We analyze the literature sources devoted to the investigation of the influence of hydrogen on the tribological behavior of metallic materials. The specific features of friction and wear in metals that do not form hydrides (iron, copper, and some alloys based on these metals) and in hydride-forming metals (niobium, zirconium, and titanium) in gaseous hydrogen-containing environments and under the conditions of electrolytic hydrogenation are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hydrogen readily diffuses in metallic materials and accumulates in them, thus affecting their structures, phase compositions, and physicomechanical properties [1–9]. This influence is ambiguous and depends on the action of numerous factors, namely, on the structure, chemical composition, and geometry of the specimens, conditions of hydrogenation, etc. As a rule, in the process of hydrogenation, the plasticity of the metals decreases. This phenomenon is called hydrogen brittleness. As a result of long-term investigations, the following basic regularities of hydrogen embrittlement of steels were established [2–4, 8]:
-
the susceptibility of steel to hydrogen embrittlement is determined by its microstructure;
-
the maximum influence of hydrogen on the mechanical properties is observed for ferritic steels and, for austenitic steels, its influence is much weaker;
-
hydrogen embrittlement may be caused even by very low concentrations of hydrogen ( <1cm3/100g ofthe metal);
-
brittleness becomes irreversible as soon as a certain critical level of hydrogen concentration is exceeded or as the time of its interaction with the metal increases;
-
the degree of embrittlement depends on the level, type, and distribution of stresses and decreases as the strain rate increases;
-
the temperature range 173–373°K proves to be most favorable for the manifestation of hydrogen embrittlement;
-
the degree of hydrogen brittleness depends on the carbon content of the metal;
-
stress concentrators enhance the action of hydrogen.
At present, there are numerous hypotheses used to explain the indicated regularities [4, 9–13]. Thus, the increase in the pressure of molecular hydrogen in internal microcavities (Zapffe and Cottrell) [10], the weakening of the interatomic bonds in the metal by dissolved hydrogen (Morlet–Johnson–Troiano) [11], and the decrease in the specific energy of the internal surfaces of cracks caused by adsorbed hydrogen (Cottrell, Popov, Moroz, and Chechulin) [10, 11] are believed to be the causes of the embrittlement of metals. It is also believed that an important role in the transfer of hydrogen and fracture of materials is played by dislocations. Thus, Cottrell hydrogen atmospheres may accelerate or retard the motion of dislocations (Bastien, Azou, Vaughan, and de Morton) [11]. The dislocation–decohesion hypothesis assumes that hydrogen is concentrated in the cores of dislocations. Therefore, in the case of accumulation of dislocations near the obstacles, the concentration of hydrogen may become sufficient for the rapid acceleration of fracture processes (Kolachov) [13]. Numerous theoretical concepts used to explain the hydrogen embrittlement of metals reveal the urgency of the problem and the absence of comprehensive knowledge of the mechanism of this phenomenon [4].
Hydrogen intensifies the process of wear of metals in the friction zone. In numerous monographs and surveys [9, 14–35], it is shown that friction creates favorable the conditions for the tribodestruction of lubricants, plastics, water, etc., and hydrogen release [whose ions are continuously accumulated in the subsurface layers of the metals with elevated amounts of defects (in the hydrophilic zone)]. As soon as a superequilibrium concentration of hydrogen ions is attained, especially in the zone of maximum temperature, we observe an exothermal reaction of formation of hydrogen molecules from ions in micro- and macrodefects of the lattice, which leads to the appearance of additional internal stresses and, finally, to the fracture of the surface layers of the metal [16]. The brittle fracture of metals (dispersion) is observed at low temperatures, whereas the ductile fracture takes place at high temperatures (1073–1273°K) [18]. On the ferrite–austenite interface, the concentration of absorbed hydrogen can be as high as several tens of percent and, as a result, the melting point of iron decreases (to 873°K), and we observe the ductile fracture of friction surfaces of the metal [3, 12].
Hydrogen-induced wear is most often observed in braking units [steel (cast iron)–plastic and titanium– bronze friction couples], piston systems of the engines (steel–bronze and steel–steel friction couples), and pumps for pumping and transportation of the oil products (18KhN3А-steel–bronze friction couples) [16].
Both domestic and foreign scientists [1–6, 9, 11–13, 15–18, 20–52] made significant contributions to the solution of the problem of the influence of hydrogen on the mechanical and tribological properties of materials [1–6, 9, 11–13, 15–18, 20–52]. However, these investigations were intensified abroad only recently in connection with the development of hydrogen power engineering [20–39, 41–45, 47–50, 52].
Influence of Hydrogen on the Tribological Behavior of Armco Iron and Steels
Wear is investigated in gaseous hydrogen and under the conditions of electrolytic hydrogenation. Cathodic saturation is realized in aqueous solutions of H2SO4 with a concentration of 2.6 vol.% containing 1–10 mg/liter As2O3 introduced to prevent the recombination of hydrogen ions on the metal surface [51–56].
The solubility of gaseous hydrogen in iron obeys the Sieverts law and, hence, is given by the formulas
for the bcc lattice of α -Fe and
for the fcc lattice of γ-Fe. According to these equations, for a hydrogen pressure of 0.1 МРа, the concentration of dissolved hydrogen does not exceed 10–5–10–4 wt.% in α-iron. At the same time, in γ-iron, it is higher by an order of magnitude. The process of dissolution runs with the absorption of energy [57].
If hydrogen is in the ionized state (in particular, in the case of cathodic polarization), then it is adsorbed by Armco iron in super-equilibrium concentrations (up to 5cm3 per 100 g of the metal) [53–55]. In this case, for a polarization current density of 1A/dm2 and a duration of the process exceeding 0.5 h, the phenomenon of blistering is observed on the surface of the metal. After hydrogenation, the gas content of the metal decreases and, after 48 h, becomes equal to 30% of its initial content. Carbon steels absorb 4–5 times more hydrogen than Armco iron, and the amount of absorbed hydrogen increases with the content of carbon. This is explained by the presence of a larger number of defects in the structure and the interaction of hydrogen with the cementite of steel accompanied by the formation of methane.
The absorption of hydrogen by iron and steels from gaseous atmospheres and its release from the metals are stimulated by friction [18, 20, 44–46]. High levels of absorption of hydrogen by carbon steel were detected in the process of grinding in the course of which the temperature in the contact zone became as high as 1123°K. The specimens immersed after this procedure in liquid intensely released hydrogen bubbles [17].
The release of hydrogen from metals in the process of friction with simultaneous decrease in the concentrations of oxygen and water vapor in the ambient atmosphere were also observed by the authors of [20]. It was shown that, in the presence of friction (under a load of 2.6 N for a sliding velocity of 42 mm/sec), hydrogen molecules are desorbed from the metallic friction surfaces, whereas oxygen and water molecules are simultaneously chemisorbed on these surfaces (Fig. 1а). The susceptibility of gases to chemisorption depends on the character of wear (for the mild friction conditions, it is higher than that under severe friction conditions). The application of magnetic fields to iron–iron friction couples intensifies both the hydrogen release and the chemisorption of oxygen and water molecules.
Adsorption and desorption of gases in the process of friction of Armco iron in air [20] (а); absorption of atmospheric hydrogen by 45 steel (b); data of mass spectrometric analysis on the variations of the chemical composition of gaseous atmospheres in the process of friction of steels in air [44] (с): (1) H2 , (2) H2O , (3) O2 , (4) stationary conditions, (5) friction.
In [44], balls made of carbon steel were rotated in a centrifuge made of aluminum oxide at a frequency of 6.67 Hz. The field of accelerations and the relative motion of the balls caused their wear. The experiments were carried out in air and in gaseous hydrogen (under a pressure of 0.1 МPа). As a result of the mass spectrometric analysis of the chemical composition of gaseous atmosphere, it was shown, that, in the case of friction in hydrogen, steel absorbs three times more hydrogen than after holding under stationary conditions (Fig. 1b).
In the case of friction of steel in air, the concentration of hydrogen increases as a result of its release from the metal (Fig. 1c, curve 1). The desorption of hydrogen is maximum in the initial stage of friction. Then its concentration in the atmosphere decreases but methane is released because hydrogen participates in the tribochemical reaction with carbon of steel. At the same time, the oxygen content of the atmosphere decreases (Fig. 1c, curve 3) as a result of its chemisorption on the friction surfaces [44].
As a rule, the influence of hydrogen on the process of wear of iron and carbon steels is negative because these materials are susceptible to hydrogen embrittlement. In [17], Matyushenko discovered that, after electrolytic hydrogenation of 45 steel in a 26% H2SO4 solution for 1–1.5 h (the polarization current density was not indicated), the microhardness of the surface layer of steel increases by 25%, which is accompanied by an insignificant increase in the wear resistance. As a result of hydrogenation for more than 2 h, the microhardness and wear resistance decrease as a result of embrittlement of the subsurface layer.
In [37], the specific features of wear of medium-carbon S45C steel (an analog of 45 steel) and SUJ2 bearing steel (an analog of ShKh15 steel) were compared both in air and in gaseous hydrogen. The friction scheme was as follows: a disk (investigated steel) and three balls (SUJ2), a load of 40 N, a velocity of 5.03 mm/sec, and a friction path of 181 m. It was shown that, in the case of friction without lubrication, the degree of wear and friction coefficients for the S45C–SUJ2 couple in hydrogen are practically equal to the values of the same parameters in air and severe defects are observed on the friction surfaces (Fig. 2). After friction in air, Fe2O3 oxides are detected in the wear products, which reveals a significant influence of the corrosion factor on the character of wear.
Friction surfaces of S45C (a, c)–SUJ2 (b, d) steel friction couples in air (a, b) and in hydrogen (c, d) [37].
Under the same friction conditions [37], the character of wear of the disk–balls couples made of the same material (SUJ2–SUJ2) is somewhat different. The microstructure of the friction surfaces in air reveals the presence of corrosion defects and oxide phases. Corrosion defects are not observed in the case of friction without lubrication in hydrogen. The friction coefficient of this couple in hydrogen is four times lower than in air and wear is practically absent (Figs. 3, 4).
Friction surfaces of a bearing ball (SUJ2–SUJ2 steel couple) in air (a) and in hydrogen (b) [37].
Wear of steels in air (□) and in hydrogen (■): SUJ2 (analog of ShKh15 steel), S45C (analog of 45 steel), SUS304L and SUS316L steels (Cr–Ni–Mo and Cr–Ni austenitic steels). The rider is made of SUJ2 steel [37].
In oxygen-containing environments, the process of wear of steels runs mainly via the corrosion processes connected with the formation and wear of the oxide phases. In hydrogen, wear is a result of the adhesive interaction of the surfaces and the embrittling effect of hydrogen. It seems likely that, under these conditions of friction, SUJ2 bearing steel is resistant to hydrogen embrittlement due to the presence of chromium and stable carbide phases. Moreover, in hydrogen, the absence of oxygen playing the role of a corrosion factor, promotes the minimization of the degree of wear.
Since austenitic steel are more resistant to hydrogen embrittlement than ferritic–pearlitic steels [1, 2], their tribological properties in gaseous [18, 37, 39, 41] and liquid (at cryogenic temperatures) hydrogen are now extensively investigated [49].
The solubility of hydrogen in austenite is 10–100 times higher than in ferrite as a result of the intensification of absorption caused by the transformation of the hexagonal structure of iron into the bcc and fcc structure s[1, 2, 57]. The denser the packing of atoms in the metal lattice, the higher its energy level, and the larger the amount of hydrogen that can be bound in this lattice in the form of protons [2, 51].
It was shown than, under the conditions of friction in hydrogen and air, after preliminary holding in gaseous hydrogen, the intensity of wear of 07Kh16N6 steel is half as large as for 40Kh steel [40]. However, it is not always possible to use austenitic steels under the conditions of rigid contact because they are plastic and susceptible to adhesion and abrasion.
In the process of friction of SUS304L (0.016% С, 0.38% Si, 1.24% Mn, 0.028% Р, 9.08% Ni, and 18.5% Cr) and SUS316L (0.012% С, 0.38% Si, 1.6% Mn, 0.031% Р, 12.6% Ni, 17.3% Cr, and 2.53% Mo) austenitic steels in hydrogen, it was discovered that their wear is more intense than in air (Fig. 4) [37]. The oxide films on the metal surface destroyed in the course of friction are not restored in hydrogen. This leads to the adhesion interaction and damage of the friction surfaces, which manifests itself in the transfer of the components of austenitic steels onto the surface of the rider.
Austenitic steels combine mechanical strength and satisfactory plasticity at extremely low temperatures and, therefore, they are used in mechanisms intended for operation at cryogenic temperatures and for the storage of liquid hydrogen (at temperatures of about 20°K). However, in moving joints (e.g., in the cars), cryogenic temperatures and hydrogen can accelerate wear, especially in the absence of lubricants, which are inapplicable under the indicated conditions. Under the conditions of deformation of steels at low temperatures, austenite may transform into martensite. The formation of martensite improves the strength properties of steel but facilitates its embrittlement, which leads to failures of machine parts.
The influence of the chemical composition of austenitic steels (in particular, of the contents of nickel, manganese, and nitrogen) on the stability of the austenitic microstructure at a temperature of 20°K in liquid hydrogen was investigated in [49]. The conditions of the experiment were as follows: a “corundum ball–disk of the investigated material” friction couple, loads of 5 and 10 N, sliding velocities of 0.06 and 0.2 m/sec, and a friction path of 1000 m.
It was shown that the high content of nickel in steels and its replacement with manganese lead to the strain-induced transformation of austenite into α -martensite. In chrome-manganese steels, the γ /ε phase transformation is possible as the load increases under the conditions of friction in liquid hydrogen.
It was discovered that the moderate alloying of chromium–manganese steels with nitrogen (up to 1.0%) increases the stability of the austenitic nanostructure and the wear resistance in hydrogen at cryogenic temperatures. Nitrogen promotes the increase in hardness and facilitates the transition from the plastic to brittle fracture of friction surfaces. On these surfaces, we observe the formation of cracks whose density depends on the loading conditions. In [49], it was shown that high loads in the presence of friction not always accelerate cracking but may also inhibit crack propagation. This means that deformation and high contact pressure do not promote the absorption of hydrogen but facilitate its release from the material. Thus, hydrogen is continuously displaced and cannot dissolve in the lattice of austenitic steel (cryogenic temperatures also favor its low solubility). The experiments demonstrate that cracks are localized in the damaged nanocrystalline layer on the friction surface (Fig. 5), which promotes the increase in the wear resistance of the metal.
Damaged contact layer and the network of cracks in austenitic steel (17.0–19.5% Cr,8.0–10.5% Ni, 2.0% Mn, < 0.07% C, and < 0.11% N) after friction in liquid hydrogen ( F = 5 N, v = 0.06 m/sec) [49]: (1) contact layer, (2) matrix material.
The localization of defects in a thin hard surface layer was also discovered in the case of friction of steels with nitrogen-containing coatings obtained by the methods of thermochemical treatment (sulfonitriding from the gaseous phase) [38, 41, 50]. Nitrided layers form an efficient barrier for the diffusion of hydrogen into the bulk of the metal due to their specific structure. Hydrogen is concentrated in a thin “white layer” with a thickness of several micrometers with residual stresses and does not penetrate into the matrix of the metal. In the process of friction, hydrogen may escape from the nitrided layer and create the effect of “self-lubrication by gas,” thus decreasing the friction coefficient [38]. On the other hand, the preliminary hydrogenation of steels prior to ion nitriding increases the thickness of the nitride zone and improves the corrosion and wear resistances of steels [62].
Thus, friction promotes the intensification of absorption and desorption of hydrogen by carbon steels: Under the conditions of friction in hydrogen, 45 steel absorbs three times more gas than in the absence of hydrogen, whereas in air, we observe the desorption of absorbed hydrogen whose maximum intensity is attained in the initial stage.
The decreased wear of the SUJ2–SUJ2 steel friction couple in hydrogen is discovered. It is explained by the absence of the corrosion action of oxygen, which plays the role of determining factor in the fracture of these steels under the conditions of friction.
The elevated wear resistance of a chrome-manganese austenitic steel alloyed with nitrogen was discovered at cryogenic temperatures in hydrogen. In the process of friction, defects are localized in a thin hard nanocrystalline surface layer.
The efficient barrier for the diffusion of hydrogen into the bulk of steels is formed by surface nitriding.
Wear of Copper-Based Alloys under the Influence of Hydrogen
Copper forms solid solutions with hydrogen. At the same time, CuH hydride is unstable and dissociates at room temperature. The solubility of gaseous hydrogen in the fcc lattice of copper obeys the Sieverts law
under pressures of up to 0.8 МРа within the temperature range 500–1083°K [57]. In this case, 10–3 at.% of hydrogen dissolves in copper under a pressure of 0.1 МPа at a temperature of 773°K. The absorbability of copper increases with the increase in its defectiveness caused by cold machining [51]. The electrolytic hydrogenation of copper alloys decreases their strength, plasticity, and microhardness for mild modes of polarization (1−2A/dm2). However, for harder modes ofpolarization (5A/dm2), σu, σy , and H μ increase by 3–5% [51]. Hydrogen ions reduce copper from its oxides [58]. Penetrating into the metal, they can react with oxides dissolved there with the formation of water vapor, which leads to the nucleation of cracks and blistering (“hydrogen disease of copper”) and affects the mechanical properties of copper.
The influence of electrolytic hydrogenation on the wear resistance of tin bronze (71.72% Cu, 26.88% Zn, 1.18% Sn, Fe, Si, and Pb < 0.1%), as a material of heat exchangers used in the chemical, petrochemical, and petroleum-refining industries, was studied in [52]. In the case of passing of a flow of hot water, pipes vibrate which leads to the appearance of sliding friction in the contact zone with partitions at low contact pressures. In this case, hydrogen releases from the medium on the surfaces of the pipes and penetrates into the material.
The cathodic hydrogenation of bronze was realized with the use of a graphite anode at room temperature in an electrolyte of the following composition: 75 vol.% methanol, 22.4 vol.% distilled water, 2.6 vol.% sulfuric acid, and 10 mg/liter arsenic dioxide (to inhibit the recombination of hydrogen on the surface).
The direct current density was equal to 2.5 A/dm2 and the time hydrogenation to 24, 48, or 72 h. The specimens were subjected to friction tests immediately after hydrogenation according to the pin–disk friction scheme. The pin and the disk were made of the same material. The friction path was up to 200 m, the normal pressure was equal to 0.16 МPа, and the sliding velocity to 11 сm/sec.
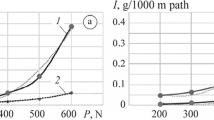
As a result of the hydrogenation of bronze, the microhardness of the surface layers (with a thickness of 0.080–0.250 mm) increases by 10–30% as the duration of polarization increases from 24 to 72 h. In the initial stages of the process of friction, the hydrogenated layers suffer intense wear accompanied by the decrease in the friction coefficient (Fig. 6). As result of wear of the hydrogenated material, the friction coefficient of bronze and its wear rate remain the same as for the intact material. Cracks are detected in the microstructure of the friction surfaces of hydrogenated materials and their number increases with the duration of hydrogenation (Fig. 7).
Dependences of the friction coefficient (a) and mass losses (b) on the friction path under a load of 200 g for specimens of tin bronze: (1) nonhydrogenated, (2) hydrogenated for 24 h, (3) hydrogenated for 48 h, (4) hydrogenated for72 h [52].
SEM micrographs of the surfaces of wear of tin bronze: (a) nonhydrogenated, (b) hydrogenated for 24 h, (c) hydrogenated for 48 h [52].
Copper and its alloys are characterized by a fairly low susceptibility to the influence of hydrogen due to the relatively low solubility of hydrogen in these alloys and the absence of hydride formation. For hydrogen contents lying within the limits of equilibrium solubility, the mechanical properties of the metal remain unchanged, whereas for its superequilibrium concentrations, the corresponding variations are insignificant, most likely due to the high plasticity of the metal.
Unlike steels, in which the procedure of electrolytic hydrogenation leads to cracking and blistering even at current densities of 1A/dm2 for 1 h, the changes in the mechanical properties of tin bronze are observed only after hydrogenation for 24 h at current densities of about 2.5 A/dm2 . These changes are localized in the subsurface layer of the material with a thickness of 0.080 mm. The process of friction leads to the formation of cracks and brittle fracture of the hydrogenated layer.
Superequilibrium concentrations of hydrogen ions can be attained in the subsurface layers of the metal as a result of electrolytic hydrogenation. Unfortunately, at present, the information available in the literature on the dependence of the absorption of hydrogen on the parameters of hydrogenation and the nature of the metal is very limited.
Influence of Hydrogen on the Wear of Zirconium and Niobium
Since all types of steels, with the exception of some steels with austenitic structure, are susceptible to hydrogen embrittlement, the tribological properties of transition metals and their alloys (more stable in gaseous hydrogen) are now extensively investigated [33, 44, 59].
The solubility of hydrogen in the bcc lattice of niobium varies from 4 to 20 аt.% at temperatures of 273–573°K.
Hydrogen forms hydrides of the following chemical composition: NbH x ( x = 0.7–1.0) with rhombic crystal lattices. Under the conditions of cathodic polarization in acids, we observe the formation of NbH2 . Due to the dissolution of hydrogen and the formation of hydride compounds, niobium is a strong absorbent of hydrogen: 1 g of the metal is able to absorb up to 104 cm3 at a temperature of 293°K. As temperature increases, the absorbability of niobium decreases to 74.4 cm3/g at 773°K and to 4.0 cm3/g at 1173°K because, in the process of heating, hydrides decompose and form finely divided metal [57, 60]. The hydride transformation in niobium increases the volume of the lattice and leads to the formation of internal stresses [42, 43].
Zirconium alloys have the hexagonal close-packed structure less permeable for hydrogen than the Feα structure and, therefore, these alloys can be used for the formation of barrier layers inhibiting the diffusion of hydrogen into steel. The maximum solubility of hydrogen in the hexagonal close-packed structure of zirconium within the temperature range 473–773°K varies from 0.2 to 5 аt.%. Zirconium and hydrogen form δ- and ε ZrH2 hydrides whose enthalpy of formation is ∼ 170 kJ/mole [57, 58]. It is known that the structure of δ ZrH2 is identical to the structure of CaF2 , which is a solid lubricant [33, 59, 61]. Hence, zirconium alloys can be wear-resistant in hydrogen if the indicated hydride is formed in the process of friction.
The parameters of wear of zirconium and niobium tribocouples in gaseous atmospheres ( H2, Не, Не + 10 vol.% H2 , and air) and vacuum were investigated in [59]. It was discovered that, under the conditions of friction in hydrogen and its mixture with helium, the friction coefficients of zirconium are lower than in air but the degrees of wear are comparable (Figs. 8а, b). It is worth noting that the tribological characteristics of the zirconium couple in a mixture of helium with 10% hydrogen are the same as in pure hydrogen. In hydrogen-containing media, ZrH2 hydrides of the ε -type were detected on the friction surfaces and wear products. The character of wear reveals the brittle fracture of the material (Figs. 9a–d).
Under the same conditions, the degrees of wear of the niobium tribocouple in hydrogen and in air are higher than in helium and in a vacuum [59]. Niobium is characterized by a high oxidability in air and the interaction with hydrogen at low temperatures. Its oxidation begins at 473°K and is accompanied by a 2.7-fold increase in volume and cracking. At 523°K, niobium intensely reacts with hydrogen and form hydrides, which also leads to embrittlement. In this connection, the elevated degrees of wear of the metal are observed in hydrogen and in air (Figs. 8c, d).
Time dependences of the friction coefficients (а, с) and wear rates (b, d) for Zr–Zr (a, b) and Nb–Nb (c, d) friction couples in gaseous atmospheres under a load of 70 N [59] for a gas pressure of 0.1 MPa or in a vacuum of 5⋅10–4 Pa : (1) vacuum, (2) helium, (3) air, (4) hydrogen, (5) Не + 10 vol.% H2; (■) wear of the disk, (□) wear of the pin.
The microstructures of the friction surface and wear particles reveal (Figs. 9e–h) the adhesive character of wear in helium and in a vacuum; cracking and brittle fracture are observed in hydrogen and in air.
Wear products of zirconium (a–d) and niobium (e–h) in hydrogen (a, e), air (b, f), vacuum (c, g), and helium (d, h) [59].
This is connected with the formation of β-NbH hydrides with orthorhombic crystal structures and Nb2O5 oxides.
Under the conditions of friction in hydrogen-containing media, the ε -ZrH2 and β -NbH hydride phases are formed on the surfaces of zirconium and niobium. This is accompanied by cracking and brittle fracture of the surface and the formation of finely divided wear products. However, the degree of wear of niobium is higher than the degree of wear of zirconium by two orders of magnitude and the friction coefficient of niobium is close to one, which is explained, in particular, by different behaviors of the hydride phases: in the first case, hydrides play the role of abrasive, whereas in the second state, they play the role of a solid lubricant.
Influence of Hydrogen on the Process of Wear of Titanium and Its Alloys
Titanium and its alloys are characterized by a very high sensitivity to hydrogen. The solubilities of hydrogen in the hexagonal lattice of the α-titanium phase and in the bcc lattice of the β -Ti phase are equal to 7 аt.%and 20 аt.%, respectively. The formation of TiH2 , ТіН, and TiH1.75 titanium hydrides is thermodynamically favorable. These hydrides are located in the form of plates on the grain boundaries and in the slip and twinning planes inside the grains. The hydride transformation in titanium has a significant volume effect and, therefore, may accelerate fracture processes in the embrittled surface layer. If the concentration of hydrogen exceeds its solubility limit, titanium and its alloys suffer embrittlement even in the absence of external loading [46–48].
In the process of friction of titanium and its alloys under relatively low pressures, the thin surface natural passivating film is destroyed. This facilitates the adsorption of hydrogen by the metal surface. The direct interaction of hydrogen with the microasperities of the friction surface leads to the formation of brittle titanium hydride on these asperities [47]. The “three sliding bars–plate” titanium friction couple was studied in air, in hydrogen, and in nitrogen under a load of 20 N for each specimen [47]. It was shown that the degree of wear of titanium after friction in pure hydrogen is twice higher than the degree of wear in nitrogen and three times higher than in dry air. The presence of hydrogen increases the degree of wear due to the formation of hydride phases and generation of internal stresses, which facilitate rapid initiation of cracks on the surfaces of asperities and their fatigue fracture (Fig. 10а). The presence of water vapor in the atmosphere intensifies wear (Fig. 10b). The adsorption of water vapor decreases the surface energy and, hence, affects the conditions of chemical reactions on the friction surfaces and their mechanical properties.
The process of friction of titanium creates the conditions [15–18] favorable for the tribodestruction of water or other hydrogen-containing materials (e.g., oil) accompanied by hydrogen release, which accelerates the process of wear of the metal. Thus, in the case of friction of Ti–5% Al alloy in the transformer oil or in water, its wear is three times more intense than in the case of unlubricated friction in air (Fig. 10с), and the concentration of hydrogen in the products of wear is quite high [18].
Hydrogen accelerates the process of wear of titanium and its alloys due to the formation of hydride phases and generation of internal stresses, which lead to the rapid initiation of cracks on the microasperities of the friction surfaces and promote their fatigue fracture.
Conclusions
According to the contemporary ideas, friction may lead to the tribodestruction of hydrogen-containing materials (water vapor and hydrocarbons) accompanied by hydrogen release. Hydrogen is absorbed on the metal surface and dissociates with chemisorption. The diffusion of atoms from the chemisorbed layer into the crystal lattice of the metal and its continuous accumulation in the subsurface layers with elevated defectiveness creates additional internal stresses, leads to the formation of cracks, and accelerates fracture processes in the surface layers of the metal.
We can mention the following principal trends in the contemporary investigations of the hydrogen-assisted wear of metals in gaseous or liquid hydrogen and after cathodic polarization:
-
the determination of the conditions of adsorption and desorption of hydrogen and other gases in the process of friction and their quantitative and qualitative analyses;
-
the choice of the materials of tribocouples guaranteeing their high wear resistance in hydrogen-containing media;
-
the investigation of the influence of the chemical composition of materials on their wear resistance and the stability of their microstructures under the conditions of friction in hydrogen;
-
the development of the procedures of formation of efficient barriers for the diffusion of hydrogen into metals.
At present, despite the presence of a great amount of the data of investigations, there is no comprehensive understanding of the general regularities of hydrogen-assisted wear of various metals, absorption and desorption of hydrogen by the metals depending on the conditions of hydrogenation and modes of friction, and the influence of the physicomechanical properties and structure of the crystal lattice of materials on their tribological behavior. The insufficient knowledge of the nature and mechanisms of hydrogen-assisted wear complicates its prediction and improvement of the existing means of protection.
References
G. V. Karpenko and R. I. Kripyakevich, Influence of Hydrogen on the Properties of Steel [in Russian], Metallurgiya, Moscow (1962).
M. M. Shved, Variations of the Operating Properties of Iron and Steel Under the Influence of Hydrogen [in Russian], Naukova Dumka, Kiev (1985).
V. I. Pokhmurs’kyi and V. V. Fedorov, Influence of Hydrogen on Diffusion Processes in Metals [in Ukrainian], Karpenko Physicomechanical Institute, Ukrainian National Academy of Sciences, Lviv (1998).
I. K. Pokhodnya and V. I. Shvachko, “Nature of hydrogen brittleness of structural steels,” Fiz.-Khim. Mekh. Mater., 37, No. 2, 87– 96 (2001); English translation: Mater. Sci., 37, No. 2, 241–251 (2001).
V. I. Pokhmurs’kyi, “Investigations of the influence of hydrogen on metals carried out at the Karpenko Physicomechanical Institute,” Fiz.-Khim. Mekh. Mater., 33, No. 4, 25–38 (1997); English translation: Mater. Sci., 33, No. 4, 421–435 (1997).
V. I. Tkachov, “Effect of hydrogen on the properties of structural steels,” Fiz.-Khim. Mekh. Mater., 41, No. 4, 107–110 (2005); English translation: Mater. Sci., 41, No. 4, 547–550 (2005).
T. C. Zhang, X. X. Jiang, and S. Z. Li, “Hydrogen-induced embrittlement wear of a high-strength, low-alloy steel in an acidic environment,” Corrosion, No. 53, 200–205 (1997).
Y. Murakami, “Basic mechanism of hydrogen embrittlement and its application to design and structural integrity,” in: Proc. Int. Hydrogen Energy Development Forum, Fukuoka (2008), pp. 109–121.
V. V. Shyrokov, O. V. Shyrokov, L. A. Arendar, and O. V. Bilous, “Hydrogen wear. A brief retrospection of the problem,” in: Proc. of the 11th Int. Industrial Conf. (February 10–14, 2011, Plav’e, Ukraine) [in Ukrainian], (2011), pp. 236–239.
P. Cotterill, The Hydrogen Embrittlement of Metals,” Pergamon Press, Oxford (1961).
V. I. Shapovalov, Influence of Hydrogen on the Structure and Properties of Iron–Carbon Alloys [in Russian], Metallurgiya, Moscow (1982).
V. I. Shapovalov and V. Yu. Karpov, “On the nature of abnormal spontaneous deformation of the quasiliquid state for some metal– hydrogen systems,” Fiz. Met. Metalloved., No. 4, 805–811 (1988).
B. A. Kolachev and R. M. Gabidullin, “On the form of manifestation of hydrogen brittleness in metals and alloys,” Fiz.-Khim. Mekh. Mater., 12, No. 5, 3–9 (1976).
M. Khebda and A. V. Chichinadze (editors), A Handbook of Triboengineering, Vol. 1: Theoretical Foundations [in Russian], Mashinostroenie, Moscow (1989).
G. P. Shpen’kov, Physical Chemistry of Friction [in Russian], Belorussian State University, Minsk (1978).
I. I. Berkovich and D. G. Gromakovskii, Tribology. Physical Foundations, Mechanics, and Engineering Applications [in Russian], Samara State Technical University, Samara (2000).
A. A. Polyakov (editor), Protection Against Hydrogen-Induced Wear in Friction Units [in Russian], Mashinostroenie, Moscow (1980).
A. A. Polyakov and D. N. Garkunov, Hydrogen-Induced Wear in Friction Units [in Russian], Nauka, Moscow (1977).
G. Slys’, V. I. Berezanskaya, I. A. Kossko, and A. P. Pomytkin, “Development of new corrosion-resistant and wear-resistant materials for use in aggressive hydrogen medium,” Int. J. Hydrogen Energy, 26, 531–536 (2001).
T. Sasada, K. Hiratsuka, and H. Saito, “Adsorption of surrounding gas molecules on pure metal surfaces during wear processes,” Wear, 135, 251–264 (1990).
H. Mishina, “Atmospheric characteristics in friction and wear of metals,” Wear, 152, 99–110 (1992).
A. F. Prisevok, G. Ya. Biliaev, Yu. Kipnis, and A. V. Timofeev, “Mechanism of metal and alloy wearing in hydrogen-containing media,” Int. J. Hydrogen Energy, 21, No. 11/12, 1005–1008 (1996).
S. Jacobson and S. Hogmark, “Surface modification in tribological contacts,” Wear, No. 266, 370–378 (2009).
E. Wandke, M. Moser, and S. Tscherny, “The influence of corrosion and hydrogen cracking on blast wear in wet media,” Wear, No. 121, 15–26 (1988).
D. Newlands, A. Olver, and N. Brandon, “Gaseous evolution of hydrogen from hydrocarbon oil and grease lubricated contacts,” Tribol. Interface Eng. Ser., 41, 719–726 (2003).
N. Kino and K. Otani, “The influence of hydrogen on rolling contact fatigue life and its improvement,” JSAE Rev., 24, 289–294 (2003).
C. Lunarska and D. Samatowicz, “The hydrogen-induced modification of the properties of the metal surface coated with oil and lubricant,” Tribol. Int., No. 33, 491–499 (2000).
J. P. Hirth, “Effects of hydrogen on the properties of iron and steel,” Metallurg. Mater. Trans. A., 11, No. 6, 861–891 (1980).
H. Attia, M. Kubota, N. Noyama, et al., “Fretting fatigue in hydrogen gas,” Tribol. Int., 39, No. 10, 1241–1247 (2006).
H. Wipf, “Electro- and thermotransport of hydrogen in metals,” Top. Appl. Phys., 29, 277–304 (1978).
C. A. Wert, “Trapping of hydrogen in metals,” Top. Appl. Phys., 29, 305–322 (1978).
M. Iino, “A more generalized analysis of hydrogen trapping,” Acta Metall., No. 30, 367–376 (1982).
K. Fukuda, S. Nagano, and J. Sugimura, “Effects of hydrogen environment on the friction and wear of the metals,” in: Proc. JAST Tribology Conf. (May 12–14, 2008, Tokyo), Tokyo (2008), pp. 61–62.
M . Okada, A. Kamegawa, J. Nakahigashi, et al., “New function of hydrogen in materials,” Mater. Sci. Eng., B, 173, No. 1–3, 253– 259 (2010).
Hydrogenation During Sliding and Hydrogen Wear Mechanism. Chapter 7. Tribol. Ser., 29, 251–309 (1995).
Y. Sawae and J. Sugimura, “Tribology in gaseous hydrogen,” J. Vacuum Soc. Jap., 53(4), 280–287 (2010).
J. Sugimura, B. Ono, M. Hashimoto, et al., “Sliding experiments of steels in gaseous hydrogen,” in: D. Dowson et al. (editors), Life Cycle Tribology, Elsevier (2005), pp. 465–473.
P. Kula, “The “self-lubrication” by hydrogen during dry friction of hardened surface layers,” Wear, No. 201, 155–162 (1996).
J. P. Hirth, “Effects of hydrogen on the properties of iron and steel,” Metall. Trans., A, 11A, No. 6, 861–891 (1980).
L. V. Bezprozvannykh, Yu. N. Ponomarev, V. I. Tkachev, and А. А. Fedorchenko, “Assessment of the influence of gaseous hydrogen on the friction and wear of steels,” Fiz.-Khim. Mekh. Mater., 23, No. 6, 103–106 (1987).
P. Kula, “The comparison of resistance to ‘hydrogen wear’ of hardened surface layers,” Wear, No. 178, 117–121(1994).
H. K. Birnbaum, “Mechanical properties of metal hydrides,” J. Less Common Met., 104, 31–41 (1984).
S. Gahr, B. J. Makenas, and H. K. Birnbaum, “Fracture of niobium hydride,” Acta Metallurg., 28, 1207–1213 (1980).
B. Frisch and W.-R. Thiele, “The tribologically induced effect of hydrogen effusion and penetration in steels,” Acta Metallurg., No. 95, 213–227 (1984).
H. Hagi, “Diffusion coefficient of hydrogen in iron without trapping by dislocations and impurities,” Mater. Trans., 35, 112–117 (1994).
V. P. Oleksandrenko and V. P. Belyanskii, “Investigation of the mechanisms of tribochemical reactions,” Probl. Tribol., No. 1, 101–110 (1996).
J. W. Jones and J. J. Wert, “The effects of gaseous environments on the wear of commercial purity titanium,” Wear, 32, 363–377 (1975).
V. A. Livanov, B. A. Kolachev, and A. A. Buhanova, “Hydrogen embrittlement of titanium and its alloys,” in: R. I. Jaffee and N. E.Promisel (editors), The Science, Technology, and Application of Titanium, Pergamon Press, Oxford (1970), pp. 561–675.
H. Ріnto, A. Pyzalla, R. Buscher, et al., “The effect of hydrogen on the deterioration of austenitic steels during wear at cryogenic temperature,” Wear, No. 259, 424–431 (2005).
P. Kula, R. Pietrasik, B. Wendler, and K. Jakubowski, “The effect of hydrogen in lubricated frictional couples,” Wear, 212, 199– 205 (1997).
A. A. Pasechnik, Foundations of the Tribology of Cutting of Structural Materials Under the Conditions of Hydrogenation [in Russian], Khmelnytskyi National University, Khmelnytskyi (2009).
A. S. El-Amoush, “Investigation of wear properties of hydrogenated tin brass heat exchanger,” J. Alloys Comp., No. 448, 257–262 (2008).
B. A. Ageev, I. M. Tsygel’nyi, and V. N. Zhitomirskii, “Kinetics of hydrogenation of steel under the conditions of cathodic polarization,” Fiz.-Khim. Mekh. Mater., 18, No. 4, 100–102 (1982).
V. H. Zakharchuk, O. T. Tsyrul’nyk, and Nykyforchyn, “Electrochemical and corrosion properties of hydrogenated 45 steel and 12Kh18N10Т steel,” Fiz.-Khim. Mekh. Mater., 41, No. 4, 66–76 (2005); English translation: Mater. Sci., 41, No. 4, 508–519 (2005).
V. I. Pokhmurs’kyi, M. M. Shved, and N. Ya. Yaremchenko, Influence of Hydrogen on the Deformation and Fracture Processes in Iron and Steel [in Russian], Naukova Dumka, Kiev (1977).
B. A. Kolochev, V. T. Talalaev, Yu. B. Egorova, and A. N. Kravchenko, “On the nature of the favorable influence of hydrogen on the cutting treatment of titanium alloys,” Nauka, Proizvod. Primen. Titana Uslov. Konversii, 2, 873–882 (1994).
E Fromm and E Gebhardt, Gase Und Kohlenstoff in Metallen, Springer, Berlin (1976).
Y. Sawada, H. Tamaru, M. Kogoma, et al., “The reduction of copper oxide thin films with hydrogen plasma generated by an atmospheric-pressure glow discharge,” Phys. D: Appl. Phys, 29, No. 10, 25–39 (1996).
T. Murakami, H. Mano, K. Kaneda, et al. “Friction and wear properties of zirconium and niobium in a hydrogen environment,” Wear, 268, 721–729 (2010).
J. F. Smith, “H–Nb (Hydrogen–Niobium),” in: T. B. Massalski, et al. (editors), Binary Alloy Phase Diagrams, ASM Internat., Materials Park (1996).
E. Zuzek, J. P. Abriata, A. San-Martin, and F. D. Manchester, “H–Zr (Hydrogen-Zirconium),” in: T. B. Massalski, et al. (editors), Binary Alloy Phase Diagrams, ASM Internat., Materials Park (1996).
M. V. Kindrachuk, N. M. Steshyna, and N. P. Mykhailiv, Influence of Preliminary Ion-Plasma Hydrogenation on the Tribological Properties of Ion-Nitrided Surfaces, http://www.nbuv.gov.ua/portal/natural/Ptz/2008_50/180-189%20Text.pdf.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Fizyko-Khimichna Mekhanika Materialiv, Vol. 48, No. 2, pp. 5–17, March–April, 2012.
Rights and permissions
About this article
Cite this article
Pokhmurs’kyi, V.I., Vasyliv, K.B. Influence of hydrogen on the friction and wear of metals (a survey). Mater Sci 48, 125–138 (2012). https://doi.org/10.1007/s11003-012-9482-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11003-012-9482-1