Abstract
Many women of reproductive age from developing countries have poor nutritional status, and the prevalence of depression during pregnancy is high. The objective of the present study was to assess the prevalence of antenatal depressive symptoms in early pregnancy, and to identify the demographic and nutritional factors associated with these symptoms in a sample of urban South Indian pregnant women. This cross-sectional study was the baseline assessment of a prospective randomized controlled trial of vitamin B12 supplementation in urban pregnant south Indian women between the ages of 18 and 40 years (www.clinicaltrials.gov: NCT00641862). 365 women in their first trimester of pregnancy were screened for depressive symptoms at an urban clinic in Karnataka, South India, using the Kessler Psychological Distress Scale (K-10). Nutritional, clinical and biochemical factors were also assessed. Mean (SD) age of the cohort was 22.6 (3.7) years and mean (SD) BMI was 20.4 (3.3) kg/m2. 121 (33 %) of the women in the 1st trimester had symptoms consistent with depression (K-10 score >6). In multivariate log binomial regression analysis, presence of antenatal depressive symptoms in the first trimester were positively associated with vomiting, prevalence ratio (PR) = 1.54 (95 % CI 1.10, 2.16) and negatively with anemia, PR = 0.67 (95 % CI 0.47, 0.96). Nutrient intakes, serum vitamin B12, methylmalonic acid, homocysteine and red cell folate levels were not associated with measures of depression. Antenatal depressive symptoms in early pregnancy are highly prevalent in urban Indian women and are more common in women with vomiting and without anemia. In this cross-sectional data, blood concentrations of vitamin B12 and folate were not associated with depressive symptoms. The relationship between nutritional status and depressive symptoms may require larger and longitudinal studies.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Depression is common during pregnancy [1, 2], with estimates ranging from 8 to 30 % [3–5]. Recent studies from the developing world report higher rates of antenatal depression (25–45 %) [6–9]. Particularly high rates of antenatal depression have been reported in the Asian sub-continent, with estimates of 28–57 % in Pakistan [10, 11] and 33 % among pregnant Bangladeshi women [12].While there are several studies of postnatal depression from India [13, 14] few have examined the prevalence and correlates of antenatal depression in Indian women [7].
One possible risk factor for antenatal depression in poor women from developing countries is inadequate nutritional status during pregnancy [15]. This risk may be mediated directly through various nutrient deficiencies such as folate, vitamin B12, iron, zinc and polyunsaturated fatty acids that impact brain functions linked to mood regulation [16, 17] or indirectly with a poorly nourished women having reduced physical capacity to cope with daily life stressors [18].
While several studies have demonstrated an association between inadequate nutrient status and a greater risk for depression in the general adult population [16], few studies have examined the association between nutrient status during pregnancy and maternal depression. Among 1,277 pregnant Korean women, depression as measured on Goldberg’s depression scale was not associated with intakes of multivitamins [19]. This study was, however, questionnaire-based and did not include biochemical markers of nutritional status. A more recent study in 86 pregnant Japanese women found no association between depression in the first trimester as measured on Center for Epidemiologic Studies Depression (CES-D) scale and folate, vitamins B6 and B12 intakes or with serum folate and plasma homocysteine concentrations [20]. However, a small but significant positive correlation was observed between higher CES-D scores and hemoglobin concentration.
Few investigators have studied the role of nutrient status in the development of post-natal depression. Corwin et al. [21] reported that women who were anemic had significantly higher depressive symptoms at day 27 postpartum compared to non-anemic women. There was a negative correlation between depression and hemoglobin concentration in the postpartum period. In a later randomized, placebo controlled study the same group of investigators reported that iron supplementation from 10 weeks to 9 months postpartum resulted in 25 % improvement in depressive symptoms among anemic mothers [22]. In a study of 865 pregnant Japanese women, there were no associations between intakes of folate, pyridoxine and cobalamin measured through self-administered diet history questionnaire and depression measured at 2–9 months postpartum [23]. However, women with riboflavin intakes in the third quartile compared to first quartile had a lower risk of postpartum depression.
In summary, the findings from the few studies examining associations of nutrient status before and after pregnancy with maternal depression are varied, and some have methodological problems. In addition, small sample sizes and absence of biochemical markers of nutrient status in some of the studies precludes any firm conclusions. We are not aware of any studies that have evaluated the relationship between nutrient status during pregnancy and maternal depressive symptoms from a developing country where a majority of women during pregnancy have inadequate nutrient intakes [24, 25]. The objective of the present study was to assess the prevalence of antenatal depressive symptoms in early pregnancy and to examine the demographic and nutritional factors associated with these symptoms in a sample of urban South Indian pregnant women. We hypothesized that poorer nutritional status, as measured by anthropometric, dietary and biochemical assessment techniques, would be associated with a higher prevalence of depressive symptoms.
Methods
This cross-sectional study was part of a prospective randomized controlled trial (RCT) of vitamin B12 supplementation in urban pregnant south Indian women between the ages of 18 and 40 years (www.clinicaltrials.gov: NCT00641862) and is the baseline-cross-sectional data of the above mentioned RCT. Recruitment and follow-up took place at Hosahalli Hospital, a Government of Karnataka facility in Bangalore. This hospital predominantly caters to the needs of the pregnant women from the lower socioeconomic strata of urban Bangalore. Pregnant women were enrolled in early pregnancy (≤ 14 weeks gestation) from December 2008 until November 2010. The Institutional Review Board at St. John’s Medical College and Harvard School of Public Health approved all study procedures. Written informed consent was obtained from each study participant at enrollment.
Study Population
All pregnant women aged between 18 and 40 years, who were ≤14 weeks of gestation and registered for antenatal screening at the Hosahalli Hospital, were invited to participate in the study. Women with multiple gestation, those with a clinical diagnosis of chronic illness (diabetes mellitus, hypertension, heart disease or thyroid disease), those who tested positive for HepBSAg, HIV or syphilis, those who anticipated moving out of the city before delivery, those who were already consuming vitamin B12 supplements, or those who were treated for infertility were excluded.
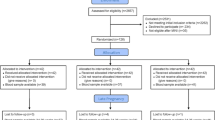
Of the 1,376 women contacted at the antenatal clinic during the study period, 958 women were excluded for the following reasons: 836 women planned to deliver outside Bangalore; 67 women wanted to terminate the pregnancy; four women were <18 years; seven women had a history of hypertension; four women with previous elective caesarean section were excluded as caesarean section was thought likely to interfere with full participation in the parent RCT and pregnancy was not confirmed among 40 women. Of the remaining 418 women, 366 women consented while 52 women declined to participate. One subject refused psychological assessment and therefore the data of 365 women are provided.
Measurements
Socio-Demographic and Anthropometric Information
Trained research assistants interviewed women in their 1st trimester to obtain socio-demographic information like age, education, occupation, income and parity. These data were collected using questionnaires developed for the study. Gestational age (in weeks) was calculated from the reported first day of the last menstrual period (LMP). Maternal body mass index was calculated as weight in kg divided by the square of height in meters (kg/m2).
Medical Morbidity
We captured maternal medical morbidity through a questionnaire adminstered at baseline and subsequently every trimester. This questionnaire had a list of 21 common medical symptoms related to respiratory, gastrointestinal, central nervous system and dermatological illnesses and the responses were recorded in a binary yes/no format. Any medical consultation that was sought for medical symptoms was noted.
Dietary Assessment
An interview-administered food frequency questionnaire (FFQ) was used to estimate the habitual dietary intake for the 3 months preceding the pregnant women’s entry into the study. The questionnaire was adapted from an earlier questionnaire developed for urban populations residing in South India [26]. We recently validated the FFQ for assessment of dietary habits in a population of south Indian pregnant women [27]. Nutrient intakes were computed by multiplying the relative frequency of consumption of each food item by nutrient content of the standard portion size. The questionnaire yielded nutrient information on 27 macro- and micro-nutrients. Energy-adjusted nutrient intakes were calculated by residual methods [28].
Biochemistry Assessments
Approximately 10 mL of blood was drawn from fasted subjects by venipuncture and collected in both ethylene diaminetetraacetate (EDTA) and plain vacutainers (BD Franklin Lakes, NJ, USA). Details of biochemical assessments have been previously described [29]. Briefly hemoglobin (Hb) and complete blood count were analyzed on whole blood samples in an automated Coulter counter (ABX Pentra C+, Horiba medicals, CA, US). Anemia was defined as Hb < 11.0 g/dL and severe anemia as Hb < 7.0 g/dL [30]. Microcytosis was defined as MCV < 80 fL [31] while microcytic anemia was defined as anemia with MCV < 80 fL. Vitamin B12 was measured using electrochemiluminescence (Elecsys 2010, Roche Diagnostics Mannheim, USA), while the measurement of homocysteine (Hcy) and methylmalonic acid (MMA) was performed by gas chromatography-mass spectrometry (GC–MS) method (Varian 3800, Palo Alto, CA, USA). The intra- and inter-day assay coefficients of variation (CV) for vitamin B12 were 0.54 and 2.44, respectively. The inter-day assay CV for MMA and Hcy was 5.57 and 5.04, respectively while the intra-day assay CV was 6.92 and 5.60, respectively. Erythrocyte folate was measured by a competitive immunoassay with direct chemiluminescence detection on an automatised immunoanalyser (ADVIA Centaurs, Bayer Health Care Diagnostics, Tarrytown, New York).
Assessment of Depressive Symptoms
Depressive symptoms were assessed on the Kessler Psychological Distress Scale (K-10). Developed for use in the US National Health Interview Survey (NHIS) to screen for depression, the K-10 consists of 10 items based on a 4-week recall period [32]. Each item has five response categories and is scored from 0 to 4 (‘0’ = ‘None of the time’, ‘1’ = ‘A little of the time’, ‘2’ = ‘Some of the time’, ‘3’ = ‘Most of the time’ and ‘4’ = ‘All of the time’) yielding a total sum of score (range 0–40). In an Indian study comparing five screening questionnaires to detect common mental disorders, the K-10 showed a high degree of correlation with other commonly used screening questionnaires in the detection of depression [33]. The K-10 was developed based on extensive psychometric analyses in large general population samples by using modern item response theory methods [32]. Its items are found to be independent of socio-cultural differences and lack meaningful gender and education biases [34]. The K-10 has been validated for screening for mental illness in the general population in the developing world. In addition, in a recent study in one hundred ninety-four women in their third trimester, we found that the K-10 compared well with the Edinburgh postnatal depression scale (EPDS) [35] in detecting depression [36]. A receiver-operating characteristic analyses showed K-10 to be good screening instrument for prenatal depression in rural South India at a cut-off of ≥6 (sensitivity = 100 %, specificity = 81.3 %, and area under the curve = 0.95) [36]. As part of this study we observed that while participants were easily able to comprehend all ten items on the K-10, some experienced difficulties with item 6 of the EPDS, “In the past 7 days, I feel like things have been getting on top of me”. Finally, K-10 is easier to administer and score as it has uniform response options for all the 10 questions unlike the EPDS, where items have different response options.
Statistical Analysis
The data were analyzed using SPSS for windows version 16. A descriptive analysis of the sample’s socio-demographic details, anthropometric characteristics, and hematological characteristics was undertaken. In the 1st trimester, subjects identified with antenatal depressive symptoms (K-10 > 6) were compared with those without depressive symptoms across various socio-demographic, hematological variables and medical morbidity using the χ2 test and independent sample t test. Nutrient intakes and biochemical markers of nutrient status were compared between those with and without depressive symptoms. In the case of nutrient intakes and food groups, we examined the association of tertiles of energy-adjusted nutrient intakes and food groups with maternal depressive symptoms using the χ2 test. We specifically examined the association between depressive symptoms and intakes of energy, protein, fat, carbohydrate, vitamin B12, folate, vitamin C and iron status derived from the food frequency questionnaire. The biomarkers of nutrient status included Hb, total blood count, microcytosis, red cell distribution width (RDW), vitamin B12, MMA, Hcy, and red cell folate. Log binomial regression analysis of depressive symptom status was carried out using the PROC GENMOD program in SAS software (version 9.2 SAS, Cary, NC) and prevalence ratios (PR) were calculated. All maternal characterisitcs with p < 0.20 in the univariate analysis were considered in the multivariate model. Statistical significance in the multivariate model was considered at p < 0.05.
Results
The mean (SD) age of the 365 pregnant women was 22.6 (3.4) years and the mean (SD) gestational age at the time of randomization in the study was 11.2 (2.4) weeks. Of the women 26 % had less than high school education. The median (interquartile range) monthly household income was USD 120 (90). The mean (SD) BMI of the sample was 20.4 (3.3) kg/m2. A majority of the women (83.8 %) were unemployed. Primiparous women made up majority of the cohort. The mean weight and height of the women were 47.8 ± 8.1 kg and 153.0 ± 5.6 cm, respectively. About half of the women in the study cohort (51.4 %) had a BMI < 19.8 and 31.3 % of the women had a BMI < 18.5.
The mean (SD) K-10 score of the cohort was 5.17 (5.46). K-10 scores were >6 during the first trimester for 121 (33 %) women, indicating the presence of antenatal depressive symptoms. Sociodemographic and anthropometric characteristics were comparable between the two groups. Among the medical symptoms, nausea and vomiting were highly prevalent and present in 128 (35.0 %) and 210 (57.7 %) participants, respectively. The prevalence of depressive symptoms was significantly higher among 128 participants who had nausea (42.4 vs. 28.3 %, p = 0.010) and 210 with vomiting (40.5 vs. 23.2 %, p = 0.001) (Table 1). The occurrence of other medical symptoms was very low and presence of depressive symptoms was not significantly different between the subjects who had and did not have other medical symptoms.
The median dietary intake of energy, protein, iron, calcium and folate among pregnant women was lower in comparison to the Indian recommended dietary allowances (RDA) for this group [37]. The median (25th, 75th percentile) dietary intake of vitamin B12 was 1.25 (0.86, 1.96) μg/day. The dietary intakes were comparable between the group with depressive symptoms and the group without depressive symptoms (Table 2). None of the women were consuming iron or folate supplements on study entry.
At baseline the mean (SD) Hb was 11.5 (1.5) g/dL and the prevalence of anemia (Hb < 11.0 g/dL) was 30.4 % (111/365) (Table 3). About one-third of the sample had microcytosis [mean corpuscular volume (MCV) < 80 fL] and 20.2 % of the women had microcytic anemia. About half (51.1 %) of the pregnant women had low plasma vitamin B12 concentrations (<150 pmol/L). MMA levels >0.26 μmol/L was observed in 75.8 % of the women. About half (43.3 %) of the pregnant women had Hcy levels >10.0 μmol/L. Nearly a quarter (22.2 %) of the pregnant women in our cohort had erythrocyte folate concentrations <283 nmol/L.
There were no significant differences between women with and without depressive symptoms among any of the nutritional biomarkers, with the exception of anemia. The prevalence of depressive symptoms was lower among pregnant women who were anemic compared to non-anemic participants (24 vs. 37 %, p = 0.021). The prevalence of depressive symptoms was lower among women with microcytosis compared to women without microcytosis. In addition, the prevalence of depressive symptoms was higher among women without microcytic anemia compared to those with microcytic anemia (37 vs. 22 %, p = 0.018). There was no significant association between deficiencies of vitamin B12 or folate with the occurrence of antenatal depressive symptoms (Table 3).
Prevalence ratios (PR) were computed from a multivariate log binomial regression model to identify factors associated with antenatal depressive symptoms in the first trimester (Table 4). Women that had vomiting had a higher prevalence ratio of being depressed (PR = 1.54; 95 % CI 1.10, 2.16) compared to women without vomiting after adjusting for education. In addition, women with anemia (PR = 0.67; 95 % CI 0.47, 0.96) had lower prevalence ratio of being depressed compared to women without anemia.
Discussion
In this cross-sectional analysis of 365 pregnant urban South Indian women, we found a high prevalence of depressive symptoms (33 %) in the first trimester of pregnancy. Vomiting during the first trimester was independently associated with depressive symptoms. Micronutrient and macronutrient intakes and demographic characteristics were not associated with antenatal depressive symptoms. Among the various biomarkers of nutritional status, only anemia was linked to antenatal depression, with depressive symptoms being less common among anemic women compared to non-anemic subjects. Serum vitamin B12, MMA, Hcy and red cell folate levels were not associated with the presence of antenatal depressive symptoms.
Studies from South East Asia have reported a high prevalence of depressive symptoms during pregnancy [38]. However, in most of the studies the assessment of depressive symptoms occurred in the third trimester. In a hospital-based study in 213 Pakistani women studied in the third trimester, 91 (42.7 %) subjects had depression as measured by the EPDS [10]. In a community-based study from Bangladesh, of the 361 pregnant women assessed on EPDS at 34–35 weeks of pregnancy, 33 % of the women had depression [12]. Our findings of a 33 % prevalence rate of depressive symptoms among Indian women are consistent with these studies. However, comparison with other studies is difficult since longitudinal studies have indicated that the prevalence of depression rates tend to decrease at later time points in pregnancy [39, 40]. High prevalence rates of depressive symptoms noted in the present study poses considerable burden on pregnant women. Previous studies have shown that sub-clinical depression is associated with considerable disability and impairment [41, 42].
Few studies have examined the prevalence of depression in the first trimester. A recent study from Japan reported a high prevalence (62 %) of depression measured on the CES-D scale in early pregnancy (6–11 weeks of gestation) but the sample size was modest (n = 86) [20]. In addition, comparison of prevalence figures across studies is rendered difficult, as some have assessed for the presence of depressive disorder, [39] while others have reported prevalence rates based on depressive and anxiety symptoms taken together [40]. Nevertheless the high prevalence of depressive symptoms noted in the present study (33 %) is in agreement with several other reports from low and middle income countries [43, 44], and is considerably higher than estimates of 7.4–12.8 % found in high income countries [45].
The high prevalence rate noted in the present study could also be a function of the screening instrument used (K-10). There is considerable overlap between symptoms of depression such as sleep disturbance, increased fatigue and changes in appetite, and physiologic changes associated with pregnancy [46]. However, in a previous study of depression in pregnant women we showed that K-10 compared well with EPDS, a scale that largely avoids biological symptoms, and thus is a valid measure of antenatal depressive symptoms [36].
Among the socio-demographic factors studied, we were unable to demonstrate any association between depressive symptoms and demographic characteristics. While some studies have reported an association between antenatal depression and various demographic characteristics [47–50] others have failed to demonstrate any association [51, 52]. In general the prevalence of prenatal depression has been reported to be high among pregnant mothers from low income countries [6–9]. However, this association may be linked to other environmental factors such as greater degree of life stressors, spousal drinking, intimate partner violence, poor marital relationship and lack of spousal and social support [38].
Our results indicate that vomiting is associated with depressive symptoms in early pregnancy. This finding is in agreement with studies of depression in early pregnancy that reported severity of emesis as one of the risk factors associated with prenatal depression [53, 54]. In a study that examined psychosocial correlates of vomiting and nausea in 113 pregnant women in their first trimester, depressive symptoms measured on CES-D scale had the highest correlation with nausea and vomiting [55]. Nausea and vomiting are common during the first trimester of pregnancy and may affect a pregnant women’s ability to consume and retain food and hydration [56]. Based on the findings from the present cross-sectional study, it is not possible to conclude a causal relationship between depressive symptoms and vomiting. However, this association is important since persistent vomiting affects a pregnant woman’s nutrient intakes in a population whose mean intakes of several macro- and micronutrients is already well below the RDA for pregnant women. This has important potential implications for fetal growth and birth outcomes. Sub-optimal maternal nutritional status during the first trimester of pregnancy increases the risk of low birth weight regardless of maternal nutritional status in the later stages of pregnancy [57, 58] .
In the present study, we did not find any association between energy adjusted micro- and macronutrient intakes and maternal depressive symptoms. In addition, there were no associations between maternal depressive symptoms in early pregnancy and various biochemical markers including serum vitamin B12, Hcy, MMA and red blood cell folate concentrations. While clinic and community based studies have reported associations between folate and vitamin B12 and depression in adults [59, 60], in a study of pregnant women in early pregnancy there were no differences in the levels of serum folate and Hcy concentrations between depressed and non-depressed subjects [20]. However, there was a modest but significant positive correlation between depression scores on CES-D and hemoglobin concentrations (r = 0.22, p < 0.05). In a recent study in 704 pregnant Chinese women, there were no correlations between various maternal iron measures including anemia in mid- or late pregnancy and post partum depression measured soon after birth and at 6 weeks [61]. The only significant correlation in this study of pregnant women was between a higher soluble transferrin receptor (sTfR) indicative of worse iron status and lower depressive symptoms. We report similar findings in our cohort of pregnant women, with depressive symptom being more prevalent among non-anemic women compared to anemic women, and fewer depressive symptoms among women with microcytic anemia. While we do not have a clear explanation, there could be several possible reasons for this finding. The first relates to the timing of assessment of depressive symptoms. Majority of the earlier studies on the association between anemia and depression have occurred in the post-partum period [21, 22, 61] and in non-pregnant women [62]. Secondly, in the present study we have relied only on hemoglobin levels to define anemia while others have used several measures of iron status [22, 61, 63]. Finally, we estimated depressive symptoms using a screening tool and have not assessed for the presence of clinical depression. Thus, the relationship between anemia and iron status, and maternal depression merits further study.
Strengths and Limitations
To our knowledge, this is the first study in India to examine the prevalence and correlates of depressed mood in early pregnancy. This study adds to the small but expanding literature on prenatal depression in early pregnancy, a period critical for fetal growth and development. This study has certain limitations. We did not assess the severity of medical symptoms though we found vomiting as positively associated with depressive symptoms. We did not evaluate past and family history of depression identified in earlier studies as risk factors for antenatal depression [64, 65]. In addition, this enquiry on prenatal depression was in the context of an ongoing nutrient intervention trial and some of the traditional psychosocial risk factors such as spousal support, social support, life stressors, intimate partner violence and pregnancy specific stressors linked to prenatal depression [66–70] were not assessed. However, we are currently following up the mother–child dyad through 3 years of age with periodic assessments of maternal depression including assessment of various psychosocial risk factors and nutritional status and their impact on birth outcomes and child development.
Conclusion
We found a high prevalence of antenatal depressive symptoms in early pregnancy among urban Indian women from a low socio-economic background. Vomiting was associated with antenatal depressive symptoms. Antenatal depressive symptoms were less prevalent among anemic women. In addition, our results suggest that dietary intake of folate and vitamin B12 were not associated with depressive symptoms in early pregnancy, nor were blood concentrations associated with depressive symptoms. However, longitudinal assessment of depression and nutrient status with evaluation at multiple time points through pregnancy and postpartum period is essential since both the prevalence of depression and nutritional status changes through the course of pregnancy [16]. Finally, this study highlights the need for systematic screening for depression during prenatal visits as pregnancy is a time when most women are likely to come into contact with health professionals and depressive symptoms during pregnancy is an independent risk factor for later development of clinical depression [38, 71, 72].
References
Chandra, P. S. (2009). The interface between reproductive health and psychiatry. Contemporary topics in womens mental health-global perspectives (pp. 189–196). UK: Wiley.
Karmaliani, R., Asad, N., Bann, C. M., et al. (2009). Prevalence of anxiety, depression and associated factors among pregnant women of Hyderabad, Pakistan. International Journal of Social Psychiatry, 55(5), 414–424.
Bowen, A., & Muhajarine, N. (2006). Prevalence of antenatal depression in women enrolled in an outreach program in Canada. Journal of Obstetric Gynecologic and Neonatal Nursing, 35(4), 491–498.
van Bussel, J. C., Spitz, B., & Demyttenaere, K. (2006). Women’s mental health before, during, and after pregnancy: A population-based controlled cohort study. Birth, 33(4), 297–302.
Bowen, A., Stewart, N., Baetz, M., et al. (2009). Antenatal depression in socially high-risk women in Canada. Journal of Epidemiology and Community Health, 63(5), 414–416.
Limlomwongse, N., & Liabsuetrakul, T. (2006). Cohort study of depressive moods in Thai women during late pregnancy and 6–8 weeks of postpartum using the Edinburgh Postnatal Depression Scale (EPDS). Archives of Women’s Mental Health, 9(3), 131–138. doi:10.1007/s00737-005-0115-7.
Patel, V., & Prince, M. (2006). Maternal psychological morbidity and low birth weight in India. The British Journal of Psychiatry, 188, 284–285. doi:10.1192/bjp.bp.105.012096.
Rahman, A., & Creed, F. (2007). Outcome of prenatal depression and risk factors associated with persistence in the first postnatal year: Prospective study from Rawalpindi, Pakistan. Journal of Affective Disorders, 100(1–3), 115–121. doi:10.1016/j.jad.2006.10.004.
Kheirabadi, G. R., Maracy, M. R., Barekatain, M., et al. (2009). Risk factors of postpartum depression in rural areas of Isfahan province Iran. Archives of Iranian medicine, 12(5), 461–467.
Imran, N., & Haider, I. I. (2009). Screening of antenatal depression in Pakistan: Risk factors and effects on obstetric and neonatal outcomes. Asia-Pacific Psychiatry, 2(1), 26–32.
Kazi, A., Fatmi, Z., Hatcher, J., et al. (2006). Social environment and depression among pregnant women in urban areas of Pakistan: Importance of social relations. Social Science and Medicine, 63(6), 1466–1476.
Gausia, K., Fisher, C., Ali, M., et al. (2009). Antenatal depression and suicidal ideation among rural Bangladeshi women: A community-based study. Archives of Women’s Mental Health, 12(5), 351–358.
Chandran, M., Tharyan, P., Muliyil, J., et al. (2002). Post-partum depression in a cohort of women from a rural area of Tamil Nadu, India Incidence and risk factors. The British Journal of Psychiatry, 181(6), 499–504.
Savarimuthu, R., Ezhilarasu, P., Charles, H., et al. (2010). Post-partum depression in the community: A qualitative study from rural south India. International Journal of Social Psychiatry, 56(1), 94–102.
Wachs, T. D., Black, M. M., & Engle, P. L. (2009). Maternal depression: A Global Threat to Children’s Health, Development, and Behavior and to Human Rights. Child Development Perspectives, 3(1), 51–59.
Bodnar, L. M., & Wisner, K. L. (2005). Nutrition and depression: Implications for improving mental health among childbearing-aged women. Biological Psychiatry, 58(9), 679–685.
Leung, B. M., & Kaplan, B. J. (2009). Perinatal depression: Prevalence, risks, and the nutrition link–a review of the literature. Journal of the American Dietetic Association, 109(9), 1566–1575.
Paulson, J. F., Dauber, S., & Leiferman, J. A. (2006). Individual and combined effects of postpartum depression in mothers and fathers on parenting behavior. Pediatrics, 118(2), 659–668. doi:10.1542/peds.2005-2948.
Cho, Y. J., Han, J. Y., Choi, J. S., et al. (2008). Prenatal multivitamins containing folic acid do not decrease prevalence of depression among pregnant women. Journal of Obstetrics and Gynaecology, 28(5), 482–484.
Watanabe, H., Suganuma, N., Hayashi, A., et al. (2010). No relation between folate and homocysteine levels and depression in early pregnant women. Bioscience Trends, 4(6), 344–350.
Corwin, E. J., Murray-Kolb, L. E., & Beard, J. L. (2003). Low hemoglobin level is a risk factor for postpartum depression. The Journal of Nutrition, 133(12), 4139–4142.
Beard, J. L., Hendricks, M. K., Perez, E. M., et al. (2005). Maternal iron deficiency anemia affects postpartum emotions and cognition. The Journal of Nutrition, 135(2), 267–272.
Miyake, Y., Sasaki, S., Tanaka, K., et al. (2006). Dietary folate and vitamins B12, B6, and B2 intake and the risk of postpartum depression in Japan: The Osaka Maternal and Child Health Study. Journal of Affective Disorders, 96(1–2), 133–138.
Yajnik, C. S., Deshpande, S. S., Jackson, A. A., et al. (2008). Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: The Pune Maternal Nutrition Study. Diabetologia, 51(1), 29–38. doi:10.1007/s00125-007-0793-y.
Toteja, G. S., Singh, P., Dhillon, B. S., et al. (2006). Prevalence of anemia among pregnant women and adolescent girls in 16 districts of India. Food and Nutrition Bulletin, 27(4), 311–315.
Bharathi, A. V., Kurpad, A. V., Thomas, T., et al. (2008). Development of food frequency questionnaires and a nutrient database for the Prospective Urban and Rural Epidemiological (PURE) pilot study in South India: Methodological issues. Asia Pacific Journal of Clinical Nutrition, 17(1), 178–185.
Dwarkanath, P., Soares, M. J., Thomas, T., et al. (2012). Food frequency questionnaire is a valid tool for the assessment of dietary habits of South Indian pregnant women. Asia-Pacific Journal of Public Health/Asia-Pacific Academic Consortium for Public Health,. doi:10.1177/1010539512459945.
Willett, W. C. (1998). Nutritional epidemiology (2nd ed.). New York: Oxford University Press.
Samuel, T. M., Thomas, T., Finkelstein, J., et al. (2012). Correlates of anaemia in pregnant urban South Indian women: A possible role of dietary intake of nutrients that inhibit iron absorption. Public Health Nutrition, 1(1), 1–9.
WHO/CDC. (2004). Joint report by World Health Organization/Centres for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level (2nd ed.). Geneva, Switzerland: World Health Organization.
Rempher, K. J., & Little, J. (2004). Assessment of red blood cell and coagulation laboratory data. AACN Clinical Issues, 15(4), 622–637. quiz 44-5.
Kessler, R. C., Barker, P. R., Colpe, L. J., et al. (2003). Screening for serious mental illness in the general population. Archives of General Psychiatry, 60(2), 184–189.
Patel, V., Araya, R., Chowdhary, N., et al. (2008). Detecting common mental disorders in primary care in India: A comparison of five screening questionnaires. Psychological Medicine, 38(2), 221–228. doi:10.1017/S0033291707002334.
Baillie, A. J. (2005). Predictive gender and education bias in Kessler’s psychological distress scale (k-10). Social Psychiatry and Psychiatric Epidemiology, 40(9), 743–748. doi:10.1007/s00127-005-0935-9.
Cox, J. L., Holden, J. M., & Sagovsky, R. (1987). Detection of postnatal depression. Development of the 10-item Edinburgh postnatal depression scale. The British Journal of Psychiatry : The Journal of Mental Science, 150, 782–786.
Fernandes, M. C., Srinivasan, K., Stein, A. L., et al. (2011). Assessing prenatal depression in the rural developing world: A comparison of two screening measures. Archives of Women’s Mental Health, 14(3), 209–216. doi:10.1007/s00737-010-0190-2.
Indian Council of Medical Research. (2010). Nutrient requirements and recommended dietary allowances for Indians. A report of the Expert Group of the ICMR, National Institute of Nutrition, Hyderabad, India.
Satyanarayana, V. A., Lukose, A., & Srinivasan, K. (2011). Maternal mental health in pregnancy and child behavior. Indian J Psychiatry, 53(4), 351–361.
Bunevicius, R., Kusminskas, L., Bunevicius, A., et al. (2009). Psychosocial risk factors for depression during pregnancy. Acta Obstetricia et Gynecologica Scandinavica, 88(5), 599–605.
Lee, A. M., Lam, S. K., Sze Mun Lau, S. M., et al. (2007). Prevalence, course, and risk factors for antenatal anxiety and depression. Obstetrics and Gynecology, 110(5), 1102–1112.
Karatas, J. C., Matthey, S., & Barnett, B. (2009). Antenatal psychosocial assessment: How accurate are we in determining ‘low-risk’ status? A pilot study. Archives of Women’s Mental Health, 12(2), 97–103.
Mariam, K. A., & Srinivasan, K. (2009). Antenatal psychological distress and postnatal depression: A prospective study from an urban clinic. Asian Journal of Psychiatry, 2(2), 71–73. doi:10.1016/j.ajp.2009.04.002.
Rahman, A., Iqbal, Z., & Harrington, R. (2003). Life events, social support and depression in childbirth: Perspectives from a rural community in the developing world. Psychological Medicine, 33(7), 1161–1167.
Hartley, M., Tomlinson, M., Greco, E., et al. (2011). Depressed mood in pregnancy: Prevalence and correlates in two cape town peri-urban settlements. Reprod Health, 8, 9.
Bennett, H. A., Einarson, A., Taddio, A., et al. (2004). Prevalence of depression during pregnancy: Systematic review. Obstetrics and Gynecology, 103(4), 698–709. doi:10.1097/01.AOG.0000116689.75396.5f.
Cott, A. D., & Wisner, K. L. (2003). Psychiatric disorders during pregnancy. International Review of Psychiatry, 15(3), 217–230. doi:10.1080/0954026031000136848.
Irfan, N., & Badar, A. (2003). Determinants and pattern of postpartum psychological disorders in Hazara division of Pakistan. Journal of Ayub Medical College Abbottabad, 15(3), 19–23.
Gotlib, I. H., Whiffen, V. E., Mount, J. H., et al. (1989). Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. Journal of Consulting and Clinical Psychology, 57(2), 269–274.
Husain, N., Parveen, A., Husain, M., et al. (2011). Prevalence and psychosocial correlates of perinatal depression: A cohort study from urban Pakistan. Archives of Women’s Mental Health, 14(5), 395–403. doi:10.1007/s00737-011-0233-3.
Klainin, P., & Arthur, D. G. (2009). Postpartum depression in Asian cultures: A literature review. International Journal of Nursing Studies, 46(10), 1355–1373.
Da Costa, D., Larouche, J., Dritsa, M., et al. (2000). Psychosocial correlates of prepartum and postpartum depressed mood. Journal of Affective Disorders, 59(1), 31–40.
Alami, K. M., Kadri, N., & Berrada, S. (2006). Prevalence and psychosocial correlates of depressed mood during pregnancy and after childbirth in a Moroccan sample. Archives of Women’s Mental Health, 9(6), 343–346.
Kitamura, T., Sugawara, M., Sugawara, K., et al. (1996). Psychosocial study of depression in early pregnancy. The British Journal of Psychiatry, 168(6), 732–738.
Reeves, N., Potempa, K., & Gallo, A. (1991). Fatigue in early pregnancy. An exploratory study. Journal of Nurse-midwifery, 36(5), 303–309.
Chou, F. H., Lin, L. L., Cooney, A. T., et al. (2003). Psychosocial factors related to nausea, vomiting, and fatigue in early pregnancy. Journal of Nursing Scholarship, 35(2), 119–125.
Tuffery, O., & Scriven, A. (2005). Factors influencing antenatal and postnatal diets of primigravid women. J R Soc Promot Health, 125(5), 227–231.
Smith, G. C., Smith, M. F., McNay, M. B., et al. (1998). First-trimester growth and the risk of low birth weight. The New England Journal of Medicine, 339(25), 1817–1822. doi:10.1056/NEJM199812173392504.
Smith, G. C. (2004). First trimester origins of fetal growth impairment. Seminars in Perinatology, 28(1), 41–50.
Alpert, J. E., & Fava, M. (1997). Nutrition and depression: The role of folate. Nutrition Reviews, 55(5), 145–149.
Tiemeier, H., van Tuijl, H. R., Hofman, A., et al. (2002). Vitamin B12, folate, and homocysteine in depression: The Rotterdam Study. American Journal of Psychiatry, 159(12), 2099–2101.
Armony-Sivan, R., Shao, J., Li, M., et al. (2012). No relationship between maternal iron status and postpartum depression in two samples in China. Journal of Pregnancy, 2012, 7. doi:10.1155/2012/521431.
Hunt, J. R., & Penland, J. G. (1999). Iron status and depression in premenopausal women: An MMPI study. Minnesota multiphasic personality inventory. Behavioral Medicine, 25(2), 62–68. doi:10.1080/08964289909595738.
Albacar, G., Sans, T., Martin-Santos, R., et al. (2011). An association between plasma ferritin concentrations measured 48 h after delivery and postpartum depression. Journal of Affective Disorders, 131(1–3), 136–142. doi:10.1016/j.jad.2010.11.006.
Rich-Edwards, J. W., Kleinman, K., Abrams, A., et al. (2006). Sociodemographic predictors of antenatal and postpartum depressive symptoms among women in a medical group practice. Journal of Epidemiology and Community Health, 60(3), 221–227.
Bloch, M., Rotenberg, N., Koren, D., et al. (2006). Risk factors for early postpartum depressive symptoms. General Hospital Psychiatry, 28(1), 3–8.
Kataoka, Y., Yukari, Y., Hiromi, E., et al. (2005). Domestic Violence against women during pregnancy. Japanese Journal of Public Health, 52(9), 785–795.
Woods, S. M., Melville, J. L., Guo, Y., et al. (2010). Psychosocial stress during pregnancy. American Journal of Obstetrics and Gynecology, 202(61), 1–7.
Rubertsson, C., Wickberg, B., Gustavsson, P., et al. (2005). Depressive symptoms in early pregnancy, 2 months and 1 year postpartum-prevalence and psychosocial risk factors in a national Swedish sample. Archives of Women’s Mental Health, 8(2), 97–104.
Bilszta, J. L., Tang, M., Meyer, D., et al. (2008). Single motherhood versus poor partner relationship: Outcomes for antenatal mental health. Australian and New Zealand Journal of Psychiatry, 42(1), 56–65.
Adewuya, A. O., Ola, B. A., Aloba, O. O., et al. (2007). Prevalence and correlates of depression in late pregnancy among Nigerian women. Depress Anxiety, 24(1), 15–21.
Davey, H. L., Tough, S. C., Adair, C. E., et al. (2011). Risk factors for sub-clinical and major postpartum depression among a community cohort of canadian women. Maternal and Child Health Journal, 15(7), 866–875.
Williams, D. (2003). Pregnancy: A stress test for life. Current Opinion in Obstetrics and Gynecology, 15(6), 465–471.
Acknowledgments
This data was presented in part at the Experimental Biology meeting 2011. We thank the women for participating in this study. We are grateful to Dr. B Nirmala in helping us obtain all the clearances to conduct the study at Hosahalli Referral Hospital. We thank the doctors, nurses and the technical staff at the hospital for their support. We acknowledge Dr. S Muthayya for her assistance during the initial part of the study. We thank Ms. P.Vijaya, Ms.S.Surekha, Ms. S.Sridevi, Ms.K.R.Shilpa, Ms.C.Darshini, Ms.P.K.Preethi and Ms.R.Poornima for technical support. This research was financially supported by the Indian Council of Medical Research, India (ICMR: 5/7/192/06-RHN) and National Institute of Health, USA (NICHD: HD052143and NICHD K24 HD058795).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lukose, A., Ramthal, A., Thomas, T. et al. Nutritional Factors Associated with Antenatal Depressive Symptoms in the Early Stage of Pregnancy Among Urban South Indian Women. Matern Child Health J 18, 161–170 (2014). https://doi.org/10.1007/s10995-013-1249-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10995-013-1249-2