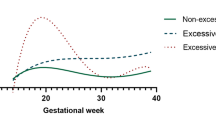
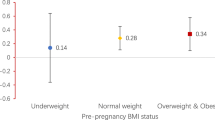
Abstract
Our objective was to test the hypothesis that intrauterine exposure to gestational diabetes [GDM] predicts childhood growth independent of the effect on infant birthweight. We conducted a prospective analysis of 28,358 mother-infant pairs who enrolled in the National Collaborative Perinatal Project between 1959 and 1965. The offspring were followed until age 7. Four hundred and eighty-four mothers (1.7%) had GDM. The mean birthweight was 3.2 kg (range 1.1–5.6 kg). Maternal characteristics (age, education, race, family income, pre-pregnancy body mass index and pregnancy weight gain) and measures of childhood growth (birthweight, weight at ages 4, and 7) differed significantly by GDM status (all P < 0.05). As expected, compared to their non-diabetic counterparts, mothers with GDM gave birth to offspring that had higher weights at birth. The offspring of mothers with GDM were larger at age 7 as indicated by greater weight, BMI and BMI z-score compared to the offspring of mothers without GDM at that age (all P < 0.05). These differences at age 7 persisted even after adjustment for infant birthweight. Furthermore, the offspring of mothers with GDM had a 61% higher odds of being overweight at age 7 compared to the offspring of mothers without GDM after adjustment for maternal BMI, pregnancy weight gain, family income, race and birthweight [OR = 1.61 (95%CI:1.07, 1.28)]. Our results indicate that maternal GDM status is associated with offspring overweight status during childhood. This relationship is only partially mediated by effects on birthweight.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A growing body of evidence suggests that predisposition to a host of chronic diseases in adulthood may be programmed in utero. “Fetal programming” stems from work by Barker and colleagues which demonstrated a relationship between birthweight and adult hypertension [1], insulin resistance [2], dyslipidemia [3] and cardiovascular disease [4–6], all of which share obesity as a common risk factor. Most of these associations have been confirmed by epidemiological studies in several different populations [7]. As a result, there has been growing interest in investigating whether obesity itself, is programmed in utero by the presence of a prenatal insult or an adverse hyperglycemic intrauterine environment [8–12].
Gestational diabetes [GDM] as diagnosed using current criteria, complicates up to 7% of U.S. pregnancies [13] and is the most common cause of sustained intrauterine hyperglycemia [14, 15]. The prevalence of GDM in the United States has increased dramatically between 1989 and 2004 along with the increasing trend in overweight status and obesity among women of reproductive age, with one study reporting a relative increase of 122% [16]. GDM is characterized by maternal insulin sensitivity and hyperglycemia. Through facilitated-diffusion, glucose is transported across the placenta resulting in fetal hyperinsulinemia, accelerated fetal growth and potential neonatal hypoglycemia at delivery [17, 18]. Results from the Hyperglycemia and Adverse Pregnancy Outcomes [HAPO] study indicate that maternal hyperglycemia even in the absence of overt diabetes, adversely affects neonatal outcomes [19]. The consequences of maternal hyperglycemia in pregnancy may extend beyond the neonatal period since high infant birthweight is independently predictive of overweight status in childhood [20, 21]—a condition which has increased at alarming rates in the Unites States. Animal studies suggest that fetal hyperinsulinemia, stimulated by maternal hyperglycemia, can alter the expression of hypothalamic neurotransmitters, leading to hyperphagia and increased weight in offspring [22, 23].
Although several epidemiologic studies have investigated the association between GDM and offspring overweight status and obesity during childhood, the results are mixed. Several prospective and retrospective cohort studies reported evidence of a positive association [17, 20, 21, 24–28], and one prospective cohort study showed no association [18]. Few studies have examined whether the effect of GDM on childhood growth is independent of infant birthweight [20, 24]. Our objectives were to (1) determine the magnitude of association of GDM with infant birth weight and anthropometric measures (weight, body mass index [BMI] and BMI z-score) during childhood at ages 3, 4 and 7 years and (2) determine whether the association was independent of infant birth weight. We conducted a prospective analysis of 28,358 mother-infant pairs who enrolled in the National Collaborative Perinatal Project [NCPP] in 1959–1965 to estimate these associations.
We hypothesized that GDM would be predictive of early childhood growth at each age interval, independent of infant birthweight. If infant birthweight mediates the association between GDM and early childhood growth, then clinicians may need to develop perinatal interventions that target infant birthweight. Alternatively, if GDM is independently predictive of early childhood growth, clinicians might focus on peri-conceptional interventions that prevent insulin sensitivity and the development of GDM.
Methods
Study Design and Population
The National Collaborative Perinatal Project [NCPP] was a prospective cohort study of pregnancy and child health. It was specifically designed to identify determinants of cerebral palsy and allied neurological defects [29]. A detailed description of the methods is described elsewhere [29]. Briefly, approximately 42,000 pregnant women were enrolled at 12 hospitals across the United States (Baltimore MD, Boston MA, Buffalo NY, Memphis TN, Minneapolis MN, New Orleans LA, New York NY [2 hospitals], Philadelphia PA, Portland OR, Providence RI and Richmond VA). Pregnant women were enrolled usually at their first prenatal visit between 1959 and 1966. Participants were deemed ineligible if there were incarcerated, if they were planning to move from the area or to give the child up for adoption or if they gave birth on the day they were recruited into the study. Records were not kept for women who refused participation at baseline. Four percent of the subjects enrolled were lost to follow up before delivery. Of the live-born children in the Collaborative Project, 71% were followed to 7 years of age.
Identification of Cohort
For the present analysis eligible mother–child pairs met the following criteria: (1) singleton live-born infants, (2) no congenital anomalies, (3) black or white race, and (4) gestational age at delivery between 36 and 42 weeks gestation. From the 34,345 initially eligible mother–child pairs, 5,987 were excluded from the analysis because of missing values for variables of interest (birth weight, gestational diabetes, family income and pregnancy weight gain and maternal BMI), producing an analytic sample of 28,358 mother–child pairs. Characteristics of the analytic sample were compared with those of the eligible cohort. Compared to the counterparts excluded from the analysis, mothers in the analytic sample were slightly older, more educated and had a higher pre-pregnancy BMI (all P < 0.05). There was no difference in pregnancy weight gain. Mothers included were more likely to be black (51% vs. 43%) and less likely to have gestational diabetes (1.7% vs. 2.6%). Among both the included and the excluded group, black mothers were less likely to have gestational diabetes (36 and 15% respectively). Due to loss to follow up, there was some attrition with increasing offspring age.
Diagnosis of GDM in the National Collaborative Perinatal Project
Several glucose tolerance tests were performed for each mother utilizing 5 specimens: a fasting specimen and specimens obtained at 30 min, 1, 2 and 3 h after oral administration of 100 g glucose in solution. In general, glucose tolerance was considered indicative of diabetes if the glucose level of the fasting blood specimen was 120 mg/dL or higher, or if it rose to over 175 mg/dL at the end of 1 h and did not return to normal in the 2- and 3-h specimens. The mother was classified as having GDM if any one of the following four conditions were met: (1) she was newly diagnosed with diabetes during pregnancy; (2) she initiated insulin during pregnancy; (3) she displayed an abnormal glucose tolerance test result; or (4) she had a blood glucose level of 200 mg or more at any time during pregnancy.
Data Collection
Maternal socio-demographic characteristics (age, sex, education, income, marital status) were obtained by interview. Maternal weight and height were measured upon enrollment; pre-pregnancy weight was self-reported. Pre-pregnancy maternal body mass index (BMI) was calculated as self reported pre-pregnancy weight in kilograms divided by height in meters squared. Just prior to delivery, the mother’s weight was measured again and used to calculate pregnancy weight gain (i.e. pregnancy weight at delivery minus self-reported pre-pregnancy weight).
At delivery, weight and length of the infant were measured. At ages 3, 4 and 7 the children’s height and weight were measured according to study protocol. BMI at ages 3, 4 and 7 were calculated as weight in kilograms divided by height in meters squared. Using Epi Info v.3.3.2, we calculated sex-specific weight for height percentiles and z-scores of BMI using the 1978 WHO/CDC growth reference curve, the earliest standard available for this group of children based on data from the Fels Longitudinal Growth Study conducted in Yellow Springs Ohio between 1929 and 1975 [30]. We classified children as being overweight if their weight for height exceeded the 85th percentile.
Statistical Analysis
Descriptive statistics, frequencies for categorical variables and means and standard deviations for continuous variables were calculated after stratification by maternal gestational diabetes status. Chi-square (χ2) statistics and Student’s t-tests with unequal variance were used to compare variables by maternal gestational diabetes status.
We constructed multiple linear regression models to assess the independent relationship between maternal gestational diabetes and weight, BMI and BMI z-score at ages 3, 4 and 7 years. We also constructed multiple logistic regression models to calculate the relative odds of overweight at ages 3, 4 and 7 for offspring of mothers with gestational diabetes compared to mothers without gestational diabetes with adjustment for maternal BMI, pregnancy weight gain, family income, race and birthweight. We used generalized estimating equations (GEE) approach to estimate the population average odds of being overweight over the 7 years of follow-up for offspring having mothers with gestational diabetes compared to those having mothers without gestational diabetes accounting for the data correlation structure for each individual. All analyses were conducted using STATA statistical software (version 9.0; Stata Corporation, College Station, Texas). All tests of significance were two-tailed. This study protocol 04-08-16-06 was approved by the Johns Hopkins Medicine Institutional Review Board.
Results
Characteristics of the Study Population
The average maternal age was 24.4 years. A little more than half of the mothers were non-white (56%) and had not completed high school (53.4%). The majority, 72%, had an annual family income less than $5000–equivalent to about $30,000 in 2005. The mean birthweight overall was 3.2 kg. By present standards, the children were fairly lean with a mean weight of 14.4 kg at age 3; 16.1 kg at age 4; and 23.4 kg at age 7. Table 1 summarizes characteristics of 28,358 mother–child pairs by maternal gestational diabetes status. Mothers with gestational diabetes were older and heavier prior to pregnancy, but gained significantly less weight during pregnancy. Compared to their non-diabetic counterparts, women with GDM were less likely to be Black and more likely to have a family income greater than $5000. Moreover, offspring of mothers with GDM had a significantly higher birthweight on average than their counterparts whose mothers did not have GDM. In addition, offspring of mothers with GDM were heavier in terms of weight and z-score of BMI at ages 4 and 7 compared to offspring of mothers without GDM. Compared to offspring of mothers without GDM, there was a higher proportion of overweight at ages 3, 4 and 7 among offspring of mothers with GDM (Table 2).
Association of GDM with Early Childhood Growth
We estimated the crude and adjusted mean difference in weight at birth and ages 3, 4, and 7 for offspring of mothers with vs. without GDM (Table 3). Offspring of mothers with GDM had a significantly higher mean birthweight even after adjustment for maternal BMI, pregnancy weight gain, family income, and race (β = 0.05 kg; 95% CI: 0.01, 0.09). There was no relationship between mothers’ GDM status and offspring weight at ages 3 and 4 after adjustment for maternal BMI, pregnancy weight gain, family income, race and birthweight. However, offspring of mothers with GDM were 0.46 kg heavier at age 7 than offspring of mothers without GDM (95%CI: 0.02, 0.91) after adjustment for maternal BMI, pregnancy weight gain, family income, race and birthweight. We repeated this analysis using BMI and BMI z-score as the outcome and the results were similar.
Association of GDM with Childhood Overweight Status
GDM was associated with an increased likelihood of being overweight [OR = 1.81 (95% CI:1.18, 2.86) and OR = 1.61 (95% CI:1.07, 1.28) at ages 4 and 7 respectively]. The associations attenuated with increasing age although there was no significant linear trend P = 0.729. Although the results did not meet statistical significance, our results suggest that on average, children of mothers with GDM were 70% more likely to be overweight [OR = 1.70 (95% CI: 0.97, 2.96)] after adjustment for maternal BMI, pregnancy weight gain, family income, race and birthweight and the correlation among multiple assessments of body weight from the same child over time. This result is based on GEE analysis using data from participants with values at each age only (Table 4).
Discussion
We conducted an analysis using data from the NCPP to better understand the effect of GDM on childhood growth. Our results expand the knowledge in this area as we found that maternal GDM status is associated with childhood growth independent of birthweight. We observed that offspring of mothers with GDM were heavier as determined by three anthropometric measures examined at age 7, compared to offspring of mothers without GDM, even after adjustment for birthweight. Also, offspring of mothers with GDM show a 61–81% higher odds of overweight status ages 7 and 4, respectively, compared to offspring of mothers without GDM.
Previous studies examined several different anthropometric measures during childhood at different points during childhood among offspring of mothers with GDM. Several studies evaluated overweight or obesity defined using either the 85th, 90th or 95th percentile of BMI or weight [20, 27], BMI z-score [18, 26, 28], BMI [21, 25, 27] and skinfold thickness [25, 26, 28]. Only one study evaluated offspring fat mass and waist circumference [26]. In addition, with the exception of birthweight, few of the studies assessed childhood anthropometry before the age of 5 [21, 28]. In one study, Gillman and colleagues [24] included birthweight in the multivariable model and unlike the results of our study, they did not find a statistically significant association between offspring exposure to GDM and overweight status in childhood among 9–14 year old participants [OR = 1.3; 95% CI 0.9, 1.9] and after the addition of mother’s BMI at the time of the child’s anthropometry assessment, the association attenuated [OR = 1.2; 95% CI 0.8, 1.7]. Whitaker and colleagues did not find an association between intrauterine exposure to GDM and overweight status in childhood defined by BMI z-score for offspring ages 8–10 years of age in a sample of medically insured, non-Hispanic white diet-treated GDM mothers even without adjustment for birthweight [18]. Our study results may differ from the null findings in previous studies due to several factors. First, the difference in results may be explained by the secular improvements in the detection and treatment of GDM. Second, our study was conducted using data from a historical cohort unexposed to the nutritional affluence of more contemporary cohorts used in other studies. Given strong influence of environmental factors and increased access and availability of calories in a contemporary cohort, these factors may be more overwhelming than the effect of the intrauterine exposure to maternal hyperglycemia. Third, overweight in these studies were assessed at older ages which allows for increased influence of the environmental factors with increasing age.
However, similar to Wright et al., we observed no significant difference in childhood growth in offspring of mothers with GDM compared to offspring of mothers without GDM at ages 3 and 4. Several factors may have contributed to the lack of association between GDM and childhood growth at ages 3 and 4. First, weight and BMI do not fully capture growth. Body composition measures, including fat free mass and lean mass which were unavailable for analysis, may be better measures of growth in childhood. Second, data from previous studies suggest that it is not until after ~5 years of age that there is a significant difference in weight of offspring of mothers with GDM compared to those whose mothers did not have GDM. These studies suggest that GDM may have a delayed influence that increases with time [17, 24, 28].
There are several mechanisms that may explain our findings. First, our findings support the idea that intrauterine exposures and programming may have long term effects on childhood growth. While the short term effect may be manifested in infant birthweight, there may be an alternative pathway linking GDM to overweight status in childhood since the observed association persisted even after adjustment for birthweight. This idea of an alternative pathway is supported by Pettit’s finding in the Pima Indians that offspring of mothers with GDM with normal birthweight had an increased risk of childhood obesity [31]. Second, there may be a combination of metabolic alterations or genetic factors that play a role. In a recent study, Catalano et al. found that newborns of mothers with mild glucose intolerance had a 20% higher body fat mass than infants born to mothers with normal glucose tolerance, suggestive of an early alteration in fat metabolism due to antenatal exposure to maternal hyperglycemia [32]. Another possible explanation for the persistent relationship between GDM and overweight status in childhood may be that offspring of mothers with GDM adopt some unhealthy dietary and physical activity patterns of their mothers and may share obesity-related genes. This explanation however, is challenged by the results of Dabelea et al. [33] which show that among Pima Indians, offspring exposed to diabetes in utero had higher BMI than their unexposed siblings. These results suggest that there may be some programming effect of GDM on the fetus, making exposed offspring more susceptible to being heavier in childhood.
The relative importance of tight glycemic control and lifestyle behavior change among mothers with GDM in the prevention of overweight status among offspring is unclear. Hillier et al. [20] found an almost twofold increased risk of elevated weight at 5–7 years among offspring of mothers with untreated GDM, whereas the association with treated GDM was weaker and similar to milder impaired glucose tolerance. However, results from a randomized controlled trial, showed that treatment of mild GDM with dietary advice, blood glucose monitoring and insulin therapy as needed, resulted in reduced incidence of macrosomia in the intervention group, but there was no effect on BMI in 4–5 year olds [34]. These results suggest that there is more to the relationship between GDM and overweight status in childhood beyond shared obesity-related genes between the mother and offspring, which is a commonly offered explanation. Further investigation of gene environment interactions in utero and postnatally are necessary to better discern these potential relationships.
Our study has several strengths. First, the study has a large diverse population-based sample though the analytic sample was reduced compared to the initial cohort. Second, we were able to examine the association of offspring exposure to GDM and weight and overweight over a 7 year time frame. Third, our study allows us to examine the association between GDM and childhood growth in a period of time with low prevalence of diabetes and obesity unlike most other observational studies using more contemporary populations or high risk populations with high prevalence of obesity and diabetes.
Nonetheless, several limitations deserve comment. First, there may be some selection bias due to missing data and differential loss to follow up such that mothers included in the analysis were more likely to be white and have completed high school. Second, there is some error in the assessment of gestational age as would be expected since this study was conducted before the implementation of routine ultrasound to determine gestational age. Gestational age in this study was determined by using self-reported date of the last menstrual period (LMP). We were conservative in our approach and limited the analytic sample births to mothers with a gestational age between 36 and 42 weeks gestation. Third, we were not able to adjust for unmeasured confounders such as family environment, diet and exercise which could be independent risk factors for both GDM and overweight status in childhood. Fourth, there may be some misclassification of GDM. However, the incidence of metabolic alterations in offspring of mothers with diabetes is not dependent on the type of diabetes. The risk of overweight and obesity during childhood and adulthood seem to follow similar profiles in offspring of mothers with Type 1 and Type 2 Diabetes [17, 35]. The OGTT thresholds used to diagnose GDM in the NCPP are higher (125 mg/dl vs. 95 mg/dl) compared to those currently recommended by the NDG or Carpenter-Coustan criteria. Thus, we may have underestimated the number of women with GDM and underestimated the magnitude of association between GDM and early childhood growth. Finally, generalizability is limited, since participants in the NCPP were enrolled over 40 years ago and were comprised largely of low income women.
We conclude that the GDM is predictor of early childhood growth independent of birthweight. Therefore clinicians might focus on peri-conceptional interventions that prevent insulin sensitivity and the development of GDM. Current evidence indicates that physical activity prior and during pregnancy results in an almost 50% reduction in the development of GDM [36, 37]. In addition, interventions to improve glucose control and increase healthy behaviors during pregnancy may impact the development of overweight and obesity in the offspring. Alternatively, since implementation of a lifestyle intervention may be challenging during pregnancy, mothers with GDM might be directed to a lifestyle intervention as part of their post partum follow-up care. Such intervention might reduce the risk of recurrent GDM with a subsequent pregnancy as well as improve lifestyle behaviors of the mother and child.
References
Barker, D. J., Bull, A. R., Osmond, C., & Simmonds, S. J. (1990). Fetal and placental size and risk of hypertension in adult life. BMJ, 301, 259–262.
Phillips, D. I., Barker, D. J., Hales, C. N., Hirst, S., & Osmond, C. (1994). Thinness at birth and insulin resistance in adult life. Diabetologia, 37, 150–154.
Barker, D. J., Martyn, C. N., Osmond, C., Hales, C. N., & Fall, C. H. (1993). Growth in utero and serum cholesterol concentrations in adult life. BMJ, 307, 1524–1527.
Barker, D. J., Winter, P. D., Osmond, C., Margetts, B., & Simmonds, S. J. (1989). Weight in infancy and death from ischaemic heart disease. Lancet, 2, 577–580.
Barker, D. J., Osmond, C., & Law, C. M. (1989). The intrauterine and early postnatal origins of cardiovascular disease and chronic bronchitis. Journal of Epidemiology and Community Health, 43, 237–240.
Barker, D. J. (1995). Fetal origins of coronary heart disease. BMJ, 311, 171–174.
Wells, J. C. (2009). Historical cohort studies and the early origins of disease hypothesis: Making sense of the evidence. Proceedings of the Nutrition Society, 68, 179–188.
Huang, J. S., Lee, T. A., & Lu, M. C. (2007). Prenatal programming of childhood overweight and obesity. Maternal and Child Health Journal, 11, 461–473.
Ravelli, A. C., Der Meulen, J. H., Osmond, C., Barker, D. J., & Bleker, O. P. (1999). Obesity at the age of 50 y in men and women exposed to famine prenatally. American Journal of Clinical Nutrition, 70, 811–816.
Ravelli, A. C., van der Meulen, J. H., Osmond, C., Barker, D. J., & Bleker, O. P. (2000). Infant feeding and adult glucose tolerance, lipid profile, blood pressure, and obesity. Archives of Disease in Childhood, 82, 248–252.
Remacle, C., Bieswal, F., & Reusens, B. (2004). Programming of obesity and cardiovascular disease. International Journal of Obesity and Related Metabolic Disorders, 28(Suppl 3), S46–S53.
Rogers, I. (2003). The influence of birthweight and intrauterine environment on adiposity and fat distribution in later life. International Journal of Obesity and Related Metabolic Disorders, 27, 755–777.
Diagnosis and classification of diabetes mellitus. (2010) Diabetes Care 33 (Suppl 1):S62–S69.
Dabelea, D., Snell-Bergeon, J. K., Hartsfield, C. L., Bischoff, K. J., Hamman, R. F., & McDuffie, R. S. (2005). Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser permanente of Colorado GDM screening program. Diabetes Care, 28, 579–584.
Jovanovic, L., & Pettitt, D. J. (2001). Gestational diabetes mellitus. JAMA, 286, 2516–2518.
Getahun, D., Nath, C., Ananth, C. V., Chavez, M. R., & Smulian, J. C. (2008). Gestational diabetes in the United States: Temporal trends 1989 through 2004. American Journal of Obstetrics and Gynecology, 198, 525.
Silverman, B. L., Rizzo, T., Green, O. C., et al. (1991). Long-term prospective evaluation of offspring of diabetic mothers. Diabetes, 40(Suppl 2), 121–125.
Whitaker, R. C., Pepe, M. S., Seidel, K. D., Wright, J. A., & Knopp, R. H. (1998). Gestational diabetes and the risk of offspring obesity. Pediatrics, 101, E9.
Metzger, B. E., Lowe, L. P., Dyer, A. R., et al. (2008). Hyperglycemia and adverse pregnancy outcomes. New England Journal of Medicine, 358, 1991–2002.
Hillier, T. A., Pedula, K. L., Schmidt, M. M., Mullen, J. A., Charles, M. A., & Pettitt, D. J. (2007). Childhood obesity and metabolic imprinting: The ongoing effects of maternal hyperglycemia. Diabetes Care, 30, 2287–2292.
Schaefer-Graf, U. M., Pawliczak, J., Passow, D., et al. (2005). Birth weight and parental BMI predict overweight in children from mothers with gestational diabetes. Diabetes Care, 28, 1745–1750.
Plagemann, A., Harder, T., Melchior, K., Rake, A., Rohde, W., & Dorner, G. (1999). Elevation of hypothalamic neuropeptide Y-neurons in adult offspring of diabetic mother rats. Neuroreport, 10, 3211–3216.
Plagemann, A., Harder, T., Janert, U., et al. (1999). Malformations of hypothalamic nuclei in hyperinsulinemic offspring of rats with gestational diabetes. Developmental Neuroscience, 21, 58–67.
Gillman, M. W., Rifas-Shiman, S., Berkey, C. S., Field, A. E., & Colditz, G. A. (2003). Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics, 111, e221–e226.
Krishnaveni, G. V., Veena, S. R., Hill, J. C., Kehoe, S., Karat, S. C., & Fall, C. H. (2010). Intrauterine exposure to maternal diabetes is associated with higher adiposity and insulin resistance and clustering of cardiovascular risk markers in Indian children. Diabetes Care, 33, 402–404.
Lawlor, D. A., Fraser, A., Lindsay, R. S., et al. (2010). Association of existing diabetes, gestational diabetes and glycosuria in pregnancy with macrosomia and offspring body mass index, waist and fat mass in later childhood: Findings from a prospective pregnancy cohort. Diabetologia, 53, 89–97.
Vaarasmaki, M., Pouta, A., Elliot, P., et al. (2009). Adolescent manifestations of metabolic syndrome among children born to women with gestational diabetes in a general-population birth cohort. American Journal of Epidemiology, 169, 1209–1215.
Wright, C. S., Rifas-Shiman, S. L., Rich-Edwards, J. W., Taveras, E. M., Gillman, M. W., & Oken, E. (2009). Intrauterine exposure to gestational diabetes, child adiposity, and blood pressure. American Journal of Hypertension, 22, 215–220.
Niswander, K. R., & Gordon, M. (1972). The women and their pregnancies (NIH) (pp 73–379). Washington, DC: US Government Printing Office, US Department of Health, Education and Welfare.
Hamill, P. V., Drizd, T. A., Johnson, C. L., Reed, R. B., & Roche, A. F. (1977). NCHS growth curves for children birth-18 years. United States. Vital Health Stat 11 i–74.
Pettitt, D. J., Knowler, W. C., Bennett, P. H., Aleck, K. A., & Baird, H. R. (1987). Obesity in offspring of diabetic Pima Indian women despite normal birth weight. Diabetes Care, 10, 76–80.
Catalano, P. M., Thomas, A., Huston-Presley, L., & Amini, S. B. (2003). Increased fetal adiposity: A very sensitive marker of abnormal in utero development. American Journal of Obstetrics and Gynecology, 189, 1698–1704.
Dabelea, D., Hanson, R. L., Lindsay, R. S., et al. (2000). Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes, 49, 2208–2211.
Gillman, M. W., Oakey, H., Baghurst, P. A., Volkmer, R. E., Robinson, J. S., & Crowther, C. A. (2010). Effect of treatment of gestational diabetes on obesity in the next generation. Diabetes Care, 33, 964–968.
Weiss, P. A., Scholz, H. S., Haas, J., Tamussino, K. F., Seissler, J., & Borkenstein, M. H. (2000). Long-term follow-up of infants of mothers with type 1 diabetes: evidence for hereditary and nonhereditary transmission of diabetes and precursors. Diabetes Care, 23, 905–911.
Dempsey, J. C., Sorensen, T. K., Williams, M. A., et al. (2004). Prospective study of gestational diabetes mellitus risk in relation to maternal recreational physical activity before and during pregnancy. American Journal of Epidemiology, 159, 663–670.
Cheung, N. W., Smith, B. J., Henriksen, H., Tapsell, L. C., McLean, M., & Bauman, A. (2007). A group-based healthy lifestyle program for women with previous gestational diabetes. Diabetes Research and Clinical Practice, 77, 333–334.
Acknowledgments
The authors are supported by the National Institutes of Health. KBR received support from the National Heart, Lung and Blood Institute (T32-HL07024). WKN received support from the National Institute for Diabetes and Digestive and Kidney Diseases (K23-DK067944), NW received support by National Institutes of Health (UL1 RR025005) & (P60 DK79637), FLB is supported by the National Institute for Diabetes and Digestive and Kidney Diseases (K24-DK6222) & (P60 DK79637).
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s10995-011-0903-9
Rights and permissions
About this article
Cite this article
Baptiste-Roberts, K., Nicholson, W.K., Wang, NY. et al. Gestational Diabetes and Subsequent Growth Patterns of Offspring: The National Collaborative Perinatal Project. Matern Child Health J 16, 125–132 (2012). https://doi.org/10.1007/s10995-011-0756-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10995-011-0756-2