by adequate folate intake [38]. Previous studies reported the TT genotype displaying increased Hcy concentration than the CC counterparts [33, 39]. In our study, it has also been shown that the TT genotype has significantly higher plasma Hcy concentrations. Multivariate regression analysis further strengthens our findings that mild HHcy (OR = 5.31, 95% CI 2.42–11.68, P = 0.0001) may be associated with an increased risk of CAD along with diabetes and hypertension. However in univariate analysis, we found that TT genotype was significantly associated with disease severity and elevated level of Hcy.
The current literature offers different suggestions with respect to the role of moderately elevated Hcy and the risk for CAD. Some studies described a linear increase of CAD risk with increasing Hcy [40], others a threshold effect [41] or no risk association at all [42]. Our data are in concordance with recent studies investigating the association of Hcy with coronary disease [43]. A recent prospective nested case–control study from Finland reported no association of Hcy with CAD [44] and the authors argued that the relatively low Hcy levels in the Finnish population might be one reason for this.
The increase in the risk associated with the TT genotype may be seen as small considering that the Hcy concentration is usually 2–4 μmol/l higher in TT than in CC subjects. Some prospective [45] and case–control [4] studies suggest a 20–40% increase in coronary disease risk caused by such an increase in Hcy. Our results show a mean increase of 13.5 μmol/l in Hcy levels in TT genotype than CC genotypes and this may be contributing significantly to the disease risk and severity. As the genetic mutation accounts only for a little variation in plasma Hcy concentration, a study with much larger sample size might be necessary to demonstrate a true picture of genotype–phenotype association.
Conclusions
A significant association was observed between Hcy levels and TT genotype. Hyperhomocysteinemia and the T allele may be affecting the progression and/or severity of the disease synergistically. Our finding supports an important role of the MTHFR gene in CAD and provides evidence of polygenic regulation of Hcy.
References
Suematsu N, Ojaimi C, Kinugawa S, Wang Z, Xu X, Koller A, Recchia FA, Hintze TH (2007) Hyperhomocysteinemia alters cardiac substrate metabolism by impairing nitric oxide bioavailability through oxidative stress. Circulation 115:255–262
Boers GH (1994) Hyperhomocysteinaemia: a newly recognized risk factor for vascular disease. Neth J Med 45:34–41
Mueller PW, Thacker SB (2002) Homocystine and cardiovascular disease: a systematic review of the evidence with special emphasis on case-control studies and nested case-control studies. Int J Epidemiol 31:59–70
Refsum U, Ueland PM, Nygård O, Vollset SE (1998) Homocysteine and vascular disease. Ann Rev Med 49:31–62
Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJH, den Heijer M, Kluijtmans LAJ, van den Heuvel LP, Rozen R (1995) A candidate genetic risk factor for vascular disease: a common mutation in methylene tetrahydrofolate reductase. Nat Genet 10:111–113
Guenther BD, Sheppard CA, Tran P, Rozen R, Matthews RG, Ludwig ML (1999) The structure and properties of methylene tetrahydrofolate reductase from Escherichia coli suggest how folate ameliorates human hyperhomocysteinemia Nat Struct Biol 6:359–365
Morita H, Taguchi J, Kurihara H, Kitaoka M, Kaneda H, Kurihara Y, Maemura K, Shindo T, Minamino T, Ohno M, Yamaoki K, Ogasawara K, Aizawa T, Suzuki S, Yazaki Y (1997) Genetic polymorphism of 5,10-methylene tetrahydrofolate reductase (MTHFR) as a risk factor for coronary artery disease. Circulation 95:2032–2036
Kluijtmans LAJ, van den Heuvel LP, Boers GHJ, Frosst P, Stevens EMB, van Oost BA, den Heijer M, Trijbels FJM, Rozen R, Blom HJ (1996) Molecular genetic analysis in mild hyperhomocysteinemia: a common mutation in the methylene tetrahydrofolate reductase gene is a genetic risk factor for cardiovascular disease. Am J Hum Genet 58:35–41
Meleady R, Ueland PM, Blom B, Whitehead AS, Refsum H, Daly LE, Vollset SE, Donohue C, Giesendorf B, Graham IM, Ulvik A, Zhang Y, Monsen ALB (2003) Thermolabile methylene tetrahydrofolate reductase, homocysteine, and cardiovascular disease risk: The European Concerted Action Project. Am J Clin Nutr 77:63–70
Chambers JC, Ireland H, Thompson E, Reilly P, Obeid OA, Refsum H, Ueland P, Lane DA, Kooner JS (2000) Methylene tetrahydrofolate reductase 677 C→T mutation and coronary heart disease risk in UK Indian Asians. Arterioscler Thromb Vasc Biol 20:2448–2452
Tsai MY, Welge BG, Hanson NQ, Bignell MK, Vessey J, Schwichtenberg K, Yang F, Bullemer FE, Rasmussen R, Graham KJ (1999) Genetic causes of mild hyperhomocysteinemia in patients with premature occlusive coronary artery disease. Atherosclerosis 143:163–170
Gardemann A, Weidemann H, Philipp M, Katz N, Tillmanns H, Hehrlein FW, Haberbosch W (1999) The TT genotype of the methylene tetrahydrofolate reductase C677T gene polymorphism is associated with the extent of coronary atherosclerosis in patients at high risk for coronary artery disease. Eur Heart J 20:584–592
Laraqui A, Allami A, Carrié A, Raisonnier A, Coiffard AS, Benkouka F, Bendriss A, Benjouad A, Bennouar N, El Kadiri N, Benomar A, Fellat S, Benomar M (2007) Relation between plasma homocysteine, gene polymorphisms of homocysteine metabolism-related enzymes, and angiographically proven coronary artery disease. Eur J Intern Med 18:474–483
Gallagher PM, Meleady R, Shields DC, Tran KS, McMaster D, Rozen R, Evans A, Graham IM, Whitehead AS (1996) Homocysteine and risk of premature coronary heart disease: evidence for a common gene mutation. Circulation 94:2154–2158
Eikelboom JW, Lonn E, Genest J Jr, Hankey G, Yusuf S (1999) Homocyst(e)ine and cardiovascular disease: a critical review of epidemiological evidence. Ann Intern Med 131:363–375
Zheng YZ, Tong J, Do XP, Pu XQ, Zhou BT (2000) Prevalence of methylene tetrahydrofolate reductase C677T and its association with arterial and venous thrombosis in the Chinese population. Br J Haematol 109:870–874
Nair KG, Nair SR, Ashavaid TF, Dalal JJ, Eghlim FF (2002) Methylene tetrahydrofolate reductase gene mutation and hyperhomocysteinemia as a risk factor for coronary heart disease in the Indian population. J Assoc Physicians India 50:S9–S15
Mukherjee M, Joshi S, Bagadi S, Dalvi M, Rao A, Shetty KR (2002) A low prevalence of the C677T mutation in the methylene tetrahydrofolate reductase gene in Asian Indians. Clin Genet 61:155–159
Refsum H, Yajnik CS, Gadkari M, Schneede J, Vollset SE, Orning L, Guttormsen AB, Joglekar A, Sayyad MG, Ulvik A, Ueland PM (2001) Hyperhomocysteinemia and elevated methylmalonic acid indicate a high prevalence of cobalamin deficiency in Asian Indians. Am J Clin Nutr 74:233–241
Karthikeyan G, Prabhakaran D, Reddy KS (2002) Plasma homocysteine levels and cardiovascular risk in Indians. J Assoc Physicians India 50:S24–S28
Graham IM, Daly LE, Refsum HM et al (1997) Plasma homocysteine as a risk factor for vascular disease. The European Concerted Action Project. J Am Med Assoc 277:1775–1781
Evans RW, Shaten BJ, Hempel JD, Cutler JA, Kuller LH (1997) Homocysteine and risk of cardiovascular disease in Multiple Risk Factor Interventional Trial. Arterioscler Thromb Vasc Biol 17:1947–1953
Ubbink JB, Fehily AM, Pickering J, Elwood PC, Vermaak WJH (1998) Homocysteine and ischaemic heart disease in the Caerphilly cohort. Atherosclerosis 140:349–356
Danesh J, Lewington S (1998) Plasma homocysteine and coronary heart disease:systematic review of published epidemiologic studies. J Cardiovasc Risk 5:217–221
Kark JD, Selhub J, Adler B (1999) Non-fasting plasma total homocysteine level and mortality in middle-aged and elderly men and women in Jerusalem. Ann Intern Med 131:321–330
Angeline T, Jeyaraj N, Tsongalis GJ (2007) MTHFR Gene polymorphisms, B-vitamins and hyperhomocystinemia in young and middle-aged acute myocardial infarction patients. Exp Mol Pathol 82:227–233
Markan S, Sachdeva M, Sehrawat BS, Kumari S, Jain S, Khullar M (2007) MTHFR 677 CT/MTHFR 1298 CC genotypes are associated with increased risk of hypertension in Indians. Mol Cell Biochem 302:125–131
Vasisht S, Gulati R, Narang R, Srivastava N, Srivastava LM, Manchanda SC, Agarwal DP (2002) Polymorphism (C677T) in the 5,10- methylene tetrahydrofolate reductase gene: a preliminary study on North Indian men. Indian J Clin Biochem 17:99–107
McAndrew PE, Brandt JT, Pearl DK, Prior TW (1996) The incidence of the gene for thermolabile methylene tetrahydrofolate reductase in African Americans. Thromb Res 83:195–198
Huh HJ, Chi HS, Shim EH, Jang S, Park CJ (2006) Gene–nutrition interactions in coronary artery disease: correlation between the MTHFR C677T polymorphism and folate and homocysteine status in a Korean population. Thromb Res 117:501–506
Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG (2002) MTHFR Studies Collaboration Group. MTHFR 677C→T polymorphism and risk of coronary heart disease: a meta-analysis. JAMA 288:2023–2031
Wald DS, Law M, Morris JK (2002) Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. Br Med J 325:1202–1209
Cronin S, Furie KL, Kelly PJ (2005) Dose-related association of MTHFR 677T allele with risk of ischemic stroke: evidence from a cumulative meta-analysis. Stroke 36:1581–1587
Mager A, Lalezari S, Shohat T, Birnbaum Y, Adler Y, Magal N, Shohat M (1999) Methylene tetrahydrofolate reductase genotypes and early-onset coronary artery disease. Circulation 100:2406–2410
Brattstrom L, Wilcken DEL, Ohrvik J, Brudin L (1998) The common methylene tetrahydrofolate reductase gene mutation leads to hyperhomocysteinemia but not to vascular disease—the results of a meta-analysis. Circulation 98:2520–2526
Weiss N (2005) Mechanisms of increased vascular oxidant stress in hyperhomocys-teinemia and its impact on endothelial function. Curr Drug Metab 6:27–36
Thambyrajah J, Townend JN (2000) Homocysteine and atherothrombosis: mechanisms for injury. Eur Heart J 21:967–974
Bockxmeer FMV, Mamotte CDS, Vasikaran SD, Taylor RR (1997) Methylene tetrahydrofolate reductase gene and coronary artery disease. Circulation 95:21–23
Lievers KJ, Kluijtmans LA, Blom HJ (2003) Genetics of hyperhomocysteinaemia in cardiovascular disease. Ann Clin Biochem 40:46–59
Bostom AG, Silbershatz H, Rosenberg IH, Selhub J, D’Agostino RB, Wolf PA, Jacques PF, Wilson PW (1999) Nonfasting plasma total homocysteine levels and all-cause and cardiovascular disease mortality in elderly Framingham men and women. Arch Intern Med 159:1077–1080
Stampfer MJ, Malinow MR, Willet WC, Newcomer LM, Upson B, Ullmann D, Tishler PV, Hennekens CH (1992) A prospective study of plasma homocyst(e)ine and risk of myocardial infarction in US physicians. J Am Med Assoc 268:877–881
Alfthan G, Pekkanen J, Jauhiainen M, Pitkaniemi J, Karvonen M, Tuomilehto J, Salonen JT, Ehnholm C (1994) Relation of serum homocysteine and lipoprotein (a) concentration to atherosclerotic disease in a prospective Finnish population based study. Atherosclerosis 106:9–19
Christen WG, Ajani UQ, Glynn RJ, Hennekens CH (2000) Blood levels of homocysteine and increased risk of cardiovascular disease. Arch Intern Med; 160:422–434
Voutilainen S, Lakka TA, Hämelahti P, Lehtimäki T, Poulsen HE, Salonen JT (2000) Plasma total homocysteine concentration and the risk of acute coronary events: the Kuopio ischemic heart disease risk factor study. J Int Med 248:217–222
Wald NJ, Watt HC, Law MR, Weir DG, McPartlin J, Scott JM (1998) Homocysteine and ischaemic heart disease. Results of a prospective study with implications regarding prevention. Arch Intern Med 158:862–867
Acknowledgments
This study was supported by the grants from Indian Council of Medical Research, Ministry of Health, Government of India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alam, M.A., Husain, S.A., Narang, R. et al. Association of polymorphism in the thermolabile 5, 10-methylene tetrahydrofolate reductase gene and hyperhomocysteinemia with coronary artery disease. Mol Cell Biochem 310, 111–117 (2008). https://doi.org/10.1007/s11010-007-9671-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-007-9671-7