Abstract
“The Pregnancy and Health Profile” (PHP) is a free prenatal genetic screening and clinical decision support (CDS) software tool for prenatal providers. PHP collects family health history (FHH) during intake and provides point-of-care risk assessment for providers and education for patients. This pilot study evaluated patient and provider responses to PHP and effects of using PHP in practice. PHP was implemented in four clinics. Surveys assessed provider confidence and knowledge and patient and provider satisfaction with PHP. Data on the implementation process were obtained through semi-structured interviews with administrators. Quantitative survey data were analyzed using Chi square test, Fisher’s exact test, paired t tests, and multivariate logistic regression. Open-ended survey questions and interviews were analyzed using qualitative thematic analysis. Of the 83 % (513/618) of patients that provided feedback, 97 % felt PHP was easy to use and 98 % easy to understand. Thirty percent (21/71) of participating physicians completed both pre- and post-implementation feedback surveys [13 obstetricians (OBs) and 8 family medicine physicians (FPs)]. Confidence in managing genetic risks significantly improved for OBs on 2/6 measures (p values ≤0.001) but not for FPs. Physician knowledge did not significantly change. Providers reported value in added patient engagement and reported mixed feedback about the CDS report. We identified key steps, resources, and staff support required to implement PHP in a clinical setting. To our knowledge, this study is the first to report on the integration of patient-completed, electronically captured and CDS-enabled FHH software into primary prenatal practice. PHP is acceptable to patients and providers. Key to successful implementation in the future will be customization options and interoperability with electronic health records.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Family health history (FHH) has been called “the cheapest genetic test” [1] or the first genetic test [2], and it has long been recognized as an essential aspect of general prenatal care [3]. FHH can inform risk assessment and management for the pregnancy (e.g., preterm delivery) [4], the infant (e.g., congenital birth defects) [5], the patient (e.g., postpartum depression) [6], and her relatives (e.g., hereditary cancers) [7].
The American College of Obstetricians and Gynecologists (ACOG) recommends screening for a variety of genetic and non-genetic conditions in the prenatal period, some of which are based on patient ethnicity, FHH, and other medical, environmental, and lifestyle risk factors [8]. A 2009 review of published ACOG and American College of Medical Genetics (ACMG) guidelines for prenatal testing found 27 genetic or FHH conditions that are appropriate for prenatal screening to assess risk for the fetus, pregnancy, and the female patient across her lifespan [9]. While most prenatal providers recognize genetic screening for aneuploidy and ethnicity-based carrier screening as part of standard practice, adherence to the relevant guidelines is variable across providers [10], often due to limited knowledge, confidence, and time [11, 12].
Point of care tools and clinical decision support (CDS) can assist in the translation of FHH and genetic risks into personalized patient management strategies [13]. CDS provides clinicians with person-specific information, intelligently filtered and presented at appropriate times, to enhance health care [14]. CDS has the added potential benefit of supplying provider and patient education [15, 16]. A limited number of genetic and FHH CDS systems have been implemented resulting in some improvements in patient outcomes and provider adherence to guidelines [17–19]. To our knowledge, FHH and genetic CDS have not been tested in the prenatal setting.
The Pregnancy and Health Profile (PHP): a Screening and Risk Assessment Tool [20] is driven by the collection of patient-entered FHH and other risk factors at the point of care and identifies patients at increased risk for genetic and other conditions. PHP includes conditions for which screening is supported in the literature and by professional organizations [9]. In addition to 27 genetic and FHH conditions, 18 additional conditions or environmental or lifestyle risk factors that confer obstetric risk were included in PHP to aid in patient intake [9]. CDS algorithms were developed for the genetic and FHH conditions based on practice guidelines and reviewed by experts.
We describe the results of a multi-level, multi-method evaluation of the clinical implementation of PHP in four diverse clinical settings that elicited feedback on the tool from providers, patients, and clinic staff. In this pilot study, PHP was utilized as a stand-alone risk assessment tool, not integrated with the site’s electronic health record (EHR).
Methods
Recruitment
Site and provider inclusion criteria: study sites with five or more participating primary care prenatal providers and a patient load of approximately 50 new pregnant patients proficient in English, per month were eligible. Diversity of the patient and provider populations was also considered to maximize ethnic/racial, socioeconomic, and geographic diversity and the inclusion of underserved populations. Providers had to be a primary care prenatal provider, such as an obstetrician (OB), nurse practitioner, certified nurse midwife, family medicine physician (FP), physician assistant, or nurse; be affiliated with one of the four clinical sites; and have a role in patient care for 25 % or greater of their time. Residents were eligible for inclusion.
Patient inclusion: women presenting for the first prenatal visit to a participating provider during the pilot period were eligible to use PHP. Patients with limited English proficiency were excluded as the tool was only available in English, as were some patients based on acute pregnancy complications (e.g., miscarriage, heavy cramping or bleeding), as determined by the site staff during the clinical encounter.
Implementation
Participating sites included: (1) Mountain Area Health Education Center, an academic and community-based obstetrics and gynecology residency program in Asheville, NC; (2) Maine Dartmouth Family Medicine Residency Program, a rural academic family medicine program in Fairfield and Augusta, ME; (3) Montefiore Medical Group—Comprehensive Family Care Center, an urban federally qualified health center with an academic affiliation in the Bronx, NY; and (4) Clearvista Women’s Care, Community Health Network, an obstetric practice that is part of a community hospital network in the suburbs of Indianapolis, IN. Clinical site partners obtained approval or exemption through their respective Institutional Review Boards.
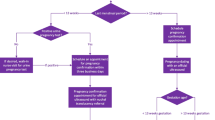
Staff met with site clinicians and clinic and IT staff prior to implementation to conduct a needs assessment, ascertain patient flow and processes, physical space, and provider educational needs and preferences and to develop an installation and implementation plan. At three sites, the tool was used with eligible patients presenting for the first prenatal visit; at one site (NY), informed consent was obtained and patients had the option to decline use of the tool (Fig. 2). In all sites, providers were not given the choice of whether or not their patients would use the tool; it was implemented as part of clinic flow, or at the NY site, offered to all eligible patients. At the first prenatal visit, patients completed an intake questionnaire on a tablet computer that collected maternal and paternal information about personal and FHH (∽17.5 min average time to completion). The tool generated the PHP report, an adaptation of the ACOG Antepartum Record, populated with patient-entered data and with the output of the CDS for the provider (Fig. 1) [20]. In two sites (NY, IN), the tool was an additional component to the existing prenatal intake process and work flow. In these sites, staff transcribed relevant data from the PHP report into the patients’ encounter in the EHR and the paper report was scanned into the EHR as a reference. In ME and NC, the tool replaced all (ME) or most (NC) of the previous intake process and documentation. Here, the paper PHP report was filed in the patient’s paper record in place of the previous form filled out by the patient.
Prior to implementation, project staff provided 1–2 h group training on tool use at each site that covered the value of FHH in prenatal care, the components of the tool, the proposed implementation plan, and workflow challenges and resolutions. Ongoing one-on-one support was provided by site coordinators as needed. The study was conducted between September 2011 and March 2012. Sites used the tool for 14–23 weeks depending on patient volume.
Data Collection
Patients presenting for their first prenatal visit were invited to complete a voluntary and anonymous 18-item paper survey after using the tool to provide feedback. Providers were asked to complete a 16-item paper baseline survey prior to training and a 33-item paper or electronic final survey 1–19 weeks after the study period that assessed confidence and knowledge. The final survey also collected provider feedback on the tool. Reminders to complete the electronic survey were sent after 2 weeks after the study period ended. Participants were given the option of completing a paper survey to increase response. The evaluation instruments were developed by the project team and tested during formative evaluation (data not shown) and included both quantitative and qualitative measures (available in supplement). Neither providers nor patients were offered a financial incentive to participate.
Semi-structured interviews with the clinic administrator were conducted at 1–3 months and at the conclusion of the pilot period.
Data Analysis
Quantitative survey data were analyzed using descriptive statistics, Chi square test or Fisher’s exact test (e.g., to compare patient characteristics by site), paired t tests (to make pre/post comparisons of provider confidence), t tests to compare perceived usefulness/helpfulness between FPs and OBs, and multivariate logistic regression (to identify patient characteristics associated with usability). Statistical tests were considered significant using Bonferroni-adjusted p values. Qualitative thematic analysis was used for responses to open-ended questions.
Results
Study Populations
Six-hundred eighteen patients used the tool during the study period. Of these, 513 (83 %) provided feedback on PHP by completing the survey (Table 1; Fig. 2). Among patients who used the tool, there was no statistically significant difference between survey responders and non-responders in terms of age, education, race, or ethnicity. In one site (IN), patients who reported a previous pregnancy were less likely to complete the survey (78 vs. 91 %, p = 0.01). The patients who completed the survey were white/Caucasian (81 %), black or African-American (11 %), and Latina (9 %). The mean age was 27 years (SD = 5.84) with a range of 15–46 years. Patients were of diverse educational levels and parity.
One hundred and sixteen physicians (36 attendings and 70 residents) were determined to be eligible at the time of implementation (Fig. 3). A total of 71 physicians used the tool. Twenty-four physicians completed both evaluation surveys and 21 self-reported they used the tool with patients, including 8 FPs (all at ME) and 13 OBs (8 NC; 1 NY; 4 IN). Among participants who completed both evaluation surveys, approximately half were residents (4 FP, 6 OB) and 67 % were female (7 FP, 7 OB).
Data from the final evaluation surveys show that OBs saw a median of 15 patients and FPs 3.5 patients who used the tool; 4 OBs (40 %) and 5 FPs (83 %) reported that they used the tool with 2–5 patients; 6 OBs (60 %) with more than 10 patients (range 10–50); and 1 FP (17 %) with 60 patients.
Patient Results
Patients overwhelmingly felt the tool was easy to use (97 %, n = 474/490) and they found the FHH questions easy to understand (98 %, 478/489). There were no significant differences in ease of use or understanding questions between sites. Multivariate logistic regression controlling for age, education, and English as first language showed patients who were “very comfortable” using computers were more likely to report that the tool was “very easy” to use (OR 3.5, 95 % CI 1.7–7.3; p = 0.001) and that the questions were “very easy” to understand (OR 4.5, 95 % CI 2.1–9.7; p < 0.001). Additionally, patients who had been pregnant before were more likely to say the questions were “very easy” to understand (OR 3.1, 95 % CI 1.5–6.2, p < 0.01). Ninety-six percent (467/486) of patients were not worried about the confidentiality of entering FHH into the tool. Only 2/513 patients reported that they were “very worried” about confidentiality. Seventy-nine percent (384/486) of patients felt the length of time it took to complete the tool was “okay” and 21 % felt the tool was “somewhat long” or “too long.” Patient attitudes about length of time varied by site, with 37 % (7/19) of patients at the ME site, 30 % (67/223) at NC, 22 % (8/37) at NY, and 10 % (20/207) at IN reporting the tool was somewhat or too long (p ≤ 0.001).
Patients were asked how willing or unwilling they were to provide their FHH through various options. Patients were equally willing to provide information in a clinical encounter by entering it into a computerized tool, 93 % (440/473), or by verbally reporting it to a provider (93 %, 437/468). These methods were preferred over completing a paper form (77 %, 362/470).
Provider Results
Seventy-six percent of eligible physicians completed the baseline survey, 61 % used the tool with patients, 21 % completed both the baseline and final surveys, and 18 % completed both surveys and self-reported they used the tool with patients. For reasons of human subjects protection, provider identity was not revealed to evaluation staff and study identification numbers were used to link baseline and final survey data, which limits our ability to comment on characteristics of survey responders versus non-responders. Residents accounted for 60 % of initially eligible providers and in some sites, these providers may have been lost to follow-up when they rotated to other clinics.
Providers answered seven knowledge questions related to FHH and identifying and managing prenatal genetic risks (survey instrument available as a supplement). FPs showed an improvement in knowledge by an average increase in knowledge score from 4.8 to 6 questions correct, although this was not statistically significant. Using the tool did not improve knowledge of OBs, who scored an average 6.2 questions correct at baseline and at the end of the study.
Providers self-reported confidence in identifying and discussing patients’ genetic risks through six different items (Table 2). OBs’ confidence significantly improved for 2 out of 6 measures whereas FPs’ confidence did not significantly improve.
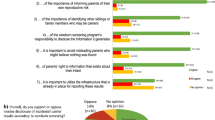
More than half of both FPs and OBs rated as useful to their clinical practice the patient questionnaire, receiving a report with pre-populated patient data, and the family history data collection/pedigrees (Fig. 4). Thematic analysis of provider responses to survey questions identified that some providers appreciate the data collection aspects of PHP that can aid in efficient clinic flow and see value in the tool’s ability to engage and educate patients during the first prenatal visit, although one provider noted that he/she missed the opportunity to develop rapport through one-on-one collection of FHH.
Providers were asked about the completeness of data collected through the tool. Combined, 53 % of physicians reported that additional questions were needed to clarify a patient response on the tool with “most” or “all” of patients. Free text responses show these were predominately to collect further detail about the patient’s personal history (e.g., details of a surgery) or the exact nature of disease in a relative. Fewer physicians (24 %) reported that they asked additional FHH questions about additional conditions that were not on the tool for all or most patients.
Physicians had a high variability in how helpful they found the CDS, with relatively equal numbers reporting positive, neutral, and negative feedback on the CDS (Fig. 5). There were no detectable trends in physicians’ perceived helpfulness of CDS when looking at subgroups (e.g., residents vs. attendings; FPs vs. OBs). Analysis of providers’ open-ended comments showed that some providers appreciate the CDS considerations list that provides recommendations on referrals and screenings tests. Negative themes from qualitative analysis included dissatisfaction with both the presentation of the CDS in the report (e.g., length and organization) and content of the CDS, with one provider expressing concerns that the tool results in over-calling of risk and an increase in unnecessary referrals. Some providers reported that the tool reduced time spent taking FHH and they could use this additional time to focus on the unique aspects of each pregnancy. Other providers reported that the tool hindered visit productivity, increased provider time spent clarifying FHH responses, and made the documentation of obstetric risks more challenging.
About half the physicians commented in open-ended responses on the length of the report and number of CDS considerations. In the survey, less than half of physicians rated the structure of the report as useful (Table 2), and their specific comments related to needing additional customization of the tool’s report to support their clinic processes. Both OBs and FPs noted that they would recommend customized changes to the report and the tool (Table 3).
At the conclusion of the pilot period, the ME site continued to use PHP in prenatal care and the other three sites did not, returning to their previous intake and management systems. The sites conveyed the duplication of data entry processes and need to focus on other EHR implementation as reasons to stop using PHP.
Impact on Clinic Flow and System
At two sites where the tool was implemented in addition to existing process (NY, IN), using the tool lengthened the clinical encounter because some work and processes were duplicated. Administrators reported initial disruptions in clinic flow, which were addressed by adjustments to the flow, and improved provider acceptance over time. Thematic analysis of qualitative interviews with administrators identified key points of customization, barriers, and facilitators (Table 4). Each site had a unique experience using the tool; however, there were some common requirements for successful implementation, including: (1) a single point person who was responsible for day-to-day management of the project; (2) a clinical champion to advocate for the project; (3) clinical and technical training for site staff; (4) technical assistance from project staff; (5) information technology resources such as a server and wireless connection, and (6) a private space, such as an exam room, in which to complete tool.
Preparing for implementation took 1–3 months and included installation and clinical staff training. IT resources required varied based on the degree of site staff participation, and may have included ~5 % FTE (more at initial installation, less once system was launched) and server space to house the database (data from initial needs assessments and administrator interviews). Site coordinators worked on this project ~25 to 50 % FTE, which included both clinical coordination and research coordination.
Discussion
Summary of Results
This observational pilot study is the first description of the impact of implementing a patient-entered, electronic genetic and pregnancy risk screening tool with CDS in the prenatal setting. We used a multi-level, multi-method approach to implement and evaluate PHP in practice to assess impact on patients, providers, and the clinic.
PHP as a Data Collection Tool
The tool showed high usability and acceptability among diverse patients in four different clinical settings. These results are consistent with other reports in the literature showing high patient acceptance and satisfaction with providing FHH and other personal health information in web-based tools [21–23]. The majority of patients who used PHP spoke English as their first language and reported that they were comfortable with computers. Not surprisingly, women who were comfortable using computers were more likely to report high usability and understanding of the tool. We also found that women who had been pregnant before were more likely to say the questions on the tool were very easy to understand. This may be due to a higher level of familiarity in general with the prenatal care process.
An important consideration in this study is the accuracy of the family history information collected by PHP. In a formative evaluation of PHP prior to implementation, we compared the FHH as collected by the tool to that collected by a genetic counselor (GC). The tool and GC were comparable in identifying accurate family structure in first- and second-degree relatives and had similar detection rates of increased risk in the family (data not shown). Various groups have studied the analytic validity of patient-reported FHH and have found a similar accuracy rate when collecting FHH compared to the standard practice of patient interviews, although the sensitivity varies based on the specific condition [24]. Providers in our study reported needing to collect additional information about patient-reported health and family conditions. This may be typical for patient-entered screening and intake forms, whether paper or computerized. We did not have a way to compare the level of additional data collection and follow-up that occurred in the clinics as compared to their other practices.
PHP as an Educational Resource for Physicians
We found that OBs’ confidence about identifying and managing genetic risks improved during the study period but not FPs’. The small sample size makes it difficult to draw definitive conclusions from our observations of FPs’ and OBs’ responses. It is possible that the difference in confidence between these two physician groups is unrelated to their subspecialties, but rather is a reflection of some unmeasured clinic or practice characteristics. The FPs were all part of one practice that had to adapt to an entirely new prenatal record system, and this adjustment may have mitigated the benefits of CDS on changing their confidence.
We did not find statistically significant changes in physician knowledge post-implementation. While the CDS report was not specifically designed to improve knowledge as an outcome—clinical behavior change was the goal—we did measure knowledge as this could be a beneficial byproduct of interacting with the CDS results over time. Qualitative data from provider open-ended comments on surveys and administrator interviews suggest that using PHP does raise awareness of relevant family history risks among providers.
Physician Perceptions on PHP Clinical Decision Support
We saw high variability in physician’s feedback on quantitative measures regarding the helpfulness of the CDS, ranging from “very helpful” to “not at all helpful.” Examining individual responses and provider open-ended comments further supports the mixed feedback about the value of the CDS and of the tool as a whole. A few physicians had very positive responses to the tool, a few had very negative responses, and the majority saw some advantages as well as some frustrations and challenges. With the small sample size, we are unable to determine if there are specific provider characteristics that affect acceptance of PHP, and this warrants future study.
Dissatisfaction with the organization, structure, or length of the report and CDS messages was a common theme among providers. Although trained on the tool, some providers were unfamiliar with the specific questions on the patient questionnaire, so there may have been confusion about exactly what was asked of the patient. The patient data populated the PHP report, which was a modified version of the ACOG Antepartum Record [25], a comprehensive prenatal care record. The PHP report was 13–17 pages long, depending on patient history, and includes templates for complete intake. We deliberately chose the Antepartum Record as a model because it is familiar to many prenatal providers, but additional study can be done to determine optimal data presentation design.
A 2006 review of physicians’ actions around CDS for drug safety found that physicians override 49–96 % of such alerts [26]. If such high rates of override are found for drug safety alerts, it is possible that CDS for non-acute occurrences, such as FHH risk, may see even higher rates of provider disregard. This study found some evidence that low-level alerts were more likely to be overridden than high-level (serious) alerts. Some physicians in our study also reported concern that there were “too many” CDS messages and one physician reported concern about the accuracy of CDS messages. Such concerns and perception of “alert overload” can negatively impact the users’ confidence in the CDS system [27], which may be a factor for those providers who were less satisfied with the tool.
An important consideration in the development of any CDS system is selection of included risks and conditions. The conditions on PHP were selected based on demonstrated evidence from the literature and professional organization guidelines [9]. All recommendations made by the CDS were a direct result of existing guidelines. The objections to the number of interventions recommended by the CDS might be considered more an objection to existing guidelines than an objection to the tool. For example, one provider-reported “a family history of hypertension is not a flag for me,” referring to the algorithm that produces a CDS message to screen for hypertension if there is a family history of it reported. Another possible factor influencing providers’ objections to the number of CDS recommendations with the system is the timing of delivery of CDS, which in this project, was all at once. A relatively simple solution would be to distribute the CDS messages over the course of multiple perinatal visits and test the impact on providers.
While the algorithms were informed by professional society guidelines and reviewed by content experts, it is possible they are not the best interpretation or that the provider’s practice is not aligned with the guidelines. Historically, many practice guidelines were developed based on expert opinion rather than evidence-based medicine, especially in genetics where the low incidence of many conditions creates challenges for conducting the large scale trials necessary to develop evidence [28]. While professional organizations continue to strive towards evidence-based practice guidelines, many of the guidelines included in the tool’s algorithms have not been tested in a clinical population to determine clinical validity and utility. Additional evaluation of the algorithms used in risk assessment and tool itself would help to determine clinical validity and utility of such screening, respectively, in the primary care population.
The observed dichotomy in patient and provider satisfaction and perception of the tool may be influenced by the general dynamics during the first obstetric visit of patients that are highly engaged to be information seekers, and providers who are pressed for time. Adding to the complexity of the visit is likely to challenge providers and be met with resistance, which could be a factor in our observations of physician acceptance. To promote both physician and patient acceptance and usability, tools such as PHP must be integrated into the clinic workflow maximally.
Implementation of PHP
Clinic flow was customized for each site based on the practices’ needs and resources, resulting in variation in IT setup, tool integration and use procedure. These different approaches impacted clinic flow and influenced provider feedback differently, which has implications for interpreting provider data. Despite developing a customized installation and implementation plan to meet each clinic’s needs, implementation and long-term maintenance of the tool required consistent monitoring, evaluation, and adjustment.
The desire for customization of the tool, CDS, and report was a theme identified by both providers and administrators. The observed variability between sites regarding implementation approaches and between providers regarding responses to PHP demonstrate that practice and provider characteristics affect the outcomes of the intervention. Our findings are consistent with the conclusions of Wilson and colleagues, who recently published a framework for developing and using FHH tools in the primary care setting. They recognized that the patient populations and clinical goals of a FHH screening program impact which attributes of the specific FHH tool are more and less important, concluding that “one size does not fit all” when considering FHH tools across primary care clinics [29]. PHP can be customized for a specific site, but this was not an option for sites during the pilot.
Kawamoto and colleagues evaluated the elements of CDS systems that influence success in achieving desired outcomes [16, 30]. These include systems that provide CDS: (1) automatically, (2) through computerized automation, and (3) at the point-of-care; and (4) recommend a clinical action (rather than simply generate a risk). Additional features that can support success include systems that (5) provide CDS as an integrated component of the health record and (6) prompt a provider to record the reason for noncompliance with CDS. PHP meets the first four core factors. PHP can be implemented as an integrated core component of the health record but in our pilot, 2 of 4 sites chose to implement it as a stand-alone risk assessment system. Future development of PHP and other tools should continue to follow these guidelines. For future implementation of PHP, we recommend integration with EHRs, a shorter report, options for customization to site needs, and additional study of the timing and delivery mechanism of CDS to promote maximum provider acceptance.
While there are aspects of prenatal care that are unique compared to other areas of medicine, we believe the implementation issues and patient and provider feedback identified in this study are translatable beyond the prenatal clinic. This project is the first to study family history and genetic CDS in the prenatal setting and our findings regarding usability are consistent with those of other studies of family history systems as well as non-genetic CDS systems [16, 29–31].
Limitations
There are a number of limitations that should be acknowledged in this study. First, caution should be used when interpreting the provider results due to the small numbers of physicians who participated in the sites and completed both surveys. This small pilot study was not designed to assess the extent to which any differences between physicians were due to differences in the demographics of their patient populations. Similar to other studies that use physician surveys, we saw a relatively low response rate, which can raise questions of nonresponse bias and the overall generalizability of the findings [32]. In addition to staff turnover, especially among residents, which we believe to be a factor influencing response rate, other potential reasons for the response rate observed in this study include the need to complete multiple surveys to be included in the analysis and a lack of monetary incentive, which as been shown to increase response rates to surveys [32]. Future evaluation of PHP and other similar tools should consider additional mixed-mode surveys and monetary incentives to increase response rates. Nurses and nurse midwives were excluded from this analysis because of small numbers, but these providers also used the tool with patients and their perspectives contribute to the overall experience of using the tool in clinical practice. Additionally, there were many factors in the clinical ecosystem that may have impacted providers’ knowledge, confidence, and perceptions of a FHH tool that we could not fully measure.
This study sought to assess the usability of PHP within a specific patient population, women who can read and speak English presenting for prenatal care in one of the participating sites. We cannot make conclusions about the usability of this tool in other populations without further study. There may also be unmeasured factors impacting patients’ experiences with the tool.
While FHH is clinically viewed as a useful tool for risk assessment [3] and there are some studies supporting clinical utility in adult populations [33, 34], there are limited data regarding the utility of FHH as a screening tool in the prenatal setting [35]. Furthermore, while the tool underwent validation testing in a small sample as part of formative evaluation and continues to undergo frequent quality control checks internally, it has not undergone a randomized control trial to assess analytic and clinical validity of the tool’s data collection and risk assessment functions. This is recommended as a future research initiative. Similarly, although we did not validate the questions and instruments via a formal study, there is some evidence to suggest they have face validity based on a formative evaluation with 12 women and 8 providers. Finally, there were CDS errors identified when deploying the tool. All errors were corrected as they were identified and communicated to clinical site staff, but such errors could have impacted the integrity and provider perceptions of the CDS.
Future Research Agenda
Additional research is indicated to further study the impact of PHP and other electronic tools on the patient, provider, and clinical system. PHP should be tested in additional patient populations, especially among non-English speaking patients, and with a larger number of providers and using diverse methods (e.g., focus groups) to determine factors associated with the impact of the tool on patient and provider outcomes and behaviors. Not included in the scope of this analysis is the long-term impact of PHP on clinical outcomes and the clinic system, which warrants further study. Future study should also examine the impact of provider and organization culture and characteristics from an implementation science framework. Finally, tools like PHP can serve as a research tool themselves, by supporting the kinds of research needed to continue to determine clinical utility and validity of FHH.
Conclusion
This is the first study to describe a computerized intervention for the identification and management of prenatal genetic risks using FHH. We found high patient satisfaction and both positive and negative feedback from providers. We present a unique study and unique CDS tool that can assist the prenatal provider and patient in shared decision-making around patient’s genetic and FHH risks and can also improve provider confidence and knowledge. Future iterations of the tool should include a Spanish-language version of the tool, customizable features for different providers and clinics, and an EHR-interoperable report.
References
Guttmacher, A. E., Collins, F. S., & Carmona, R. H. (2004). The family history–more important than ever. New England Journal of Medicine, 351(22), 2333–2336.
Pyeritz, R. E. (2012). The family history: the first genetic test, and still useful after all those years? Genetics in Medicine, 14(1), 3–9.
ACOG. (2011). Committee Opinion No. 478: Family history as a risk assessment tool. Obstetrics and Gynecology, 117(3), 747–750.
Bhattacharya, S., Raja, E. A., Mirazo, E. R., et al. (2010). Inherited predisposition to spontaneous preterm delivery. Obstetrics and Gynecology, 115(6), 1125–1133.
Oyen, N., Poulsen, G., Boyd, H. A., et al. (2009). Recurrence of congenital heart defects in families. Circulation, 120(4), 295–301.
Forty, L., Jones, L., Macgregor, S., et al. (2006). Familiality of postpartum depression in unipolar disorder: Results of a family study. American Journal of Psychiatry, 163(9), 1549–1553.
Hampel, H., Frankel, W. L., Martin, E., et al. (2005). Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). New England Journal of Medicine, 352(18), 1851–1860.
ACOG. (2012). Practice bulletin: Clinical management guidelines for obstetrician-gynecologists. http://www.acog.org/~/media/List%20of%20Titles/PBListOfTitles.pdf.
Lin, B. K., Edelman, E. A., McInerney, J. D., et al. (2013). Personalizing prenatal care using family health history: Identifying and integrating a panel of conditions into a novel screening tool. Personalized Medicine, 10(3), 307–318.
Darcy, D., Tian, L., Taylor, J., et al. (2011). Cystic fibrosis carrier screening in obstetric clinical practice: Knowledge, practices, and barriers, a decade after publication of screening guidelines. Genet Test Mol Biomarkers, 15(7–8), 517–523.
SACGHS. (2011). Genetics education and training: Report of the secretary’s advisory committee on genetics, health, and society. Bethesda, MD: Department of Health and Human Services.
Nippert, I., Harris, H. J., Julian-Reynier, C., et al. (2011). Confidence of primary care physicians in their ability to carry out basic medical genetic tasks-a European survey in five countries-Part 1. Journal of Community Genetics, 2(1), 1–11.
Hoffman, M. A., & Williams, M. S. (2011). Electronic medical records and personalized medicine. Human Genetics, 130(1), 33–39.
Osheroff, J. A., Teich, J. M., Middleton, B., et al. (2007). A roadmap for national action on clinical decision support. Journal of the American Medical Informatics Association, 14(2), 141–145.
Litvin, C. B., Davis, K. S., Moran, W. P., et al. (2012). The use of clinical decision-support tools to facilitate geriatric education. Journal of the American Geriatrics Society, 60(6), 1145–1149.
Welch, B. M., & Kawamoto, K. (2013). Clinical decision support for genetically guided personalized medicine: A systematic review. Journal of the American Medical Informatics Association, 20(2), 388–400.
Rubinstein, W. S., Acheson, L. S., O’Neill, S. M., et al. (2011). Clinical utility of family history for cancer screening and referral in primary care: A report from the family healthware impact trial. Genetics in Medicine, 13(11), 956–965.
Ozanne, E. M., Loberg, A., Hughes, S., et al. (2009). Identification and management of women at high risk for hereditary breast/ovarian cancer syndrome. The Breast Journal, 15(2), 155–162.
Orlando, L. A., Hauser, E. R., Christianson, C., et al. (2011). Protocol for implementation of family health history collection and decision support into primary care using a computerized family health history system. BMC Health Services Research, 11, 264.
NCHPEG. (2013). The pregnancy and health profile: A screening and risk assessment tool. Accessed July 15, 2013, from http://www.nchpeg.org/index.php?option=com_content&view=article&id=410&Itemid=277.
Ralston, J. D., Carrell, D., Reid, R., et al. (2007). Patient web services integrated with a shared medical record: Patient use and satisfaction. Journal of the American Medical Informatics Association, 14(6), 798–806.
Arar, N., Seo, J., Abboud, H. E., et al. (2011). Veterans’ experience in using the online surgeon general’s family health history tool. Personalized Medicine, 8(5), 523–532. doi:10.2217/pme.11.53.
Westman, J., Hampel, H., & Bradley, T. (2000). Efficacy of a touchscreen computer based family cancer history questionnaire and subsequent cancer risk assessment. Journal of Medical Genetics, 37(5), 354–360.
Facio, F. M., Feero, W. G., Linn, A., et al. (2010). Validation of my Family health Portrait for six common heritable conditions. Genetics in Medicine, 12(6), 370–375.
ACOG. (2013). ACOG Resource Center. Accessed February 4, 2013, from fromhttp://www.acog.org/About_ACOG/ACOG_Departments/Resource_Center.
van der Sijs, H., Aarts, J., Vulto, A., et al. (2006). Overriding of drug safety alerts in computerized physician order entry. Journal of the American Medical Informatics Association, 13(2), 138–147.
Shah, N. R., Seger, A. C., Seger, D. L., et al. (2006). Improving acceptance of computerized prescribing alerts in ambulatory care. Journal of the American Medical Informatics Association, 13(1), 5–11.
Toriello, H. V., & Goldenberg, P. (2009). Evidence-based medicine and practice guidelines: Application to genetics. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 151C(3), 235–240.
Wilson, B. J., Carroll, J. C., Allanson, J., et al. (2012). Family history tools in primary care: Does one size fit all? Public Health Genomics, 15(3–4), 181–188.
Kawamoto, K., Houlihan, C. A., Balas, E. A., et al. (2005). Improving clinical practice using clinical decision support systems: A systematic review of trials to identify features critical to success. BMJ, 330(7494), 765.
Bauer, N. S., & Carroll, A. E. (2013). Downs SM. Journal of the American Medical Informatics Association: Understanding the acceptability of a computer decision support system in pediatric primary care.
VanGeest, J. B., Johnson, T. P., & Welch, V. L. (2007). Methodologies for improving response rates in surveys of physicians: A systematic review. Evaluation and the Health Professions, 30(4), 303–321.
Ruffin, M. T., Jr Nease, D. E., Sen, A., et al. (2011). Effect of preventive messages tailored to family history on health behaviors: The family healthware impact trial. The Annals of Family Medicine, 9(1), 3–11.
Qureshi, N., Armstrong, S., Dhiman, P., et al. (2012). Effect of adding systematic family history enquiry to cardiovascular disease risk assessment in primary care. Annals of Internal Medicine, 156(4), 253–262.
NIH. (2009). Family history and improving health. National Institutes of Health State of the Science Consensus Conference; Bethesda, Maryland.
Acknowledgments
The Pregnancy and Health Profile was developed by the National Coalition for Health Professional Education in Genetics, Genetic Alliance, HughesRiskApps.net, the March of Dimes, and the Health Resources and Services Administration (HRSA) and is funded by Grant #U33MC12786 from HRSA. The views expressed in this publication are solely the opinions of the authors and do not necessarily reflect the official policies of the US Department of Health and Human Services or the Health Resources and Services Administration, nor does mention of the department or agency names imply endorsement by the US government. We are grateful to the women, providers, and clinic staff that participated in this project, especially the study coordinators and investigators from our collaborating clinical sites for their effort and continued partnership. We also acknowledge the project’s national advisory committee for members’ advice and time, and Joseph McInerney, Michele Lloyd Puryear, and Penny Kyler for their leadership and vision at early stages of this project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Edelman, E.A., Lin, B.K., Doksum, T. et al. Evaluation of a Novel Electronic Genetic Screening and Clinical Decision Support Tool in Prenatal Clinical Settings. Matern Child Health J 18, 1233–1245 (2014). https://doi.org/10.1007/s10995-013-1358-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10995-013-1358-y