Abstract
Singlet oxygen is a highly reactive form of molecular oxygen that may harm living systems by oxidizing critical cellular macromolecules and it also promotes deleterious processes such as cell death. Recently, we demonstrated that the control of redox balance and the cellular defense against oxidative damage are the primary functions of cytosolic NADP+-dependent isocitrate dehydrogenase (IDPc) through supplying NADPH for antioxidant systems. In this report, we demonstrate that modulation of IDPc activity in HL-60 cells regulates singlet oxygen-induced apoptosis. When we examined the protective role of IDPc against singlet oxygen-induced apoptosis with HL-60 cells transfected with the cDNA for mouse IDPc in sense and antisense orientations, a clear inverse relationship was observed between the amount of IDPc expressed in target cells and their susceptibility to apoptosis. The results suggest that IDPc plays an important protective role in apoptosis of HL-60 cells induced by singlet oxygen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Singlet molecular oxygen (1O2), an electronically excited state of oxygen, which results from the promotion of an electron to high-energy orbitals, is produced in mammalian cells under normal and pathophysiological conditions [1]. The photodynamic action of some drugs and pigments is also mediated through singlet oxygen [2]. 1O2 is a highly reactive form of molecular oxygen that may harm living systems by oxidizing critical cellular macromolecules, including lipids, nucleic acids, and proteins, and it also promotes deleterious processes such as lipid peroxidation, membrane damage, and cell death [3].
Biological systems have evolved an effective and complicated network of defense mechanisms, which enable cells to cope with lethal oxidative environments. These defense mechanisms involve antioxidant enzymes, such as superoxide dismutases (SOD), which catalyze the dismutation of O −2 to H2O2 and O2 [4], catalase, and peroxidases, which remove hydrogen peroxide and hydroperoxides [5]. The isocitrate dehydrogenases (ICDHs; EC1.1.1.41 and EC1.1.1.42) catalyze oxidative decarboxylation of isocitrate to α-ketoglutarate and require either NAD+ or NADP+, producing NADH and NADPH, respectively [6]. NADPH is an essential reducing equivalent for the regeneration of GSH by glutathione reductase and for the activity of NADPH-dependent thioredoxin system [7, 8], both are important in the protection of cells from oxidative damage. Therefore, ICDH may play an antioxidant role during oxidative stress. We recently reported that cytosolic ICDH (IDPc) is involved in the supply of NADPH needed for GSH production against oxidative damage [9].
In the present report, we demonstrate that modulation of IDPc activity in HL-60 cells regulated apoptosis induced by photoactivated rose bengal, which generates singlet oxygen. These results suggest that IDPc has an important protective role in singlet oxygen-induced apoptosis, presumably, through acting as an antioxidant enzyme.
Materials and methods
Materials
Rose bengal (RB), β-NADP+, isocitrate, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), propidium iodide (PI), and 4′,6-diamidino-2-phenylindole (DAPI) were from Sigma Chemical Company (St. Louis, MO, USA). Electrophoreses reagents and Bio-Rad protein assay kit were purchased from Bio-Rad (Hercules, CA, USA). 2′,7′-Dichlorofluoroscin diacetate (DCFHDA), t-butoxycarbonyl-Leu-Met-7-amino-4-chloromethylcoumarin (CMAC), and rhodamine 123 dye were purchased from Molecular Probes (Eugene, OR, USA). Antibodies against Bcl-2, Bax, lamin B, cleaved caspase-3, and cleaved poly(ADP-ribose) polymerase (PARP) were purchased from Santa Cruz (Santa Cruz, CA). A purified mouse IDPc was used to prepare polyclonal anti-IDPc antibodies in rabbits.
Cell culture
The HL-60 cell lines with stable transfections with the cDNA for mouse IDPc in sense or antisense orientation, which was directed by the cytomegalovirus promoter, were prepared as described [9]. The HL-60 cell line transfected with LNCX-vector alone was used as a control. HL-60 cells were grown in RPMI 1640 culture medium supplemented with 10% (v/v) fetal bovine serum (FBS) (Hyclone, Logan, USA), 50 U/ml penicillin, and 50 μg/ml streptomycin at 37°C in an incubator under 5% CO2. Cells treated with 3 μM RB were irradiated with white light from a 100 W tungsten bulb at 30 cm from the petri dish.
Enzyme assay
Cells were collected at 1,000g for 10 min at 4°C and were washed once with cold PBS. Briefly, cells were homogenized with a Dounce homogenizer in sucrose buffer (0.32 M sucrose, 10 mM Tris–Cl, pH 7.4). Cell homogenate was centrifuged at 1,000g for 5 min and the supernatants further centrifuged at 15,000g for 30 min. The supernatants were added by 1/10 volume of 10× PBS containing 1% Triton-X100, which finally made the solution 1× PBS containing 0.1% Triton-X100. The supernatants were used to measure the activities of several cytosolic enzymes. The protein levels were determined by the method of Bradford using reagents purchased from Bio-Rad. Catalase activity was measured with the decomposition of hydrogen peroxide, which was determined by the decrease in absorbance at 240 nm. SOD activity in cell extracts was assayed spectrophotometrically using a pyrogallol assay, where 1 unit of activity is defined as the quantity of enzyme which reduces the superoxide-dependent color change by 50%. Glutathione reductase activity was quantified by the GSSG-dependent loss of NADPH as measured at 340 nm (ε = 6.67 mM−1 cm−1). Reaction mixture contained 0.1 mM NADPH, cell-free extract, 1 mM GSSG, 1 mM EDTA, and 0.1 M potassium phosphate, pH 7.4 in a final volume of 1.5 ml. Glucose 6-phosphate dehydrogenase (G6PD) activity was measured by following the rate of NADP+ reduction at 340 nm. Glutathione peroxidase activity was measured by the standard indirect method based on NADPH oxidation by t-butyl hydroperoxide in the presence of excess glutathione and glutathione reductase, as previously described [9]. The activity of ICDH was measured by the production of NADPH at 340 nm. The reaction mixture for ICDH activity contained 50 mM MOPS, pH 7.2, 5 mM threo-DS-isocitrate, 35.5 mM triethanolamine, 2 mM NAD+, 1 mM ADP, 2 mM MgCl2, and 1 μg/ml rotenone. One unit of ICDH activity is defined as the amount of enzyme catalyzing the production of 1 μmol of NADPH/min.
Immunoblotting analysis
Proteins were separated on 10–12.5% SDS-polyacrylamide gel, transferred to nitrocellulose membranes, and subsequently subjected to immunoblot analysis using appropriate antibodies. Immunoreactive antigen was then recognized by using horseradish peroxidase-labeled anti-rabbit IgG and an enhanced chemiluminescence detection kit (Amersham Pharmacia Biotech).
DAPI staining
DAPI staining was used for apoptotic nuclei determination. HL-60 cells were collected at 2,000g for 5 min, washed once with cold PBS, fixed in ice-cold methanol/acetic acid (1:1, v/v) for 5 min, and stained with 0.8 μg/ml DAPI in the dark state. The morphological changes of apoptotic cells were analyzed by the Zeiss Axiovert 200 microscope at fluorescence DAPI region (excitation, 351 nm; emission, 380 nm).
FACS
To determine the portion of apoptotic cells, cells were analyzed with PI staining. Untreated or RB/light-treated HL-60 transfectant cells were collected at 2,000g for 5 min and washed once with cold PBS, fixed in 70% ethanol, decant ethanol by centrifuge and stained with 1 ml of solution containing 50 mg/ml PI, 1 mg/ml RNase A, 1.5% Triton X-100 for at least 1 h in the dark at 4°C. Labeled nuclei were subjected to flow cytometric analysis and then gated on light scatter to remove debris, and the percentage of nuclei with a sub-G1 content was considered as apoptotic cells.
Cellular redox status
Intracellular peroxide production was measured using the oxidant-sensitive fluorescent probe DCFH-DA with confocal microscopy [9]. Cells were grown at 2 × 106 cells per 100-mm plate containing slide glass coated with poly-l-lysine and maintained in the growth medium for 24 h. Cells were treated with 10 μM DCFH-DA for 15 min and exposed to RB/light. Cells on the slide glass were washed with PBS and a cover glass was put on the slide glass. DCF fluorescence (excitation, 488 nm; emission, 520 nm) was imaged on a laser confocal scanning microscope (DM/R-TCS, Leica) coupled to a microscope (Leitz DM REB). NADPH was measured using the enzymatic cycling method as described by Zerez et al. [10]. Briefly, the reaction mixture, which combined 100 mM Tris (pH 8.0), 5 mM EDTA, 2 mM phenazine ethosulfate, 0.5 mM MTT, 1.3 unit glucose 6-phosphate dehydrogenase, and appropriate amounts of the cell extracts was preincubated for 5 min at 37°C. The reaction was started by the addition of 1 mM glucose 6-phosphate. The absorbance at 570 nm was measured for 3 min. The intracellular GSH level was determined by using a GSH-sensitive fluorescent dye CMAC [11]. HL-60 cells (1 × 106 cells/ml) were incubated with 5 μM CMAC cell tracker for 30 min. The images of CMAC cell tracker fluorescence by GSH was analyzed by the Zeiss Axiovert 200 inverted microscope at fluorescence DAPI region (excitation, 351 nm; emission, 380 nm).
MPT
Mitochondrial membrane potential transition (MPT) was measured by the incorporation of rhodamine 123 dye into the mitochondria, as previously described [12]. Cells (1 × 106) grown on poly-l-lysine coated slide glasses were exposed to ionizing radiation. Cells were then treated with 5 μM rhodamine 123 for 15 min and excited at 488 nm with an argon laser. Cells were double-stained with 100 nM MitoTracker Red, which is a morphological marker of mitochondria. The fluorescence images at 520 nm were simultaneously obtained with a laser confocal scanning microscope.
Quantitation of relative fluorescence
The averages of fluorescence intensity from fluorescence images were calculated as described [13].
Replicates
Unless otherwise indicated, each result described in the paper is representative of at least three separate experiments.
Results
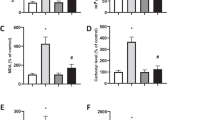
To study the relationship between IDPc activity and singlet oxygen-induced apoptotic cell death, the three kinds of HL-60 transfectant cells were constructed. The HL-60 cells were transfected with the LNCX containing either a IDPc gene as a sense orientation, IDPc(+), as an antisense orientation, IDPc(−), or the LNCX alone, control. Chromosomal integration of the transfected IDPc constructs was confirmed by polymerase chain reaction (PCR) (data not shown). The IDPc activity of IDPc(+) cells was increased 2 to 3-fold compared with that of the control cells. In contrast, IDPc(−) cells exhibited 30% less IDPc activity when compared with that of the control (Fig. 1A). Immunoblot analysis using anti-IDPc antibody further confirmed the correlation between the amount of IDPc enzyme measured in cell extracts by immunoreaction and the corresponding levels of enzyme activity (Fig. 1A). Increased expression of IDPc(+) or reduced expression of IDPc(−) did not significantly alter the activities of other antioxidant enzymes such as SOD, catalase, G6PD, glutathione peroxidase, and glutathione reductase (Fig. 1B), suggesting that the transfection of IDPc cDNA did not affect the activities of other enzymes involved in antioxidation.
(A) Activity of IDPc in HL-60 transfectant cells. Control, IDPc(+) and IDPc(−) denote the cells expressing LNCX-vector alone, LNCX-sense IDPc, and LNCX-antisense IDPc, respectively. The results shown are the means ± SD of three separate experiments. *P < 0.01 compared to control cells exposed to singlet oxygen. The cytosolic fraction (20 μg protein) from cultured cells were separated on 10% SDS-polyacrylamide gel, transferred to nitrocellulose membrane, and the subjected to immunoblot analysis using anti-IDPc antibodies. (B) Activity of antioxidant enzymes in HL-60 transfectant cells. Activity of untreated cells is expressed as 100%. The results shown are the means ± SD of five separate experiments. SOD, superoxide dismutase; Cat, catalase; G6PD, glucose 6-phosphate dehydrogenase; GR, glutathione reductase; Gpx, glutathione peroxidase
Exposure of HL-60 cells to 3 μM RB with 15 min of illumination, the singlet oxygen generator, caused shrinkage of the cell and plasma membrane blebbing that was apparent by light microscopy (Fig. 2A). To assess whether these changes were attributable to apoptotic changes, nuclear morphology was assessed by fluorescence microscopy using DAPI and flow cytometry using PI. As shown in Fig. 2B, nuclear condensation and fragmentation were apparent in IDPc(−) cells treated with RB/light when compared to control and IDPc(+) cells. Figure 3 shows a typical cell cycle plot of HL-60 transfectant cells that were untreated or treated with RB (3 μM)/light (15 min). Apoptotic cells were estimated by calculating the number of subdiploid cells in the cell cycle histogram. When cells were exposed to singlet oxygen, apoptotic cells were increased markedly in IDPc(−) cells as compared to IDPc(+) cells.
(A) Morphology features of HL-60 transfectant cells exposed to singlet oxygen. IDPc transfectant HL-60 cells were exposed to 3 μM RB for 15 min with illumination and morphological changes were observed with light microscopy. (B) Singlet oxygen-induced nuclear condensation and fragmentation in HL-60 transfectant cells. IDPc transfectant HL-60 cells were exposed to 3 μM RB for 15 min with illumination, and then harvested, fixed, permeabilized, and loaded with 0.8 μg/ml DAPI for 5 min. The morphological changes of cells were analyzed by fluorescence microscopy (excitation, 351 nm; emission, 380 nm). Arrows indicate condensed or fragmented nuclei
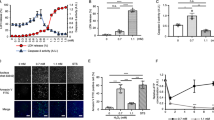
To investigate the role of IDPc in cellular defense against singlet oxygen-induced oxidative stress, we determined the cellular redox status in HL-60 transfectants unexposed or exposed to singlet oxygen. The levels of intracellular peroxides in HL-60 cells were evaluated with an oxidant-sensitive probe DCFH-DA. As shown in Fig. 4A, an increase in DCF fluorescence was observed in IDPc(−) cells when they were exposed to RB (3 μM)/light (15 min). The increase in fluorescence was significantly reduced in IDPc(+) cells. NADPH, required for GSH generation by glutathione reductase, is an essential factor for the cellular defense against oxidative damage. The ratio for [NADPH]/[NADP+ + NADPH] was significantly decreased in IDPc(−) cells treated with singlet oxygen, however, the decrease in this ratio was much less pronounced in IDPc(+) cells (Fig. 4B). GSH is one of the most abundant intracellular antioxidants and determination of changes in its concentration provides an alternative method of monitoring oxidative stress within cells. It has been shown that GSH-sensitive fluorescent dye CMAC can be employed as a useful probe to evaluate the level of intracellular GSH [11]. Cellular GSH levels in IDPc(−) cells exposed to RB/light were significantly decreased compared to that in IDPc(+) cells (Fig. 4C). These results strongly suggest that diminished IDPc activity in IDPc(−) cells resulted in the perturbation of cellular antioxidant mechanisms by the depletion of GSH presumably through the decrease in NADPH generation.
Cellular redox status of HL-60 transfectant cells exposed to singlet oxygen. (A) Measurement of in vivo molecular oxidation. DCF fluorescence was recorded at an excitation wavelength of 504 nm and an emission wavelength of 524 nm. (B) Ratios of NADPH vs. total NADP pool in HL-60 transfectant cells. (C) Fluorescence image of CMAC-loaded cells was obtained under microscopy. The averages of fluorescence intensity were calculated as described [13]. The results shown are the means ± S.D. of five separate experiments. *P < 0.01 and **P < 0.05 compared to control cells exposed to singlet oxygen. Open and shaded bars represent the cells unexposed and exposed to singlet oxygen, respectively
Mitochondrial changes occur in cells undergoing apoptosis, and some of the alterations may be causally involved in the cell death process. MPT is associated with the opening of large pores in the mitochondrial membranes [14]. To answer whether IDPc modulates the MPT upon exposure to singlet oxygen, we determined the change in MPT by intensity of fluorescence emitting from a lipophilic cation dye, rhodamine 123. Significantly less rhodamine 123 dye was taken up by the mitochondria of IDPc(−) cells, compared with IDPc(+) cells (Fig. 5).
MPT of HL-60 transfectant cells was measured by the incorporation of rhodamine 123 dye into the mitochondria. (A) Typical patterns of rhodamine 123 fluorescence are presented for transfected cells untreated or treated with 3 μM RB for 15 min with illumination. (B) The average of fluorescence intensity was calculated as described [13]. The results shown are the means ± SD of three separate experiments. *P < 0.01 and **P < 0.05 compared to control cells exposed to singlet oxygen. Open and shaded bars represent the cells unexposed and exposed to singlet oxygen, respectively
Caspase-3 activation in HL-60 cells was assessed by immunoblot analysis of lysates from cells that had been exposed to singlet oxygen. As shown in Fig. 6, the appearance of the apoptotic-cleaved product of caspase-3 was identified in HL-60 cells treated with RB/light, and the cleavage was more pronounced in IDPc(−) cells when compared to that in IDPc(+) cells. Singlet oxygen also induced the formation of fragments, which represents proteolytic fragments of PARP and lamin B, indicates an oncoming apoptotic process. The cleaved products of PARP and lamin B increased markedly in IDPc(−) cells compared to IDPc(+) cells upon exposure to RB/light. Taken together, singlet oxygen-induced cleavage of procaspase-3 into the active form of caspase-3 and caspase-3 induces degradation of PARP or lamin B. The results also indicate that IDPc exhibits a protective effect on the singlet oxygen-induced apoptosis. The role of mitochondrial pathway of apoptosis in the singlet oxygen-induced death of HL-60 cells were examined by immunoblot analysis of the abundance of Bcl-2, an antiapoptotic protein, and of Bax, an proapoptotic protein. As shown in Fig. 6, the abundance of Bcl-2 in HL-60 cells was significantly decreased in IDPc(−) cells as compared to that of IDPc(+) cells when exposed to singlet oxygen. The amount of Bax was increased after treatment with singlet oxygen, and it was significantly increased in IDPc(−) cells as compared to that of IDPc(+) cells.
Immunoblot analysis of various apoptosis-related proteins in HL-60 transfectant cells untreated or treated with 3 μM RB for 15 min with illumination. Cell extracts were subjected to 10–12.5% SDS-PAGE and immunoblotted with antibodies against cleaved caspase-3, cleaved PARP, lamin B, Bcl-2, and Bax. Equal loading protein was confirmed by β-actin
Discussion
In the present study, we examined the protective role of antioxidant enzyme IDPc in singlet oxygen-induced apoptosis in HL-60 cells. Upon exposure to RB/light, the singlet oxygen generator, perturbation of redox status, and apoptotic cell death were observed. When we examined the protective role of IDPc against singlet oxygen-induced apoptosis with HL-60 cells transfected with the cDNA for mouse IDPc in sense and antisense orientations, a clear inverse relationship was observed between the amount of IDPc expressed in target cells and their susceptibility to apoptosis.
It is well established that 1O2 can be generated in cells such as under conditions of oxidative stress [7], from decomposition of lipid peroxides or by spontaneous dismutation of superoxide [15, 16]. In addition, both naturally occurring compounds such as riboflavin [17] and many xenobiotics, such as psoralene [18], porphyrins [19], and tetracyclins [20] can generate 1O2 inside cells when irradiated by visible light. Singlet oxygen is most often generated in vitro by photosensitization reactions. Irradiation of the sensitizer dye, such as RB, mediates photoreactions through an excited triplet state which acts either by hydrogen atom or electron transfer reactions (type I) or by transferring the excited energy, forming singlet oxygen, which then reacts with the target molecules (type II) [16]. Light induced a few diseases including erythropoietic protoporphyria, pellagra and cataractogenesis have been attributed in part to the toxicity of 1O2 [21, 22]. On the other hand, it has been suggested that singlet oxygen is the primary active species in a novel cancer treatment modality known as photodynamic therapy [23].
When cells are grown in air, NADPH must be used to maintain a level of GSH as well as reduced thioredoxin to combat oxidative damage. Glutathione reductase converts GSSG to GSH in the cell using NADPH as a reductant [3]. The oxidized form of thioredoxin, with a disulfide bridge between the half-cystines, is reduced by NADPH in the presence of a flavoprotein, thioredoxin reductase [24]. Reduced thioredoxin may provide reducing equivalents to several enzymes including thioredoxin peroxidases and methionine sulfoxide reductase, presumably involving the defense against oxidative stress. Recently, a family of antioxidant proteins from eucaryotes and procaryotes were purified and these proteins removed hydrogen peroxide using hydrogen provided by the NADPH-dependent thioredoxin system, and thus were called thioredoxin peroxidases [8, 25]. Reduced thioredoxin may also provide reducing power to methionine sulfoxide reductase, which can reactivate proteins damaged by previous oxidation of their methionine residues [26].
The pentose phosphate pathway is considered to be a major source of cellular reducing power, with G6PD catalyzing the key NADPH-producing step. It is well documented that this pathway, specifically G6PD, plays a protective role during oxidative stress [27, 28]. However, it is possible that other enzymes, which generate NADPH, may have roles in oxidative stress resistance. ICDH in the cytosol of rat liver has been proven to have an approximately 20 times higher specific activity than G6PD [29]. We recently reported that the control of cellular redox balance and oxidative damage is one of the primary functions of ICDH in cytosol of NIH3T3 cells [9].
GSH is a well-known antioxidant, which is usually present as the most abundant low-molecular-mass thiol in most organisms. It has various functions in the defense against oxidative stress and xenobiotic toxicity [30]. It can act as the electron donor for glutathione peroxidase in animal cells, and also directly reacts with ROS. GSH is readily oxidized to glutathione disulfide (GSSG) by the glutathione peroxidase reaction, as well as the reaction with ROS, which may subsequently cause the reduction of GSH level. In our study, singlet oxygen induced by RB/light directly affected the glutathione redox status and the intracellular ROS was increased in the same condition. These results indicate that singlet oxygen is able to modulate the cellular redox balance presumably by depleting GSH. Consequently, the perturbation of the balance between oxidants and antioxidants leads to a pro-oxidant condition.
Mitochondria are vulnerable to oxidants because they are the major source of free radicals in the cells and are limited in their ability to cope with oxidative stress [31]. Therefore, improving the mitochondrial status could cause a significant decrease in the level of oxidants in the cells [32]. It is well established that mitochondrial dysfunction is directly and indirectly involved in a variety of pathological states. The changes caused by singlet oxygen are compatible with mitochondrial failure, encompassing decline of rhodamine 123 fluorescence which reflect mitochondrial swelling or changes in the mitochondrial inner membrane. A clear enhancement of such damage by reduction of IDPc activity indicates that IDPc prevents a deterioration of bioenergetic state.
Cleavage of caspase-3 and its target proteins such as PARP and lamin B, a signature event of apoptosis, was induced by singlet oxygen. In the meantime, the Bcl-2 protein is a suppressor of apoptosis that homodimerizes with itself and forms heterodimers with a homologous protein Bax, a promoter of cell death [33]. Reduction of IDPc activity in IDPc(−) cells significantly enhanced programmed cell death by increasing apoptotic features including caspase activation, decreasing anti-apoptotic molecules (Bcl-2), and increasing pro-apoptotic molecules (Bax), presumably via perturbation of redox status.
In conclusion, IDPc plays an important protective role in singlet oxygen-induced apoptosis of HL-60 cells which may contribute to various pathologies associated with singlet oxygen-mediated oxidative stress.
References
Kanofsky JR (1989) Singlet oxygen production by biological systems. Chemico-Biol Interact 70:1–28
Niziolek M, Korytowski W, Girotti AW (2005) Self-sensitized photodegradation of membrane-bound protoporphyrin mediated by chain lipid peroxidation: inhibition by nitric oxide with sustained singlet oxygen damage. Photochem Photobiol 81:299–305
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine. Oxford Press, Oxford
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Chance B, Sies H, Boveris A (1979) Hydroperoxide metabolism in mammalian organs. Physiol Rev 59:527–605
Koshland DE Jr, Walsh K, LaPorte DC (1985) Sensitivity of metabolic fluxes to covalent control. Curr Top Cell Regul 27:13–22
Kirsch M, De Groot H (2001) NAD(P)H, a directly operating antioxidant? FASEB J 15:1569–1574
Nakamura H (2005) Thioredoxin and its related molecules: update 2005. Antioxid Redox Signal 7:823–828
Lee SM, Koh HJ, Park DC, Song BJ, Huh TL, Park JW (2002) Cytosolic NADP+-dependent isocitrate dehydrogenase status modulates oxidative damage to cells. Free Radic Biol Med 32:1185–1196
Zerez CR, Lee SJ, Tanaka KR (1987) Spectrophotometric determination of oxidized and reduced pyridine nucleotides in erythrocytes using a single extraction procedure. Anal Biochem 164:367–373
Tauskela JS, Hewitt K, Kang LP, Comas T, Gendron T, Hakim A, Hogan M, Durkin J, Morley P (2001) Evaluation of glutathione-sensitive fluorescent dyes in conical culture. Glia 30:329–341
Pastorino JG, Simbula G, Yamamoto K, Glascott PA, Rothman RJ, Farber JL (1996) The cytotoxicity of tumor necrosis factor depends on induction of the mitochondrial permeability transition. J Biol Chem 271:29792–29798
Sundaresan M, Yu ZX, Ferrans CJ, Irani K, Finkel T (1995) Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270:296–299
Kroemer G, Zamzami N, Susin SA (1997) Mitochondrial control of apoptosis. Immunol Today 18:44–51
Krinsky NI (1974) Membrane photochemistry and photobiology. Photochem Photobiol 20:532–535
Foote CS (1976) Photosensitized oxidation and singlet oxygen: consequences in biological systems. In: Pyror WA (ed) Free radicals in biology, vol 2. Academic Press, New York, pp 85–133
Joshi PC (1985) Comparison of the DNA-damaging property of photosensitised riboflavin via singlet oxygen (1O2) and superoxide radical O −. 2 Mechanisms. Toxicol Lett 26:211–217
de Mol NJ, van Henegouven GMJB, van Beele B (1981) Singlet oxygen formation by sensitization of furocoumarins complexed with, or bound covalently to DNA. Photochem Photobiol 34:661–666
Weishaupt KR, Gomer CJ, Dougherty YJ (1976) Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res 36:2326–2329
Hasan T, Khan AU (1986) Phototoxicity of the tetracyclines: photosensitized emission of singlet delta dioxygen. Proc Natl Acad Sci USA 83:4604–4606
Mathews-Roth MM (1986) Beta-carotene therapy for erythropoietic protoporphyria and other photosensitivity diseases. Biochimie 68:875–884
Egorov SI, Babizhaev MA, Krasnovskii AA, Shredova AA (1987) Photosensitized generation of singlet molecular oxygen by endogenous substances of the eye lens. Biofizika 32:169–171
Henderson BW, Dougherty TJ (1992) How does photodynamic therapy work? Photochem Photobiol 55:145–157
Holmgren A (2000) Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid Redox Signal 2:811–820
Rhee SG, Chae HZ, Kim K (2005) Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med 38:1543–1552
Schallreuter KU, Rubsam K, Chavan B, Gillbro JM, Spencer JD, Wood JM (2006) Functioning methionine sulfoxide reductase A and B are present in human epidermal melanocytes in the cytosol and in the nucleus. Biochem Biophys Res Commun 342:145–152
Kletzien RF, Harris PKW, Foellmi LA (1994) Glucose-6-phosphate dehydrogenase: a “housekeeping” enzyme subject to tissue-specific regulation by hormones, nutrients, and oxidant stress. FASEB J 8:174–181
Pandolfi PP, Sonati F, Rivi R, Mason P, Grosveld F, Luzzatto L (1995) Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J 14:5209–5215
Veech RL, Eggleston LV, Krebs HA (1969) The redox state of free nicotinamide-adenine dinucleotide phosphate in the cytoplasm of rat liver. Biochem J 115:609–619
Meister A, Anderson ME (1983) Glutathione. Ann Rev Biochem 52:711–760
de Grey AD (2000) The reductive hotspot hypothesis: an update. Arch Biochem Biophys 373:295–301
Atamna H, Paler-Martinez A, Ames BN (2000) N-t-Butyl hydroxylamine, a hydrolysis product of α-phenyl-N-t-butyl nitrone, is more potent in delaying senescence in human lung fibroblasts. J Biol Chem 275:6741–6748
Yang E, Korsmeyer SJ (1996) Molecular thanatopsis: a discourse on the Bcl-2 family and cell death. Blood 88:386–401
Acknowledgment
This work was supported by a grant from the Korea Research Foundation (KRF-2005-070-C00100).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, S.Y., Lee, S.M., Tak, J.K. et al. Regulation of singlet oxygen-induced apoptosis by cytosolic NADP+-dependent isocitrate dehydrogenase. Mol Cell Biochem 302, 27–34 (2007). https://doi.org/10.1007/s11010-007-9421-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-007-9421-x