Abstract
The objective of this study was to explore pregnant and recently pregnant women’s perceptions of influenza vaccine and antivirals during the 2009 H1N1 pandemic. We conducted 18 focus groups with pregnant and recently pregnant women in three US cities in September 2009. Participants were segmented into groups by insurance status (no or public insurance vs. private insurance), vaccine attitudes (higher vs. lower likelihood of acceptance of any vaccines, not only influenza vaccines), and parity (first child vs. other children in the home) based on information they provided on the screening questionnaire at the time of recruitment. We found that women are not well informed about influenza vaccinations and antiviral medicine and have significant concerns about taking them during pregnancy. An interest in their infant’s well-being, however, can be strong motivation to adopt preventive recommendations, including vaccination. A woman’s health care provider is a highly trusted source of information about the 2009 H1N1. Pregnant women have unique communication needs for influenza. Messages directing pregnant women to adopt public health recommendations, particularly for vaccination or prophylactic medication should include a detailed description of the benefits or lack of risk to the fetus and the safety of breastfeeding. Additionally, messages should recognize that pregnant women are taught to be selective about taking medication and provide a clear rationale for why the medicine or vaccine is necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple factors contribute to an increased risk of morbidity and mortality from influenza among pregnant women [1–4]. These factors include changes to physiology and anatomy, the immune system, and the respiratory and cardiovascular systems during pregnancy [2, 5, 6]. In addition, fever, a common symptom of influenza, is associated with an increased risk for miscarriages, stillbirths, and neural tube defects [3].
The increased risk for severe illness in pregnant women has been reported during past influenza pandemics [6, 7] and was again evident in the pandemic (H1N1) 2009 virus. During the first wave of the outbreak, an estimated 5% of deaths were among pregnant women [8], and hospitalization rates were estimated to be 4 times higher for pregnant women than for the general population [9]. Because of the increased risk of severe influenza, the Centers for Disease Control and Prevention (CDC) recommends that all pregnant women receive an annual influenza vaccine, and during the recent 2009 HINI influenza outbreak, pregnant women were identified as a high-priority group for vaccination [10].
Despite being at increased risk for complications from influenza, pregnant women have low seasonal influenza vaccination rates, ranging from 15 to 25% [10]. Studies examining determinants of influenza vaccination provide insights related to reasons for these low influenza coverage rates, including lack of awareness about influenza prevention guidelines during pregnancy [11] and the belief that the vaccine itself might cause influenza symptoms [12]. In addition, worldwide increases in autism rates have fueled parental concerns about the safety of childhood vaccines [13, 14]. However, it is unclear whether these concerns influence influenza vaccination during pregnancy.
Pregnant women’s increased risk for serious complications because of influenza, and their historically low influenza vaccination rates, called for additional formative research regarding influenza risk perceptions and likelihood of adopting recommended preventive behaviors. This paper presents findings from exploratory research among pregnant and recently pregnant women regarding their perceptions about the 2009 H1N1 and seasonal influenza vaccinations. The paper further identifies needed information intended to improve communication strategies to encourage the 2009 H1N1 and seasonal influenza vaccinations and potentially future influenza pandemic vaccines.
Theoretical Framework
Because little is known about pregnant women’s views regarding the 2009 H1N1 pandemic, the Protection Motivation Theory (PMT) [15] served as a guide to this research. The PMT posits that the motivation to adopt certain protective behaviors is a function of an individual’s threat appraisal, which is a person’s perceived vulnerability to and assessment of a threat, and his or her coping appraisal. Coping appraisal involves the confidence in being able to carry out the protective recommendation (self-efficacy); his or her belief that these behaviors will be effective protection from the threat (response efficacy); and the perceived benefits of and barriers to the recommendation. Constructs from this theory led to the following three research questions, which are addressed in this paper:
-
Threat appraisal: what are pregnant women’s perceptions regarding their vulnerability to and severity of 2009 H1N1 and seasonal influenza, and how do these perceptions influence preventive behaviors?
-
Coping appraisal: what are the perceived benefits and costs (barriers) to adopting key influenza recommendations?
-
Get the 2009 H1N1 vaccination.
-
Take antiviral medicines if your doctor recommends them.
-
-
Trusted sources: what are the trusted sources of health information for pregnant women?
Methods
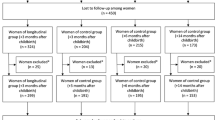
In September 2009, 18 focus groups were conducted with pregnant or recently pregnant (within 6 months postpartum) women in Atlanta, Georgia; Dallas, Texas; and Portland, Oregon. Focus groups were selected as the method of data collection since they can provide formative insights into subjects or topics about which little is known [16]. Data from focus group testing are not intended to be generalized but rather to provide guidance for additional research and to inform theory and practice.
Participants were recruited by a professional recruitment firm and community partners in each city and provided with a $70 incentive. Participants were segmented into groups by insurance status (no or public insurance vs. private insurance), vaccine attitudes (higher vs. lower likelihood of acceptance of any vaccines, not only influenza vaccines), and parity (first child vs. other children in the home) based on information they provided on the screening questionnaire at the time of recruitment. These segmentation variables were selected because in the theoretical framework, these factors influence threat appraisal and coping appraisal. To obtain a diverse representation of participants regarding two of these segmentation variables (insurance status and attitudes on vaccine) and to consider potential for exposure to 2009 H1N1 based on regional influenza activity, the focus groups were conducted in 3 different cities.
Data Collection
Prior to beginning the focus group, participants completed an informed consent form and an intake questionnaire that captured their demographic characteristics and likelihood of adopting key influenza prevention behaviors. A trained moderator led the discussion using a guide containing semi-structured questions and probes that covered perceptions and awareness of 2009 H1N1, influenza vaccinations and antiviral medicines, and trusted sources of information. In addition, participants were shown video clips of news broadcasts that focused on the April 2009 H1N1 outbreak to further elicit discussion. The focus groups lasted approximately 90 min, and were audio recorded and later professionally transcribed. Note takers attended the groups and recorded key responses and group dynamics. All data collection was approved by the institutional review board of Danya International.
Data Analysis
Focus group transcripts were coded and analyzed using a coding structure that reflected the conceptual framework and segmentation scheme (i.e., site, parity, insurance, and vaccine attitudes). Established procedures were followed to ensure the reliability and validity of qualitative analysis [17]. Initially, the first two transcripts were double coded by separate analysts to assess the coding structure reliability. Then, after one third of the transcripts were coded, the analytic team convened to review non-concurrences and multiple coding of similar text and refine the coding structure to mitigate these issues. The software used for this analysis was NVivo 8 software (QSR International, Doncaster, Victoria, Australia).
Limitations
The timeframe of the analysis did not allow for the entire set of transcripts to be double coded so a thorough reliability assessment could not be carried out. Also, the complex segmentation scheme across 18 groups limited the degree of data saturation on all key findings although similarities in responses were evident across groups.
Results
Findings presented in this paper reflect pregnant women’s 2009 H1N1 threat appraisal, coping appraisal, and trusted sources of influenza information. Results were also noted where key differences were identified by segmentation variables.
A total of 144 pregnant and recently pregnant women participated in 18 focus groups. As shown in Table 1, more than half of the participants (56%) were postpartum at the time of the focus groups, and 44% were currently pregnant. The majority of participants (62%) were aged 25–34 years; 26% were aged 18–24 years, and 12% were aged 35–44 years. Participants were mostly white (65.5%), followed by 28.1% black. Nine percent of participants described themselves as Hispanic. Dallas had the highest percentage of participants of Hispanic background (10.9%).
Approximately 60% of the participants were placed in the higher vaccine acceptance focus groups based on perceptions of vaccine safety and their intention to receive a seasonal influenza vaccine while pregnant as determined by their responses to the intake questionnaire. Fifty three percent of participants had private insurance, and approximately 57% of participants had one child or more living in the home, excluding the current pregnancy or newborn.
H1N1 Threat Appraisal
Perceived Severity of 2009 H1N1
Most participants reported having limited understanding of the potential severity of the H1NI subtype infection during pregnancy and were confused about the differences between seasonal influenza and the 2009 H1N1. A primary source of confusion was the lack of consistent messaging regarding 2009 H1N1, particularly from the media. Participants heard two conflicting messages: (a) that 2009 H1N1 should be taken quite seriously, but (b) that 2009 H1N1 is no more severe than seasonal influenza. The latter view was reinforced by the fact that recommendations for seasonal influenza and the 2009 H1N1 were the same (e.g., hand washing, cough etiquette). As a result, most participants indicated that they would approach the 2009 H1N1 as they do seasonal influenza and without much extra concern. As one participant said, “It’s not necessarily worse. It’s just a different trend. If you take care of it the way you would take care of the other flu, then you should be okay.” Some participants, particularly those in the lower vaccine acceptance groups and the no or public insurance groups, blamed the media for generating “mass hysteria” and doubted the outbreak was as severe as reported.
Perceived Susceptibility to the 2009 H1N1
Participants expressed wide variability in their perceived susceptibility to the 2009 H1N1 on the intake questionnaire (Table 2). Only 17.3%Footnote 1 were extremely worried and one quarter of participants (25.2%) were not worried at all. Concern was the highest in Atlanta, where 2009 H1N1 was most active (29.3% were extremely worried), compared with Dallas (15.6%) and Portland (9.4%), where the outbreak had not yet peaked (see Fig. 1).
In focus group discussions, some participants were aware that pregnant women are at higher risk for complications from 2009 H1N1 infection, and several stated that their pregnancy was the primary reason they were concerned about the 2009 H1N1. However, some participants believed that pregnant women have stronger immune systems because they are more careful with their health (e.g., take prenatal vitamins, eat well, and exercise) and are, therefore, less vulnerable to the 2009 H1N1. As one participant stated, “Your immune system [when pregnant] is definitely stronger because I was able to fight the flu.”
Notably, although many participants initially did not perceive the 2009 H1N1 to be either severe or personally threatening, their views shifted during group discussions and exposure to the news media clips. Discussions about the severity of 2009 H1N1 infection during pregnancy raised the participants’ level of concern for being infected with the virus.
Coping Appraisal: Reactions to 2009 H1N1 Prevention Recommendations
Influenza Vaccination
The majority of participants in both the lower and higher vaccine acceptance groups expressed uncertainty about whether they should get the 2009 H1N1 vaccine while pregnant. On the exit questionnaire, fewer than half of the participants (48.5%) reported being likely to get a 2009 H1N1 vaccine (Table 3). According to one participant, “I don’t think I can get it [2009 H1N1 vaccine], especially while pregnant. I just don’t know what the side effects are. It’s a risk I’m just not willing to take.”
Predictably, the participants in the higher vaccine acceptance groups expressed a greater willingness to accept the 2009 H1N1 vaccine despite their uncertainty than those in the lower vaccine acceptance groups. However, almost all participants raised concerns about the vaccine being untested and lack of information about potential side effects, particularly if side effects include long-term effects in the developing fetus. Views on seasonal influenza vaccination did not necessarily correlate to behavioral intention for the 2009 H1N1 vaccination. For some, the seasonal flu vaccine was seen as safe and tested compared with the 2009 H1N1 vaccine which was seen as new and potentially unsafe. As one participant from the lower vaccine acceptance group said, “I wouldn’t choose the swine flu shot over the regular flu shot because I’ve been taking the regular flu shot for a long time and I trust that one. I don’t want them to inject nothing swine in me.”
Protecting the fetus was the key motivating factor informing intentions to get vaccinated. Despite the high levels of uncertainty reported by the majority of participants, a few participants, mostly in the higher vaccine acceptance groups, indicated they would strongly consider getting the 2009 H1N1 vaccine during pregnancy to protect themselves and their babies. Most participants indicated that the obstetrician’s or pediatrician’s recommendation regarding the 2009 H1N1 vaccine (either for or against the vaccine) would be a key influence in the decision-making process. However, a physician’s recommendation held no influence among the few participants who stated they definitely would not get the 2009 H1N1 vaccine. One of these participants said, “My OBG, she told me that I should get the flu shot and the swine vaccine that’s coming out, I don’t know. I’m not gonna do it, absolutely not.”
Antiviral Medicines
Unfamiliarity with antiviral medicine was an important factor influencing their acceptability among the participants. Consistently throughout the groups, participants were unaware of the term “antiviral” or how an antiviral works. For example, some participants thought that antiviral medicines only relieve symptoms and do not provide any substantive, clinical benefits. Participants also tended to confuse antiviral medicines with antibiotics and to some extent, with vaccines. Some participants recalled being prescribed a “Z-pack” when previously sick with influenza.
Despite this confusion, most participants said that they would be willing to take antiviral medicine if infected with the 2009 H1N1 virus while pregnant but others were more hesitant because of concerns about potential side effects on the fetus. Notably the acceptance of antiviral medicines was not uniform for 2009 H1N1 and seasonal influenza. Consistently throughout the groups, most participants said they would not take antiviral medicine for seasonal influenza primarily because they considered the seasonal influenza less dangerous than 2009 H1N1. One participant (from the higher vaccine acceptance group) said and others agreed, “I would probably be more likely to take it for swine flu ‘cause it’s a scarier thing. I’ll try what I can to get it [seasonal influenza] out of my system, I guess. A [seasonal] flu, I can ride through a little bit.”
The primary factor influencing acceptance or rejection of antiviral medicines for 2009 H1N1, as it was for influenza vaccine, was concern for the fetus. Because pregnant women are routinely taught to be careful about taking medications, many participants reported being very cautious about taking medications during pregnancy, even if they are prescribed. Some, however, expressed a willingness to take Tylenol to control a high fever as recommended by their physicians. Some women knew that antiviral medicine was contraindicated for young children and were concerned about transmission of the medication through breast milk. As one woman reported, “I don’t know what I would do because I don’t know if I can risk taking it [antiviral medicine] and giving it to him [infant].”
Trusted and Distrusted Sources of Health Information
Participants described several trusted sources of health information, which tended to vary by health issue. For example, many participants said they look for general health information on the web (e.g., WebMD), but look to pregnancy-specific websites (e.g., www.babycenter.com), call nurse hotlines or doctor’s offices, and talk to friends and family for pregnancy information. Physician offices and government health agencies, including the CDC, state and/or local health departments, were the most cited sources for the 2009 H1N1-related information. There was some regional variation in trusted sources. In Atlanta, participants most frequently mentioned CDC as a trusted source, while in Dallas, it was health departments. Some participants, particularly from the no or public insurance groups, distrusted sources like the media, which they perceived were benefiting financially from the 2009 H1N1 outbreak. In some cases, this distrust extended to government officials because of participants’ beliefs in conspiracy theories and other forms of misinformation.
Many participants reported that health care providers, including obstetricians, midwives, and pediatricians, are a deeply trusted source and have the capacity to help pregnant women and new mothers make sound decisions about vaccine and antiviral use. However, a small subset of participants, mostly in the lower vaccine acceptance groups, reported having actively researched health topics on their own and routinely weighing their own findings against the advice of their providers. Some of these participants thought that providers had financial incentives to overprescribe; others recognized that providers were still learning about the 2009 H1N1 and did not have all the answers.
Opinions varied about the best communication mediums for reaching pregnant women. The Internet was a preferred medium for participants because it enables immediate access to information and is low cost. Likewise, social networks were a popular medium for similar reasons. Participants with older children in the home recommended schools as a helpful medium. Some participants agreed that, regardless of the medium, information must be reliable and come from a trusted source. They explained that people have different ways of accessing information, so information should be disseminated in multiple ways, through many channels.
Discussion
The views and perceptions held by pregnant women about 2009 H1N1 as described here have implications for public health and patient-provider communication. A recent, post-pandemic study indicated that vaccination during the H1N1 pandemic was low (approximately 38%) given the reports of pregnant women’s death and serious illness [18]. They suggest that further research is needed to examine the barriers and facilitators of vaccine-related behavior. Findings from our study shed some light on these drivers. Our findings suggest that risk perceptions of 2009 H1N1 and intentions to follow recommended actions are not fixed but diverse and malleable. The majority of participants initially expressed low concern about the 2009 H1N1 but showed a willingness to reassess their views after exposure to engaged discussion. The willingness to reconsider previous views suggests that low-risk perceptions might have been based, at least in part, on lack of information and knowledge of the 2009 H1N1. Possibly, the participants’ understanding of the 2009 H1N1 was shaped by less trustworthy sources of information, like the media and family and, thus, would be amenable to change when presented with information from more credible sources.
Several constructs from our conceptual framework highlight areas where improved communication might affect motivations to adopt protective actions. For example, Protection Motivation Theory posits that the participants who reported misperceptions of susceptibility might benefit from targeted messages that emphasize the physiological factors that increase susceptibility in pregnant women. Similarly, the confusion over the potential severity of 2009 H1N1 infection in pregnant women and its relationship to seasonal influenza needs to be considered in future influenza communication. If the motivation to adopt recommendations is influenced by differential perceptions of severity among multiple influenza strains, this could pose a potential challenge to framing a coherent influenza message. At the time these focus groups were conducted, a seasonal influenza vaccination with the 2009 HINI was not available, so it is not known whether these differential perceptions would still be a factor to adoption.
In addition, communication with pregnant women needs to address perceived barriers to vaccine and antiviral use. The CDC recommended that all pregnant women receive the H1N1 vaccine due to their heightened risk of complications if infected [19]. However, participants across all groups balanced serious concerns about the safety of the 2009 H1N1 vaccines and antiviral medicines for the fetus against the risk of contracting/treating 2009 H1N1. These concerns extended to the postpartum period, as participants questioned the safety of taking antiviral medicine while breastfeeding. Reluctance toward influenza medicines was tied to routine guidance which cautions medication use in pregnancy.
Identifying trusted sources of information for pregnant women is important because it might improve the acceptance of behavior change recommendations [20]. Although other sources were mentioned, health care providers are a deeply trusted source for most pregnant women and should be engaged in delivering key messages. Participants also relied on external sources on the Internet, such as CDC and pregnancy-related and general medical information websites. Schools also might be an effective channel for reaching pregnant women with school-aged children in their homes. Our findings support the conclusion that the provider–patient dyad is important in making decisions about protective actions; however, other information sources are needed to reinforce provider messages.
Participants’ concern, confusion, and lack of knowledge regarding 2009 H1N1 and their willingness to change initial views highlight a critical role for education. The following section highlights practical implications for provider-patient and public health communication efforts.
Conclusions
Findings suggest that pregnant women have unique communication needs for influenza. Providers and public health practitioners should take into account these needs when communicating with pregnant women regarding influenza prevention.
-
The safety of the infant is a key factor in pregnant women’s motivation to adopt recommendations. Any recommendations directing pregnant women to adopt public health recommendations, particularly vaccination, should include a detailed description of the benefits or lack of risk to the fetus.
-
Pregnant women are taught to be selective about taking medications. Recommendations for antiviral medicine or vaccine use should acknowledge this belief and provide a clear rationale for why antiviral medicine, vaccine, or an over-the-counter medication, such as Tylenol, to control fever is necessary, particularly if the medication is perceived as new.
-
Postpartum women have concerns about taking medications while breastfeeding. Recommendations that interfere with breastfeeding are barriers for pregnant and postpartum women. Messages should include specific information on the safety of medications or other interventions while breastfeeding.
-
Influenza vaccination and antiviral medicine are not well understood. Basic knowledge of these terms and what they mean is a clear need for pregnant and postpartum women that can be addressed by providers and other trusted sources of information. In particular, the confusion between antiviral medicine and antibiotics should be a focus of education.
Notes
Quantitative results are intended to provide context and a point of reference and are not statistically significant.
References
Centers for Disease Control and Prevention. (1999). Office of Minority Health and Health Disparities. Eliminate disparities in infant mortality. Atlanta: CDC. Available from: http://www.cdc.gov/omhd/AMH/factsheets/infant.htm. cited March 23, 2009.
Farrell, R. M., & Beigi, R. H. (2009). Pandemic influenza and pregnancy: an opportunity to reassess maternal bioethics. American Journal of Public Health, 99(Suppl 2), S231–S235.
Hayes, C. E. (2008). Prevention of influenza. Journal of Midwifery and Womens Health, 53(3), 268–271.
Tamma, P. D., Ault, K. A., del Rio, C., Steinhoff, M. C., Halsey, N. A., & Omer, S. B. (2009). Safety of influenza vaccination during pregnancy. American Journal of Obstetrics and Gynecology, 201(6), 547–552.
Naleway, A. L., Smith, W. J., & Mullooly, J. P. (2006). Delivering influenza vaccine to pregnant women. Epidemiologic Reviews, 28, 47–53.
Rasmussen, S. A., Jamieson, D. J., & Bresee, J. S. (2008). Pandemic influenza and pregnant women. Emerging Infectious Diseases, 14(1), 95–100.
Rebmann, T. (2008). Preparing for pandemic influenza. The Journal of Perinatal and Neonatal Nursing, 22(3), 191–202. quiz 3–4.
Siston, A. M., Rasmussen, S. A., Honein, M. A., Fry, A. M., Seib, K., Callaghan, W. M., et al. (2010). Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. Journal of American Medical Association, 303(15), 1517–1525.
Jamieson, D. J., Honein, M. A., Rasmussen, S. A., Williams, J. L., Swerdlow, D. L., Biggerstaff, M. S., et al. (2009). H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet, 374(9688), 451–458.
Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, et al. (2009). Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. Morbidity and Mortality weekly Recommendations and Reports. 58(RR-8):1–52.
Harris Interactive. (2008). Flu shot & pregnancy survey report. Red Bank (NJ): The National Women’s Health Resource Center.
Tong, A., Biringer, A., Ofner-Agostini, M., Upshur, R., & McGeer, A. (2008). A cross-sectional study of maternity care providers’ and women’s knowledge, attitudes, and behaviours towards influenza vaccination during pregnancy. Journal of Obstetrics and Gynaecology Canada, 30(5), 404–410.
Gerber, J. S., & Offit, P. A. (2009). Vaccines and autism: A tale of shifting hypotheses. Clinical Infectious Diseases, 48(4), 456–461.
Wallis, D. H., Chin, J. L., Sur, D. K., & Lee, M. Y. (2006). Increasing rates of influenza vaccination during pregnancy: A multisite interventional study. Journal of the American Board of Family Medicine, 19(4), 345–349.
Rogers, R. W. (1975). A protection motivation theory of fear appeals and attitude change. Journal of Psychology, 91, 93–114.
Patton, M. Q. (2002). Qualitative research and evaluation methods (3rd edn ed.). Thousand Oaks (CA): Sage.
Krueger, R. A., & Casey, M. A. (2000). Focus groups: A practical guide for applied research. Thousand Oaks (CA): Sage.
Fridman, D, Steinberg E, Azhar E, Weedon J, Wilson TE. (2011). Predicators of H1N1 vaccination in pregnancy. American Journal of Obstetrics and Gynecology. 204(6)S1:S124–127.
Centers for Disease Control and Prevention. (2009). H1N1 vaccination recommendations. Atlanta: CDC. Available from: http://www.cdc.gov/h1n1flu/vaccination/acip.htm.
U.S. Public Health Service. (1995). Risk communication: working with individuals and communities to weigh the odds. Prevention report. Washington, DC: U.S. Department of Health and Human Services, Public Health Service. Available from: http://odphp.osophs.dhhs.gov/pubs/prevrpt/Archives/95fm1.htm. cited December 17, 209.
Acknowledgments
The authors wish to thank Kitty MacFarlane of the CDC and Colleen Carr and Laurie Brockman of Danya International for their contributions to the development and review of this manuscript. We also wish to thank Jackie Amoozegar, Joey Horne, and Genny Cromwell of RTI International for their analytic support. The research was funded under a contract with the Centers for Disease Control and Prevention No. 0210637 Task Order 9.
Author information
Authors and Affiliations
Corresponding author
Additional information
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Rights and permissions
About this article
Cite this article
Lynch, M.M., Mitchell, E.W., Williams, J.L. et al. Pregnant and Recently Pregnant Women’s Perceptions about Influenza A Pandemic (H1N1) 2009: Implications for Public Health and Provider Communication. Matern Child Health J 16, 1657–1664 (2012). https://doi.org/10.1007/s10995-011-0865-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10995-011-0865-y