Abstract
Overuse of antibiotics is one of the important factors that contribute to developing antimicrobial resistance. Many studies have been conducted to find out promising solutions to overcome the problems. Antimicrobial peptides (AMPs) are fundamental components of human innate immunity. They have an important role in the treatment of a wide range of diseases, including cancer, allergies, and also in warding off invading pathogens. In the case of infectious disease, the AMPs exhibit broad-spectrum activity against a wide range of pathogens including Gram-positive and -negative bacteria, yeasts, fungi, and enveloped viruses. These peptides have been isolated from various sources such as microorganisms, plants, invertebrates, and vertebrates. The peptides show distinct physicochemical and structural properties but most of them are small cationic peptides with amphipathic properties. In this review, an overview of the classification, antimicrobial activities, mode of action, and mechanism of resistance of human AMPs will be provided. These peptides are categorized into three main groups. The defensins are cationic peptides containing six cysteine residues with three intramolecular disulfide bridges. In humans, two classes of defensins could be found, α-defensins and β-defensins. The second group is cathelicidins that only one AMP, LL-37, has been found in humans. This peptide is derived from proteolytic digestion of the C-terminal of human CAP18 protein. The third group is the family of histatins that are small cationic histidine-rich peptides, and mainly present in human saliva. The last two groups have random coil conformation in hydrophilic environments and α-helices in a hydrophobic environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antibacterial resistance by multidrug-resistant (MDR) pathogens has become a global health challenge and threatens the health of societies. The emergence of resistant infections leads to existing antibacterial drugs becoming less effective or even ineffective and therefore, there is an urgent need for the development of new antibacterial agents (Khameneh et al. 2016). The death by drug-resistant bacterial infections in each year for USA, EU, and India was 23,000, 25,000, and 58,000, respectively (Chaudhary 2016). Additionally, without developing novel approaches to combat MDR pathogens, many fields of medicine such as surgery, cancer chemotherapy, and transplantation medicine will be affected severely (Worthington and Melander 2013). Whenever the antibacterial resistance has emerged, the problem has been tackled with various approaches such as repurposing non-antibiotic drugs, modification of the existing antibiotic classes with limited cross-resistance, developing new classes of antibiotics, antibiotic combinations, development of adjuvant therapy, developing a novel formulation of antibiotic, and using natural compounds (Bazzaz et al. 2019; Farhadi et al. 2019; Khameneh et al. 2015a, 2019a, b; Soheili et al. 2019; Theuretzbacher et al. 2020).
During the past years, the efforts for discovering the novel antimicrobial agents have been focused on developing groups of short polypeptides, up to 40 residues, namely antimicrobial peptides (AMPs) (Fox 2013; Javia et al. 2018). AMPs can be obtained from both natural and synthetic sources and show a broad spectrum of targeted organisms including viruses to parasites (Bahar and Ren 2013; Zasloff 2002). These peptides are categorized based on different properties such as similarities in charge, sequence, functional and 3-dimensional structure. Based on the charge of AMPs, they are grouped into anionic and cationic peptides. Anionic ones are small peptides (721.6–823.8 Da) and present in bronchoalveolar lavage, fluid surfactant extracts, and airway epithelial cells, while, cationic peptides are mainly found in all living species. These latter AMPs contain 12–50 amino acid residues with a net positive charge of + 2 to + 7. They have an excess of basic amino acid residues such as arginine, lysine, and histidine in comparison with acidic residues. Cationic AMPs have a diverse range of cellular targets (Bahar and Ren 2013; Brogden et al. 2003; Bulet et al. 2004; Huang et al. 2010).
AMPs show their antimicrobial properties via different mechanisms. They showed weak antimicrobial actions but have wide and strong immune-modulatory properties. Taken together, they have been considered as alternative agents for traditional antibiotics. AMPs have been successfully used against resistant infections such as infectious biofilm or MDR bacteria (Boparai and Sharma 2020; Park et al. 2011).
In contrast to an antibiotic peptide that is synthesized through a specific metabolic pathway, the amino acid sequence of AMPs is encoded naturally by the genetic materials of the host organisms (Boparai and Sharma 2020). Upon pathogen invasion, transcription and translation processes are started and followed by post-translational modifications including precursor protein cleavage, C-terminus amidation, and disulfide bond formation for approximately 50% of the known AMPs. Moreover, glycosylation modification may happen in uncommon cases (Toke 2005). These AMPs were first detected in plant organisms, then in a wide range of other organisms like humans (Toke 2005). So far, the number of detected AMPs has reached more than one million (Boparai and Sharma 2020).
AMPs show a broad variety of secondary structures like β-strands, α-helices with extended structures, loops, and one or more disulfide bridges (Edwards et al. 2017; Hwang and Vogel 1998). These observed structural differences are necessary for their antimicrobial activities in a broad spectrum (Hancock 2001). Additionally, particular crucial factors like peptide self-association to biological membranes, amphipathic stereo-geometry, hydrophobicity, charge, and also size contribute to their antimicrobial activities (Boparai and Sharma 2020). Moreover, the smaller size of antimicrobial peptides facilitates the fast secretion and diffusion of peptide outside the cells that is important for inducing defense responses against pathogenic fungi and bacteria (Brogden 2005). This article aims to review the classification of AMPs based on the structure and composition and their mode of action. Moreover, the essential aspects of human AMPs that have revealed their significance will be discussed.
Classification of AMPs
Knowing about the three-dimensional structure of AMPs leads to a better understanding of their mode of actions. Nuclear magnetic resonance (NMR) has been used extensively for analyzing the structures of most of the AMPs. Because of the small size of AMPs in comparison with other biopharmaceuticals, the three-dimensional structures could be obtained by conventional two-dimensional NMR methods (Reddy et al. 2004; Zhang and Falla 2006). Based on the NMR data, AMPs could be classified into five groups.
α-Helical AMPs
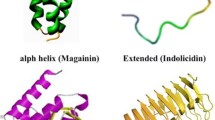
Abundant classes of AMPs are those with amphipathic properties that have α-helical domains. Their particularly successful structural arrangements help them for innating defense. Most of them are short (< 40 residues) and therefore can be synthesized easily. Because of the structure, they can be characterized by circular dichroism (CD) or NMR spectroscopies, simply. It should be noted that these types of AMPs are active against a wide range of pathogens, including both Gram‐positive and ‐negative bacteria, fungi, and protozoa (Giangaspero et al. 2001; Reddy et al. 2004). Some of them are illustrated in Fig. 1.
Cysteine-Rich AMPs
Cysteine-rich AMPs are diverse and widely distributed in animal and plant tissues. They have an important role in host defense systems (Dimarcq et al. 1998). The human neutrophil peptides HNP-1, -2, and -3 were the first of these AMPs which isolated from the human granules (Ganz et al. 1985). These α-defensins compose of 30 amino acid residues which are rich in cysteine and could be found in a wide variety of organisms (Reddy et al. 2004). Most of these AMPs contain six cysteine residues and all of them participate in forming three intramolecular disulfide bonds (Dimarcq et al. 1998). These disulfide bridges are mostly between C1–C4, C2–C5, and C3–C6 (Dimarcq et al. 1998; Hill et al. 1991). Based on the NMR data, α-defensin has three-stranded antiparallel β-sheets (Pardi et al. 1992). Some of them are illustrated in Fig. 2.
β-Sheet AMPs
The third group of AMPs is peptides with β-sheets structure. Most of them are further stabilized by one or more disulfide bonds. Based on their cysteine content and structural properties, they can be divided into two groups: (i) β-hairpin peptides; and (ii) α-defensin peptides (Koehbach and Craik 2019). β-hairpin peptides encompass approximately 20 residues containing one or two disulfide bonds. Examples of β-hairpin peptides are Horseshoe crab (Limulus polyphemus) peptides, tachyplesins, and polyphemusin II, which are stabilized via two disulfide bridges; thanatin from insects (one disulfide bond); and protegrin-1, peptides isolated from porcine leukocytes. These molecules form an antiparallel β-sheet connected to a β-turn and are composed of disulfide bonds (Kawano et al. 1990; Tamamura et al. 1993). As shown by NMR studies, lactoferricin B, containing 25 amino acids, presents a β-sheet structure that stabilized by a single disulfide bridge (Hwang et al. 1998). Some of these AMPs are illustrated in Fig. 3.
AMPs Rich in Regular Amino Acids
Some of the AMPs contain high numbers of regular amino acid residues and therefore their structures are different from the regular α-helical or β-sheet peptides. For example, histatin, a peptide isolated from human saliva is histidine-rich AMPs and also effective against Candida albicans (Xu et al. 1991). Cathelicidins, a family of endogenous AMPs, are proline-rich and show irregular structures (Tomasinsig and Zanetti 2005). Indolicidin, a cationic AMP that consists of only 13 amino acids, has the highest tryptophan content, and tritripticin like indolicidin is rich in tryptophan (Bacalum et al. 2017; Falla et al. 1996). Bactenecins are a class of arginine-rich AMPs of bovine neutrophil granules (Ciociola et al. 2018).
AMPs with Unnatural Amino Acids
Some of the AMPs contain unnatural amino acids such as peptides from bacteria themselves. Nisin, a lantibiotic, is a notable example of AMPs from this group. It is produced by Lactococcus lactis and contains rare amino acids like lanthionine, 3-methyllanthionine, dehydroalanine, and dehydrobutyrine (de Vos et al. 1993; Zhang et al. 2014). Nisin Q, consisting of 34 amino acids, is a ribosomally-synthesized AMP and contains post-translationally modified residues such as lanthionine and dehydroalanine (Fukao et al. 2008). Leucocin A is another peptide that composes of 37 amino acids and isolated from Leuconostoc gelidum. It can form an amphiphilic conformation and could interact with cell membranes (Fregeau Gallagher et al. 1997). These AMPs undergo post-translational modifications and after that, their conformations could not see in other classes of AMPs. For example, subtilin is a ribosomally synthesized AMP that composes of several unusual amino acids as a result of post-translational modifications (Liu and Hansen 1993). Gramicidin is another example that contains several DH-amino acids that result in forming an unusual cyclic β-hairpin (Gibbs et al. 1998). Cbf-14, derived from cathelicidin-BF, is effective against antibiotic-resistant bacteria. In this AMP, lysine or leucine was substituted with similar unnatural amino acids such as ornithine (Orn) and norleucine (Ile) to generate AMPs with enhanced antibacterial activities. The data indicated that the mutant of Cbf-14 possesses potent antibacterial activities against penicillin-resistant bacteria (Kang et al. 2017).
The Mode of AMPs Action
It was shown that the main mechanism of AMPs is presumably to include the establishment of membrane pore or ion channel, without stereo-special interactions with chiral receptors (Toke 2005). These observations were also confirmed by others that showed D enantiomers of melittin, magainin 2 amide, and cecropin A were assayed and discovered to show the identical hemolytic and antibacterial efficacies as their L counterparts that naturally occurred. Furthermore, the compounds all generated one channel conductance in lipid bilayers, with the L, as well as D analogs, inducing the identical extent of conductivity (Boparai and Sharma 2020; Wade et al. 1990). AMPs attain dynamic interchanges in their topologies and structures upon interacting with cell membranes in microorganisms (Sansom 1998).
The outer surface of eukaryotic cells is composed of sphingomyelin phospholipids and zwitterionic phosphatidylcholine, whereas the outer surface of prokaryotic cells is negatively charged, because of the existence of teichoic acid or lipopolysaccharides (Dolis et al. 1997). The electrostatic interactions of AMPs with the negatively charged molecules on membranes seem to be the preliminary mechanism responsible for antimicrobial activities (Boparai and Sharma 2020). AMPs apply their activities in host cells through translocating across the cell membranes and prevent fundamental cellular processes like cell wall synthesis, enzymatic activities, as well as nucleic acid and protein synthesis (Brogden 2005). Particular other factors like outer membrane fluidity, molecular architecture, the concentration of negatively charged molecules, as well as outer membrane charge and magnitude also are important for the transport of AMPs across the biological membranes (Kondejewski et al. 1999). The membrane fluidity was determined to regulate the insertion and adsorption of antimicrobial peptides into the cell membranes.
According to the mechanisms of action, AMPs are classified widely into non-membrane acting and membrane acting peptides. Three mechanisms were suggested for pore-forming by membrane acting peptides (Boparai and Sharma 2020). The barrel-stave mechanism refers to insert AMPs into the biological membrane hydrophobic substance that flip inward and create pores through producing trans-membrane helical bundles (Peters et al. 2010). In the carpet-like mechanism, AMPs destruct the cell membranes assembly via their collaborative activity. In this path, AMPs self-associate onto the acidic phospholipids-rich areas located at lipid bilayer, and as soon as their concentrations approach specific thresholds, they can permeate into biological membranes. This phenomenon is facilitated via escalating in the positive potential of lipid bilayers (Mookherjee and Hancock 2007). Toroidal refers to build toroidal pore in a lipid bilayer by the AMPs. Pore constructions are administrated through the helix bundles and lipid polar head groups, which vertically orient to the external section of membranes. In other words, the AMPs cumulate and impel the lipid monolayers for continuous bending through the pore so that both lipid head groups as well as inserted peptides line the water core (Rahnamaeian 2011). The related mechanisms were illustrated in Fig. 4.
The permeabilizing non-membrane peptides are mostly represented by the capability for translocating across the cell membranes without permeabilizing the membrane, whereas the permeabilizing membrane peptides are cationic peptides with the ability to form the transient pores on the biological membranes (Pushpanathan et al. 2013). Particular AMPs inducing trans-membrane pores on the membrane of target cells comprise LL-37 (Harder et al. 2007), magainins (Hallock et al. 2003), melittin (Yang et al. 2001), and defensin (Schneider et al. 2005a). AMPs like mersacidin (Brotz et al. 1995), pyrrhocidin (Kragol et al. 2001), indolicidin (Friedrich et al. 2001), pleurocidin (Patrzykat et al. 2002), HNP-1 (Lee et al. 2002), dermaseptin (Patrzykat et al. 2002), and buforin II (Park et al. 2000) get translocated across the biological membranes and suppress important cellular processes that result in cells death. Besides, some AMPs like lactoferrin (Patrzykat et al. 2002), histatin (Kavanagh and Dowd 2004), melittin (Park and Lee 2010), and papiliocin (Hwang et al. 2011) apply their antimicrobial activity by the formation of reactive oxygen species.
Human AMPs
Human AMPs protect the human from microbial infection by various mechanisms. They have been identified in a variety of tissues or surfaces such as eyes, skin, ears, mouth, lungs, intestines, and also the urinary tract (Wang 2014). Some of the most important ones with more details are summarized here. Some important features of these AMPs are summarized in Table 1.
Human Defensins
α-Defensins were the first human group of AMPs to be characterized which were isolated from human blood. They are mostly cationic peptides containing between 29 and 35 amino acid residues. They have six cysteine residues that form three disulphide bonds. The cysteine bridges are between C1–C6, C2–C4, and C3–C5 that form cyclic peptides with a typical structure of a triple-stranded β-sheet and a β-hairpin loop (Ryley 2001; Wang 2014). Based on the source, property, and size, these peptides α-defensins are categorized into four groups: (I) HNP-1, HNP-2, and HNP-3; these three AMPs have almost identical amino acid sequences. In comparison with HNP-2, HNP-1 and HNP-3 contain only one additional residue at the N-terminus: alanine in HNP-1 and aspartate in HNP-3. (II) The fourth human neutrophil defensin, HNP-4, has a distinct peptide sequence with 33 amino acid residues. (III) HD-5 was obtained from human Paneth cells and present in the female reproductive tract. (IV) HD-6 ones are only expressed in the Paneth cells of human intestines (Schneider et al. 2005; Wang 2014). The human β-defensin family (hBD) was different from α-defensins. The disulfide bonds of β-defensins are between C1–C5, C2–C4, and C3–C6. Also, these AMPs have a slightly longer sequence than α-defensins that leads to form an additional helical region. The related structures are illustrated in Fig. 5.
Spectrum of Activity
They are active against a wide spectrum of both Gram-positive and -negative bacteria, mycobacteria, fungi, and some enveloped viruses (Winter and Wenghoefer 2012). For example, at concentrations higher than 100 μg/ml, HNP-1 and HNP-2 could kill the bacteria, including both intracellular and extracellular microorganisms (Lehrer et al. 1993). HNP-1 exerts potent in vitro microbicidal activity against a wide range of human pathogens, such as Staphylococcus aureus. Based on the evidence, HNP-1 could target and disrupt the bacterial membrane (Xiong et al. 1999). HNP1–4 and HD-5 show antibacterial activities against Gram-positive bacteria, such as S. aureus, and also Gram-negative bacteria as Enterobacter aerogenes and Escherichia coli (Ericksen et al. 2005). HNP1 was also highly effective against Mycobacterium tuberculosis (Sharma et al. 2000, 2001). HNP1–3 by binding to the lethal factor of the anthrax pathogen, Bacillus anthracis, was able to inhibition of its enzymatic activity (Verma et al. 2007). HD-5 shows potent antimicrobial activities against a wide range of pathogens such as E. coli, Listeria monocytogenes, Salmonella typhimurium, and C. albicans, whereas minimal inhibitory concentration (MIC) values are in the nanomolar range (Porter et al. 1997). hBD-1, -2, -3 showed antibacterial activities against E. coli and S. aureus in a dose-dependent manner (Chen et al. 2005). hBD-3 showed a broad spectrum of antimicrobial activities against different pathogenic microbes such as multiresistant S. aureus, vancomycin-resistant Enterococcus faecium, Pseudomonas aeruginosa, Klebsiella pneumonia, Streptococcus pneumoniae, and Burkholderia cepacia. This AMP has also antifungal activities against Candida glabrata and synergistic effects with fluconazole were observed (Dhople et al. 2006; Harder et al. 2001; Inthanachai et al. 2020; Pazgier et al. 2006b). The in vitro activities of hBD-3 alone or combined with other antimicrobial agents were investigated and the results showed that it was effective against Streptococcus mutans, Streptococcus sanguinis, Streptococcus sobrinus, Lactobacillus acidophilus, and Porphyromonas gingivalis. The bactericidal activities were enhanced in combination with the antimicrobial agents (Maisetta et al. 2003). Antimicrobial effect of hBD-4 on Fusobacterium nucleatum and P. gingivalis has been studied and the results showed antibacterial activity (Zhai et al. 2019). hBD‐4 exhibits potent antibacterial activity against P. aeruginosa (MIC = : 4.1 μg/ml) (Garcia et al. 2001). These AMPs also showed the anti-viral activities and at natural levels they could provide a minimal level of defense against viral infections (Park et al. 2018).
Mechanism of Action
It was assumed that the antibacterial activities the defensins are composed of a two-stage mode of action. It should be noted that there is the same mechanism of action for both types of defensins. At first, they bond to the outer membrane of the bacteria that leads to access to the inner (cytoplasmic) membrane. Then, by internalization into the cytoplasmic membrane, they form channels in the bacterial surfaces (Ryley 2001).
In more detail, these AMPs interact with the divalent cationic binding sites such as Ca2+ and Mg2+ in the lipopolysaccharide of bacterial surface and displacing these cations. In the case of Gram-positive bacteria, AMPs could interact with the anionic lipoteichoic acid of the cell resulting in access to the cytoplasmic membrane (Malanovic and Lohner 2016). It should be noted that the size of AMPs has direct influences on the distortion of the outer layer and then access to the underlying cytoplasmic layer (White et al. 1995).
The second stage is similar for both Gram-positive and -negative bacteria. The cationic residues interact with the negatively charged membrane and then because of high electrical potential, the AMPs being inserted into the membrane. Following the aggregation of AMPs in the membrane, they could form the channels, and finally, leads to membrane permeabilization and disruption (Ryley 2001).
Mechanism of Bacterial Resistance
The mechanisms of antibacterial resistance are poorly understood, however, it was assumed that changing in LPS structure leads to make it less susceptible to AMPs, and therefore binding of peptides is restricted and antibacterial resistance will be developed (Ryley 2001).
Cathelicidins
Cathelicidins, along with defensins belong to the group of cationic AMPs with amphipathic properties and recognize as an integral part of the immune system (De Smet and Contreras 2005). They are mainly stored in neutrophil and macrophage granules and show a direct antimicrobial activity against a wide range of microbial pathogens via the oxygen-independent activities (Bals and Wilson 2003; Tomasinsig and Zanetti 2005). Despite a large number of cathelicidin family members in animals, to date, only a single cathelicidin, LL-37, has been known in humans (Ryley 2001). This AMP is encoded by the cathelicidin gene (CAMP) which is composed of 39 residues, two leucine residues at N-terminal, and 37 residues long and has a molecular weight of 18 kDa (Agerberth et al. 1995). Subsequently proteolysis of the precursor, possibly by elastase digestion, in the neutrophil and missing the terminal residues, a 37 amino acid peptide was released and consequently termed LL-37 (Gudmundsson et al. 1996).
LL-37 has an α-helix structure that lacks cysteine. Therefore, it has a linear structure without disulphide bonds. It is expressed in leukocytes such as neutrophils, monocytes, NK cells, T cells, and B cells, and also, like hBD-2, in human epithelial tissue such as testis, skin, and the gastrointestinal and respiratory tracts in the presence of inflammation and is possibly related to the interleukin-6 production (De Smet and Contreras 2005; Frohm Nilsson et al. 1999).
Spectrum of Activity
LL-37 shows a wide spectrum of antibacterial activities against both Gram-positive and negative bacteria, with the MIC values lower than those of defensins (Turner et al. 1998). However, unlike defensins, it presents little activity against C. albicans or herpesviruses. This AMP is also inactive against some bacteria, like B. cepacia, that is naturally resistant to cationic peptides (Ryley 2001). In vitro antibacterial activities, LL-37 against Legionella pneumophila was tested and the results indicated that the AMP displayed broad-spectrum and in vitro activity against L. pneumophila (Birteksoz-Tan et al. 2019). Synergic enhancement of activity was observed between LL-37 and alpha-defensin against both E. coli and S. aureus (Nagaoka et al. 2000). In a study, the antibacterial effects of this peptide were evaluated against methicillin-resistant S. aureus (MRSA) and multidrug-resistant P. aeruginosa. Additionally, the antipseudomonal activities of colistin or imipenem combined to LL-37 were also studied. The results of the study revealed the rapid antibacterial effects of LL-37 against both antibiotic susceptible and resistant bacterial strains. When antibiotics were combined with LL-37, the MIC values of colistin and imipenem decreased up to eight-fold and four-fold, respectively (Geitani et al. 2019). In a study, the in vitro anti-Helicobacter pylori activity of LL-37 in simulated gastric juice was assessed and the results showed that after incubation the antibacterial activity was not retained (Leszczynska et al. 2009). It was shown that LL-37 and its fragments exerted antibacterial activities against drug-resistant Acinetobacter baumannii strains. This AMP and two of its fragments also could inhibit biofilm formation by A. baumannii (Feng et al. 2013). Microscopy studies show that the treatment of fluconazole-resistant Candida strains with LL-37 causes Candida cells to undergo surface changes that indicating surface membrane damage (Durnas et al. 2016). LL-37 is also effective against mycobacteria and can kill Mycobacterium smegmatis, Mycobacterium bovis BCG, and Mycobacterium tuberculosis under in vitro growth conditions, additionally, this AMP reduced the intracellular survival of the mycobacteria remarkably (Sonawane et al. 2011).
Mechanism of Action
They act as antimicrobial agents by either directly killing the pathogen or indirectly by binding to the bacterial exopolysaccharides, outer bacterial cell wall components such as the lipopolysaccharide (LPS) layer in the Gram-negative or the teichoic acids in the Gram-positive bacteria (Ramanathan et al. 2002; Xhindoli et al. 2016). Amphipathicity is an important factor for effective antibacterial properties. This attitude is more pronounced for α-helical cathelicidins such as LL-37. These peptides can form ion channels or aqueous pores in the bacterial membrane and rapidly permeabilize the membranes of microbial pathogens (Gennaro et al. 1998; Oren et al. 1999). Additionally, it was observed that LL-37 could strongly bind to zwitterionic or acidic phospholipid membranes of the vesicles and leads to leakage of vesicular content (Oren et al. 1999). Then the peptide forms quite sizeable toroidal pores. In more detail, at the first step, LL-37 is electrostatically attracted by membranes followed by assembly and partial integration. In the next step and after full integration into the lipid bilayer, the peptide forms channels based on peptide-peptide and peptide-lipid interactions. These interactions initiate the conformational changes and the formation of fiber-like oligomers on the inner membrane. The fibers lead to increasing the local concentration of the peptide that finally will interfere with the bacterial membrane stability. These transient pores allow the peptide to translocate into the bacterium. At this site, the peptide may interfere with internal targets such as DNA and also vital processes such as transcription (Brogden 2005; Wang 2008; Xhindoli et al. 2016). Moreover, in Gram-negative bacteria, LPS may be translocated apart from the bacterial cell wall to build holes for the LL-37 translocation into the periplasmic space (Vandamme et al. 2012).
Mechanism of Bacterial Resistance
It was assumed that the changes in the structure of LPS in Gram-negative bacteria affect the binding properties of LL-37. For example, surface structures containing phosphorylcholine, a component of eukaryote cell membranes, have been found in some respiratory pathogens such as Haemophilus influenza. The Mutants that do not express this structure were 1000-fold more sensitive to LL-37 than those having this lipid in their membrane (Lysenko et al. 2000). It was also shown that Neisseria gonorrhoeae has a type of efflux pump system that can reduce the susceptibility of the bacteria to LL-37 (Miyasaki et al. 1990). In Bordetella pertussis, d-alanine incorporation induces resistance to the AMPs. It was demonstrated that the dra operon of B. pertussis is responsible for the d-alanylation of the outer membrane component. Mutation in this operon results in increasing sensitivity of bacteria to LL-37, and other AMPs (Taneja et al. 2013).
Additionally, the antibacterial resistance against AMPs might be due to the production of degradation enzymes such as proteases AMPs. Bacterial strains can promote bacterial resistance to the AMPs by their proteolytic degradation properties (Abdi et al. 2019).
Histatin
Histatins are a family of cationic small AMPs that are largely composed of histidine-rich repeats. They have a molecular weight of about 3–4 kDa and are produced by the submandibular, sublingual, and parotid glands and secreted into human saliva (De Smet and Contreras 2005).
These AMPs consist of several members, however, among them, histatin 1, 3, and 5 are the most important ones. They have linear structures and composed of 38, 32, and 24 amino acid residues for histatin 1, 3, and 5, respectively and each of them contains seven histidine residues. Histatin 1 and 3 are encoded by two highly related genes, HIS1 and HIS2, respectively and others are produced by the cleavage of these two peptides. For example, histatin 5 was proteolytic digestion of histatin 3 (Wang 2014). Histatin 5 has a unique secondary structure. This AMP forms a random coil structure in aqueous solvents while in non-aqueous solvents the structure converts to α-helix (Helmerhorst et al. 1997; Raj et al. 1998).
Spectrum of Activity
These peptides possess some bactericidal activities and, more importantly, fungicidal properties. It should be noted that of all histatins, histatin 5 has the strongest antimicrobial activity, and most of the research on histatins has focused on this peptide (Khurshid et al. 2016; Oppenheim et al. 1988). The in vitro study indicated that histatin 5 could inhibit Candida species (C. albicans, C. glabrata, C. krusei) at a certain concentration (15–30 μM). It was also demonstrated that the analog of histatin 3 with the shorter amino acid sequences has the same candidacidal activity with respect to the full-length molecule (Raj et al. 1990).
These AMPs possess their anti-fungal activities in different ways such as growth inhibitory actions on C. albicans or inhibition of the conversion of C. albicans yeast growth into hyphal growth (Moffa et al. 2015).
The antimicrobial activities against other pathogens Cryptococcus neoformans and Aspergillus fumigatus has also been reported (Helmerhorst et al. 1999b). Also, histatin 5 virucidal activity and only histatin 5 derived peptides affect HIV-1 (Wang 2014). Inhibitory and bactericidal activities of histatins have also been reported against various bacteria, including S. mutans, P. gingivalis, P. aeruginosa, E. coli, and S. aureus (Gusman et al. 2001; MacKay et al. 1984; Sajjan et al. 2001).
Mechanism of Action
Unlike other AMPs, it is well-known that all targets of histatins are intracellular and the primary mode of actions is not related to lyse lipid membranes (Puri and Edgerton 2014; Ryley 2001; Wang 2014). It has been found that the massive non-lytic release of ATP along with the decrease in intracellular ATP levels is the main mechanism of actions of histatins (Helmerhorst et al. 1999a; Koshlukova et al. 1999). It was shown that histatin 5 could bind to the cell wall proteins and glycans of C. albicans and then is taken up by the fungal polyamine transporters in the cells in an energy-dependent manner. Inside the fungal cells, the AMP may affect the mitochondrial functions and leads to oxidative stress. However, cell death is the result of other processes such as volume dysregulation and ion imbalance caused by osmotic stress. Additionally, the metal-binding ability of histatin 5 is the other mentioned mechanism (Edgerton and Koshlukova 2000; Khurshid et al. 2017a; Komatsu et al. 2011; Puri and Edgerton 2014). Taken together, the key steps in the histatin 5 antifungal activity involve a bioenergetic collapse of C. albicans, consequently decreasing the mitochondrial ATP synthesis.
The other reported fungistatic and fungicidal mechanisms include disruption of the plasma membrane, which leads to the loss of intracellular constituents (Oppenheim et al. 1988). It was also found that these AMPs were also effective in killing the yeast cells by damaging their membranes and releasing potassium ions. These effects were related to the binding to the Trk1 potassium transporter and then the loss of intracellular components (Swidergall and Ernst 2014). The other modifications caused by histatin 5 in C. albicans included organelles in disarray, intracellular membrane disarrangement, and central cavities with deformed structures displaced to the cell periphery (Isola et al. 2007).
It was found that the histatins, like other AMPs, could interact with extracellular actin and the extracellular actin might regulate histatin anti-fungal activities in the oral cavity. In a study, it was demonstrated that both histatin 3 and 5 interact with actin, however, and affect the actin structure (Blotnick et al. 2017).
Mechanism of Microbial Resistance
It was shown that cellular accumulation of the peptides is necessary for anti-fungal activities and also that accumulation of histatins depends on the availability of cellular energy. Therefore, respiratory mutants of the microorganisms which have undergone mutations in mitochondrial DNA exhibited resistance against histatins (Gyurko et al. 2000). Therefore, it was assumed that the altered membrane energetics is an important factor for developing resistance (Yeaman and Yount 2003). Additionally, histatin 5 is transported out of C. albicans cells by the Flu1 and Mrr1 efflux pumps (Hampe et al. 2017; Li et al. 2013). The multidrug resistance transporters CgTpo1_1 and CgTpo1_2 have critical roles in the virulence of C. glabrata infections such as resistance to histatin 5 (Santos et al. 2017). It was demonstrated that the upregulation of CgCDR efflux pumps could develop the resistance to histatin 5 in C. glabrata (Helmerhorst et al. 2006).
AMPs Challenges
Although AMPs often present antibacterial activities, own several unfavorable features for clinical applications, including (I) the pharmacokinetic profiles of antimicrobial peptides are not well known, and only a few clinical research have been performed with AMPs; (II) sensitivity to proteolysis process derived by bacterial proteases; and (III) cytotoxicity to the eukaryotic cell, which could result in neurotoxicity, nephrotoxicity, and hemolysis (Craik et al. 2013; Grassi et al. 2017). Nevertheless, several types of research have been conducted for improving our knowledge of the above-mentioned challenges (Chou et al. 2008). In comparison to conventional antibiotics, AMPs are also expensive for being synthesized on mass scales (Otvos and Wade 2014). Various strategies have been persuaded to overcome these limitations. For this scenario, employing nanocarriers, developing pegylated AMPs, and covalent immobilization of AMPs on surfaces have been mentioned (Long et al. 2017; Manteghi et al. 2020).
Covalent attachment of the polyethylene glycol polymer to peptides and proteins (PEGylation) could improve the bioavailability of AMPs and enhance their pharmacokinetic properties such as bio-distribution and rate of clearance. Additionally, the proteolytic degradation of AMPs might be decreased by protecting their C- and N-terminus (Gong et al. 2015; Gonzalez-Valdez et al. 2012; Khameneh et al. 2015b).
The progress in nanotechnology has permitted various nanoparticles to work as a favorable approach for minimizing the unfavorable features of synthetic as well as natural AMPs (Umerska et al. 2017). The researchers have suggested that AMPs in nanoparticles represent increased efficiency, decreased degradation, and lower toxicity (Wang et al. 2017). As a result, nanoparticles could contribute to the manufacturing of AMPs and their applications in the industry. Nano-carriers are considered as a drug delivery system that presents many profits, like improvement of drug pharmacokinetic profile, treatment selectivity, and the protection of AMPs against extracellular degradations (Sadat et al. 2016). Over the last 50 years, multiple drug delivery systems have been used for encapsulating drugs and other biomolecules, including polymeric nanoparticles, dendrimers, micelles, and liposomes (Malaekeh-Nikouei et al. 2020).
Nanobiotechnology has proposed two major procedures to encapsulate AMPs. The passive delivery, as non-directed procedures, includes traditional nano-delivery systems that do not own surface modifications for guiding the nano-carriers; this could be manipulated by control the nano-carriers shape as well as size (Makowski et al. 2019). Active targeting, as directed delivery, includes surface modifications of the nano-carriers with various ligands and/or other moieties for permitting interaction of nano-carriers and the target sites (Biswaro et al. 2018). However, developing viable drug delivery systems for clinical trials remains a challenge (Wimley and Hristova 2011). There are two procedures of nano-delivery systems that have some advantages and disadvantages (Makowski et al. 2019). Passive systems attend to own fewer agents in their combination in contrast to active systems (Biswaro et al. 2018). As passive systems possess fewer agents in their combination, this causes it easier for preparing them (Makowski et al. 2019). Besides, active systems contain modified surface-carrying ligands and/or other moieties for facilitating its interactions with infected cells and increase the drug transports at such particular sites (Biswaro et al. 2018), while passive systems involve encapsulating peptides without making available extra surface modifications (Hunter et al. 2012). Given the above-mentioned, the terms nano-delivery system as well as nano-carriers have been suggested (Makowski et al. 2019).
Conclusion
Clinical overuse of antibiotics unavoidably results in the growing emergence of drug-resistant strains of microbial pathogens. Consequently, the development of a new class of antibiotics is an urgent need. During the last decades, considerable efforts have been conducted for investigating the possible use of AMPs or synthetic derivatives as therapeutic antibacterial agents. They are implicated in several biological processes, and also have an important role in the innate immune system. The AMPs protect the host against both systemic and topical infections either alone or in combination with conventional antibiotics. Human AMPs are an important class of these peptides which attract attention for the treatment of various disease and some of them have entered clinical trials for use in clinical applications such as the treatment of diabetic ulcers as topical anti-infective agents for the treatment of microbial infections. The ongoing discovery and development of new AMPs are promising approaches in the fight against increasingly resistant pathogens.
Data Availability
This review was based on data extracted from published papers available in all relevant databases without limitation up to 1st July 2020.
References
Abdi M, Mirkalantari S, Amirmozafari N (2019) Bacterial resistance to antimicrobial peptides. J Pept Sci 25:e3210
Agerberth B, Guðmundsson G (2006) Host antimicrobial defence peptides in human disease. Antimicrobial peptides and human disease. Springer, Berlin, pp 67–90
Agerberth B, Gunne H, Odeberg J, Kogner P, Boman HG, Gudmundsson GH (1995) FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci USA 92:195–199
Bacalum M, Janosi L, Zorila F, Tepes AM, Ionescu C, Bogdan E et al (2017) Modulating short tryptophan- and arginine-rich peptides activity by substitution with histidine. Biochim Biophys Acta 1861:1844–1854
Bahar AA, Ren D (2013) Antimicrobial peptides. Pharmaceuticals (Basel) 6:1543–1575
Bals R, Wilson JM (2003) Cathelicidins—a family of multifunctional antimicrobial peptides. Cell Mol Life Sci 60:711–720
Bazzaz BSF, Fakori M, Khameneh B, Hosseinzadeh H (2019) Effects of omeprazole and caffeine alone and in combination with gentamicin and ciprofloxacin against antibiotic resistant Staphylococcus aureus and Escherichia coli strains. J Pharmacopuncture 22:49–54
Birteksoz-Tan AS, Zeybek Z, Hacioglu M, Savage PB, Bozkurt-Guzel C (2019) In vitro activities of antimicrobial peptides and ceragenins against Legionella pneumophila. J Antibiot 72:291–297
Biswaro LS, da Costa Sousa MG, Rezende TMB, Dias SC, Franco OL (2018) Antimicrobial peptides and nanotechnology, recent advances and challenges. Front Microbiol 9:855
Blotnick E, Sol A, Bachrach G, Muhlrad A (2017) Interactions of histatin-3 and histatin-5 with actin. BMC Biochem 18:3
Boparai JK, Sharma PK (2020) Mini review on antimicrobial peptides, sources, mechanism and recent applications. Protein Pept Lett 27:4–16
Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3:238–250
Brogden KA, Ackermann M, McCray PB Jr, Tack BF (2003) Antimicrobial peptides in animals and their role in host defences. Int J Antimicrob Agents 22:465–478
Brotz H, Bierbaum G, Markus A, Molitor E, Sahl HG (1995) Mode of action of the lantibiotic mersacidin: inhibition of peptidoglycan biosynthesis via a novel mechanism? Antimicrob Agents Chemother 39:714–719
Bulet P, Stocklin R, Menin L (2004) Anti-microbial peptides: from invertebrates to vertebrates. Immunol Rev 198:169–184
Chaudhary AS (2016) A review of global initiatives to fight antibiotic resistance and recent antibiotics discovery. Acta Pharm Sin B 6:552–556
Chen X, Niyonsaba F, Ushio H, Okuda D, Nagaoka I, Ikeda S et al (2005) Synergistic effect of antibacterial agents human beta-defensins, cathelicidin LL-37 and lysozyme against Staphylococcus aureus and Escherichia coli. J Dermatol Sci 40:123–132
Chou HT, Kuo TY, Chiang JC, Pei MJ, Yang WT, Yu HC et al (2008) Design and synthesis of cationic antimicrobial peptides with improved activity and selectivity against Vibrio spp. Int J Antimicrob Agents 32:130–138
Ciociola T, Giovati L, Giovannelli A, Conti S, Castagnola M, Vitali A (2018) The activity of a mammalian proline-rich peptide against Gram-negative bacteria, including drug-resistant strains, relies on a nonmembranolytic mode of action. Infect Drug Resist 11:969–979
Craik DJ, Fairlie DP, Liras S, Price D (2013) The future of peptide-based drugs. Chem Biol Drug Des 81:136–147
Cunliffe RJ (2003) α-Defensins in the gastrointestinal tract. Mol Immunol 40:463–467
De Smet K, Contreras R (2005) Human antimicrobial peptides: defensins, cathelicidins and histatins. Biotechnol Lett 27:1337–1347
de Vos WM, Mulders JW, Siezen RJ, Hugenholtz J, Kuipers OP (1993) Properties of nisin Z and distribution of its gene, nisZ, in Lactococcus lactis. Appl Environ Microbiol 59:213–218
Dhople V, Krukemeyer A, Ramamoorthy A (2006) The human beta-defensin-3, an antibacterial peptide with multiple biological functions. Biochim Biophys Acta 1758:1499–1512
Dimarcq JL, Bulet P, Hetru C, Hoffmann J (1998) Cysteine-rich antimicrobial peptides in invertebrates. Biopolymers 47:465–477
Dolis D, Moreau C, Zachowski A, Devaux PF (1997) Aminophospholipid translocase and proteins involved in transmembrane phospholipid traffic. Biophys Chem 68:221–231
Durnas B, Wnorowska U, Pogoda K, Deptula P, Watek M, Piktel E et al (2016) Candidacidal activity of selected ceragenins and human cathelicidin LL-37 in experimental settings mimicking infection sites. PLoS ONE 11:e0157242
Dürr UH, Sudheendra U, Ramamoorthy A (2006) LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochem Biophys Acta 1758:1408–1425
Edgerton M, Koshlukova SE (2000) Salivary histatin 5 and its similarities to the other antimicrobial proteins in human saliva. Adv Dent Res 14:16–21
Edwards IA, Elliott AG, Kavanagh AM, Blaskovich MAT, Cooper MA (2017) Structure-activity and -toxicity relationships of the antimicrobial peptide tachyplesin-1. ACS Infect Dis 3:917–926
Ericksen B, Wu Z, Lu W, Lehrer RI (2005) Antibacterial activity and specificity of the six human {alpha}-defensins. Antimicrob Agents Chemother 49:269–275
Falla TJ, Karunaratne DN, Hancock RE (1996) Mode of action of the antimicrobial peptide indolicidin. J Biol Chem 271:19298–19303
Farhadi F, Khameneh B, Iranshahi M, Iranshahy M (2019) Antibacterial activity of flavonoids and their structure-activity relationship: an update review. Phytother Res 33:13–40
Feng X, Sambanthamoorthy K, Palys T, Paranavitana C (2013) The human antimicrobial peptide LL-37 and its fragments possess both antimicrobial and antibiofilm activities against multidrug-resistant Acinetobacter baumannii. Peptides 49:131–137
Fox JL (2013) Antimicrobial peptides stage a comeback. Nat Biotechnol 31:379–382
Fregeau Gallagher NL, Sailer M, Niemczura WP, Nakashima TT, Stiles ME, Vederas JC (1997) Three-dimensional structure of leucocin A in trifluoroethanol and dodecylphosphocholine micelles: spatial location of residues critical for biological activity in type IIa bacteriocins from lactic acid bacteria. Biochemistry 36:15062–15072
Friedrich CL, Rozek A, Patrzykat A, Hancock RE (2001) Structure and mechanism of action of an indolicidin peptide derivative with improved activity against gram-positive bacteria. J Biol Chem 276:24015–24022
Frohm Nilsson M, Sandstedt B, Sorensen O, Weber G, Borregaard N, Stahle-Backdahl M (1999) The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect Immun 67:2561–2566
Fukao M, Obita T, Yoneyama F, Kohda D, Zendo T, Nakayama J et al (2008) Complete covalent structure of nisin Q, new natural nisin variant, containing post-translationally modified amino acids. Biosci Biotechnol Biochem 72:1750–1755
Ganz T (2001) Defensins in the urinary tract and other tissues. J Infect Dis 183:S41–S42
Ganz T, Selsted ME, Szklarek D, Harwig SS, Daher K, Bainton DF et al (1985) Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest 76:1427–1435
Garcia JR, Krause A, Schulz S, Rodriguez-Jimenez FJ, Kluver E, Adermann K et al (2001) Human beta-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J 15:1819–1821
Geitani R, Ayoub Moubareck C, Touqui L, Karam Sarkis D (2019) Cationic antimicrobial peptides: alternatives and/or adjuvants to antibiotics active against methicillin-resistant Staphylococcus aureus and multidrug-resistant Pseudomonas aeruginosa. BMC Microbiol 19:54
Gennaro R, Scocchi M, Merluzzi L, Zanetti M (1998) Biological characterization of a novel mammalian antimicrobial peptide. Biochim Biophys Acta 1425:361–368
Giangaspero A, Sandri L, Tossi A (2001) Amphipathic alpha helical antimicrobial peptides. Eur J Biochem 268:5589–5600
Gibbs AC, Kondejewski LH, Gronwald W, Nip AM, Hodges RS, Sykes BD et al (1998) Unusual beta-sheet periodicity in small cyclic peptides. Nat Struct Biol 5:284–288
Gong Y, Leroux JC, Gauthier MA (2015) Releasable conjugation of polymers to proteins. Bioconjug Chem 26:1172–1181
Gonzalez-Valdez J, Rito-Palomares M, Benavides J (2012) Advances and trends in the design, analysis, and characterization of polymer-protein conjugates for “PEGylaided” bioprocesses. Anal Bioanal Chem 403:2225–2235
Grassi L, Maisetta G, Esin S, Batoni G (2017) Combination strategies to enhance the efficacy of antimicrobial peptides against bacterial biofilms. Front Microbiol 8:2409
Gudmundsson GH, Agerberth B, Odeberg J, Bergman T, Olsson B, Salcedo R (1996) The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J Biochem 238:325–332
Gusman H, Travis J, Helmerhorst EJ, Potempa J, Troxler RF, Oppenheim FG (2001) Salivary histatin 5 is an inhibitor of both host and bacterial enzymes implicated in periodontal disease. Infect Immun 69:1402–1408
Gyurko C, Lendenmann U, Troxler RF, Oppenheim FG (2000) Candida albicans mutants deficient in respiration are resistant to the small cationic salivary antimicrobial peptide histatin 5. Antimicrob Agents Chemother 44:348–354
Hallock KJ, Lee DK, Ramamoorthy A (2003) MSI-78, an analogue of the magainin antimicrobial peptides, disrupts lipid bilayer structure via positive curvature strain. Biophys J 84:3052–3060
Hampe IAI, Friedman J, Edgerton M, Morschhäuser J (2017) An acquired mechanism of antifungal drug resistance simultaneously enables Candida albicans to escape from intrinsic host defenses. PLoS Pathog 13:e1006655
Hancock RE (2001) Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect Dis 1:156–164
Harder J, Bartels J, Christophers E, Schroder JM (2001) Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J Biol Chem 276:5707–5713
Harder J, Glaser R, Schroder JM (2007) Human antimicrobial proteins effectors of innate immunity. J Endotoxin Res 13:317–338
Helmerhorst EJ, Van’t Hof W, Veerman EC, Simoons-Smit I, Nieuw Amerongen AV (1997) Synthetic histatin analogues with broad-spectrum antimicrobial activity. Biochem J 326(Pt 1):39–45
Helmerhorst EJ, Venuleo C, Sanglard D, Oppenheim FG (2006) Roles of cellular respiration, CgCDR1, and CgCDR2 in Candida glabrata resistance to histaten 5. Antimicrob Agents Chemother 50:1100–1103
Helmerhorst EJ, Breeuwer P, van’t Hof W, Walgreen-Weterings E, Oomen LC, Veerman EC et al (1999) The cellular target of histatin 5 on Candida albicans is the energized mitochondrion. J Biol Chem 274:7286–7291
Helmerhorst EJ, Reijnders IM, van’t Hof W, Simoons-Smit I, Veerman EC, Amerongen AV (1999) Amphotericin B- and fluconazole-resistant Candida spp., Aspergillus fumigatus, and other newly emerging pathogenic fungi are susceptible to basic antifungal peptides. Antimicrob Agents Chemother 43:702–704
Hill CP, Yee J, Selsted ME, Eisenberg D (1991) Crystal structure of defensin HNP-3, an amphiphilic dimer: mechanisms of membrane permeabilization. Science 251:1481–1485
Huang Y, Huang J, Chen Y (2010) Alpha-helical cationic antimicrobial peptides: relationships of structure and function. Protein Cell 1:143–152
Hunter AC, Elsom J, Wibroe PP, Moghimi SM (2012) Polymeric particulate technologies for oral drug delivery and targeting: a pathophysiological perspective. Maturitas 73:5–18
Hwang PM, Vogel HJ (1998) Structure-function relationships of antimicrobial peptides. Biochem Cell Biol 76:235–246
Hwang PM, Zhou N, Shan X, Arrowsmith CH, Vogel HJ (1998) Three-dimensional solution structure of lactoferricin B, an antimicrobial peptide derived from bovine lactoferrin. Biochemistry 37:4288–4298
Hwang B, Hwang JS, Lee J, Kim JK, Kim SR, Kim Y et al (2011) Induction of yeast apoptosis by an antimicrobial peptide, Papiliocin. Biochem Biophys Res Commun 408:89–93
Inthanachai T, Thammahong A, Edwards SW, Virakul S, Kiatsurayanon C, Chiewchengchol D (2020) The inhibitory effect of human beta-defensin-3 on Candida Glabrata isolated from patients with Candidiasis. Immunol Invest. https://doi.org/10.1080/08820139.2020.1755307
Isola R, Isola M, Conti G, Lantini MS, Riva A (2007) Histatin-induced alterations in Candida albicans: a microscopic and submicroscopic comparison. Microsc Res Tech 70:607–616
Javia A, Amrutiya J, Lalani R, Patel V, Bhatt P, Misra A (2018) Antimicrobial peptide delivery: an emerging therapeutic for the treatment of burn and wounds. Ther Deliv 9:375–386
Kalmodia S, Son K-N, Cao D, Lee B-S, Surenkhuu B, Shah D et al (2019) Presence of histatin-1 in human tears and association with aqueous deficient dry eye diagnosis: a preliminary study. Sci Rep 9:1–10
Kang W, Liu H, Ma L, Wang M, Wei S, Sun P et al (2017) Effective antimicrobial activity of a peptide mutant Cbf-14-2 against penicillin-resistant bacteria based on its unnatural amino acids. Eur J Pharm Sci 105:169–177
Kavanagh K, Dowd S (2004) Histatins: antimicrobial peptides with therapeutic potential. J Pharm Pharmacol 56:285–289
Kawano K, Yoneya T, Miyata T, Yoshikawa K, Tokunaga F, Terada Y et al (1990) Antimicrobial peptide, tachyplesin I, isolated from hemocytes of the horseshoe crab (Tachypleus tridentatus). NMR determination of the beta-sheet structure. J Biol Chem 265:15365–15367
Khameneh B, Iranshahy M, Ghandadi M, Ghoochi Atashbeyk D, Fazly Bazzaz BS, Iranshahi M (2015) Investigation of the antibacterial activity and efflux pump inhibitory effect of co-loaded piperine and gentamicin nanoliposomes in methicillin-resistant Staphylococcus aureus. Drug Dev Ind Pharm 41:989–994
Khameneh B, Jaafari MR, Hassanzadeh-Khayyat M, Varasteh A, Chamani J, Iranshahi M et al (2015) Preparation, characterization and molecular modeling of PEGylated human growth hormone with agonist activity. Int J Biol Macromol 80:400–409
Khameneh B, Diab R, Ghazvini K, Fazly Bazzaz BS (2016) Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb Pathog 95:32–42
Khameneh B, Iranshahy M, Soheili V, Fazly Bazzaz BS (2019) Review on plant antimicrobials: a mechanistic viewpoint. Antimicrob Resist Infect Control 8:118
Khameneh B, Iranshahy M, Vahdati-Mashhadian N, Sahebkar A, Fazly Bazzaz BS (2019) Non-antibiotic adjunctive therapy: a promising approach to fight tuberculosis. Pharmacol Res 146:104289
Khurshid Z, Naseem M, Sheikh Z, Najeeb S, Shahab S, Zafar MS (2016) Oral antimicrobial peptides: types and role in the oral cavity. Saudi Pharm J 24:515–524
Khurshid Z, Najeeb S, Mali M, Moin SF, Raza SQ, Zohaib S et al (2017a) Histatin peptides: pharmacological functions and their applications in dentistry. Saudi Pharm J 25:25–31
Khurshid Z, Najeeb S, Mali M, Moin SF, Raza SQ, Zohaib S et al (2017b) Histatin peptides: Pharmacological functions and their applications in dentistry 25:25–31
Koehbach J, Craik DJ (2019) The vast structural diversity of antimicrobial peptides. Trends Pharmacol Sci 40:517–528
Komatsu T, Salih E, Helmerhorst EJ, Offner GD, Oppenheim FG (2011) Influence of histatin 5 on Candida albicans mitochondrial protein expression assessed by quantitative mass spectrometry. J Proteome Res 10:646–655
Kondejewski LH, Jelokhani-Niaraki M, Farmer SW, Lix B, Kay CM, Sykes BD et al (1999) Dissociation of antimicrobial and hemolytic activities in cyclic peptide diastereomers by systematic alterations in amphipathicity. J Biol Chem 274:13181–13192
Koshlukova SE, Lloyd TL, Araujo MW, Edgerton M (1999) Salivary histatin 5 induces non-lytic release of ATP from Candida albicans leading to cell death. J Biol Chem 274:18872–18879
Kragol G, Lovas S, Varadi G, Condie BA, Hoffmann R, Otvos L Jr (2001) The antibacterial peptide pyrrhocoricin inhibits the ATPase actions of DnaK and prevents chaperone-assisted protein folding. Biochemistry 40:3016–3026
Lee MK, Cha L, Lee SH, Hahm KS (2002) Role of amino acid residues within the disulfide loop of thanatin, a potent antibiotic peptide. J Biochem Mol Biol 35:291–296
Lee C-C, Sun Y, Qian S, Huang HW (2011) Transmembrane pores formed by human antimicrobial peptide LL-37. Biophys J 100:1688–1696
Lehrer RI, Lichtenstein AK, Ganz T (1993) Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol 11:105–128
Leszczynska K, Namiot A, Fein DE, Wen Q, Namiot Z, Savage PB et al (2009) Bactericidal activities of the cationic steroid CSA-13 and the cathelicidin peptide LL-37 against Helicobacter pylori in simulated gastric juice. BMC Microbiol 9:187
Li R, Kumar R, Tati S, Puri S, Edgerton M (2013) Candida albicans Flu1-mediated efflux of salivary histatin 5 reduces its cytosolic concentration and fungicidal activity. Antimicrob Agents Chemother 57:1832–1839
Liu W, Hansen JN (1993) The antimicrobial effect of a structural variant of subtilin against outgrowing Bacillus cereus T spores and vegetative cells occurs by different mechanisms. Appl Environ Microbiol 59:648–651
Long T, Li H, Wang X, Yue B (2017) A comparative analysis of antibacterial properties and inflammatory responses for the KR-12 peptide on titanium and PEGylated titanium surfaces. RSC Adv 7:34321–34330
Lysenko ES, Gould J, Bals R, Wilson JM, Weiser JN (2000) Bacterial phosphorylcholine decreases susceptibility to the antimicrobial peptide LL-37/hCAP18 expressed in the upper respiratory tract. Infect Immun 68:1664–1671
MacKay BJ, Pollock JJ, Iacono VJ, Baum BJ (1984) Isolation of milligram quantities of a group of histidine-rich polypeptides from human parotid saliva. Infect Immun 44:688–694
Maisetta G, Batoni G, Esin S, Luperini F, Pardini M, Bottai D et al (2003) Activity of human beta-defensin 3 alone or combined with other antimicrobial agents against oral bacteria. Antimicrob Agents Chemother 47:3349–3351
Makowski M, Silva IC, Pais do Amaral C, Goncalves S, Santos NC (2019) Advances in Lipid And Metal Nanoparticles For Antimicrobial Peptide Delivery. Pharmaceutics 11:588
Malaekeh-Nikouei B, Fazly Bazzaz BS, Mirhadi E, Tajani AS, Khameneh B (2020) The role of nanotechnology in combating biofilm-based antibiotic resistance. J Drug Delivery Sci Technol 60:101880
Malanovic N, Lohner K (2016) Antimicrobial peptides targeting Gram-positive bacteria. Pharmaceuticals (Basel) 9:59
Manteghi R, Pallagi E, Olajos G, Csoka I (2020) Pegylation and formulation strategy of anti-microbial peptide (AMP) according to the quality by design approach. Eur J Pharm Sci 144:105197
Miyasaki KT, Bodeau AL, Ganz T, Selsted ME, Lehrer RI (1990) In vitro sensitivity of oral, gram-negative, facultative bacteria to the bactericidal activity of human neutrophil defensins. Infect Immun 58:3934–3940
Moffa EB, Mussi MC, Xiao Y, Garrido SS, Machado MA, Giampaolo ET et al (2015) Histatin 5 inhibits adhesion of C. albicans to reconstructed human oral epithelium. Front Microbiol 6:885
Mookherjee N, Hancock RE (2007) Cationic host defence peptides: innate immune regulatory peptides as a novel approach for treating infections. Cell Mol Life Sci 64:922–933
Nagaoka I, Hirota S, Yomogida S, Ohwada A, Hirata M (2000) Synergistic actions of antibacterial neutrophil defensins and cathelicidins. Inflamm Res 49:73–79
Neville F, Cahuzac M, Konovalov O, Ishitsuka Y, Lee KYC, Kuzmenko I et al (2006) Lipid headgroup discrimination by antimicrobial peptide LL-37: insight into mechanism of action. Biophys J 90:1275–1287
Oppenheim FG, Xu T, McMillian FM, Levitz SM, Diamond RD, Offner GD et al (1988) Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J Biol Chem 263:7472–7477
Oren Z, Lerman JC, Gudmundsson GH, Agerberth B, Shai Y (1999) Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem J 341(Pt 3):501–513
Otvos L Jr, Wade JD (2014) Current challenges in peptide-based drug discovery. Front Chem 2:62
Pardi A, Zhang XL, Selsted ME, Skalicky JJ, Yip PF (1992) NMR studies of defensin antimicrobial peptides. 2. Three-dimensional structures of rabbit NP-2 and human HNP-1. Biochemistry 31:11357–11364
Park C, Lee DG (2010) Melittin induces apoptotic features in Candida albicans. Biochem Biophys Res Commun 394:170–172
Park CB, Yi KS, Matsuzaki K, Kim MS, Kim SC (2000) Structure-activity analysis of buforin II, a histone H2A-derived antimicrobial peptide: the proline hinge is responsible for the cell-penetrating ability of buforin II. Proc Natl Acad Sci USA 97:8245–8250
Park SC, Park Y, Hahm KS (2011) The role of antimicrobial peptides in preventing multidrug-resistant bacterial infections and biofilm formation. Int J Mol Sci 12:5971–5992
Park MS, Kim JI, Lee I, Park S, Bae J-Y, Park M-S (2018) Towards the application of human defensins as antivirals. Biomol Ther 26:242
Patrzykat A, Friedrich CL, Zhang L, Mendoza V, Hancock RE (2002) Sublethal concentrations of pleurocidin-derived antimicrobial peptides inhibit macromolecular synthesis in Escherichia coli. Antimicrob Agents Chemother 46:605–614
Pazgier M, Hoover D, Yang D, Lu W, Lubkowski JJC (2006a) & CMLS MLS. Human β-defensins 63:1294–1313
Pazgier M, Hoover DM, Yang D, Lu W, Lubkowski J (2006b) Human beta-defensins. Cell Mol Life Sci 63:1294–1313
Peters BM, Shirtliff ME, Jabra-Rizk MA (2010) Antimicrobial peptides: primeval molecules or future drugs? PLoS Pathog 6:e1001067
Porter EM, van Dam E, Valore EV, Ganz T (1997) Broad-spectrum antimicrobial activity of human intestinal defensin 5. Infect Immun 65:2396–2401
Puri S, Edgerton M (2014) How does it kill?: understanding the candidacidal mechanism of salivary histatin 5. Eukaryot Cell 13:958–964
Pushpanathan M, Gunasekaran P, Rajendhran J (2013) Antimicrobial peptides: versatile biological properties. Int J Pept 2013:675391
Rahnamaeian M (2011) Antimicrobial peptides: modes of mechanism, modulation of defense responses. Plant Signal Behav 6:1325–1332
Raj PA, Edgerton M, Levine MJ (1990) Salivary histatin 5: dependence of sequence, chain length, and helical conformation for candidacidal activity. J Biol Chem 265:3898–3905
Raj PA, Marcus E, Sukumaran DK (1998) Structure of human salivary histatin 5 in aqueous and nonaqueous solutions. Biopolymers 45:51–67
Ramanathan B, Davis EG, Ross CR, Blecha F (2002) Cathelicidins: microbicidal activity, mechanisms of action, and roles in innate immunity. Microbes Infect 4:361–372
Reddy KV, Yedery RD, Aranha C (2004) Antimicrobial peptides: premises and promises. Int J Antimicrob Agents 24:536–547
Ryley HC (2001) Human antimicrobial peptides. Rev Med Microbiol 12:177–186
Sadat SM, Jahan ST, Haddadi A (2016) Effects of size and surface charge of polymeric nanoparticles on in vitro and in vivo applications. J Biomater Nanobiotechnol 7:91
Sajjan US, Tran LT, Sole N, Rovaldi C, Akiyama A, Friden PM et al (2001) P-113D, an antimicrobial peptide active against Pseudomonas aeruginosa, retains activity in the presence of sputum from cystic fibrosis patients. Antimicrob Agents Chemother 45:3437–3444
Sansom MS (1998) Peptides and lipid bilayers: dynamic interactions. Curr Opin Colloid Interface Sci 3:518–524
Santos R, Costa C, Mil-Homens D, Romão D, de Carvalho CCCR, Pais P et al (2017) The multidrug resistance transporters CgTpo1_1 and CgTpo1_2 play a role in virulence and biofilm formation in the human pathogen Candida glabrata. Cell Microbiol 19:e12686
Schneider JJ, Unholzer A, Schaller M, Schafer-Korting M, Korting HC (2005) Human defensins. J Mol Med (Berl) 83:587–595
Shah D, Ali M, Pasha Z, Jaboori AJ, Jassim SH, Jain S et al (2016) Histatin-1 expression in human lacrimal epithelium. PLoS ONE 11:e0148018
Sharma S, Verma I, Khuller GK (2000) Antibacterial activity of human neutrophil peptide-1 against Mycobacterium tuberculosis H37Rv: in vitro and ex vivo study. Eur Respir J 16:112–117
Sharma S, Verma I, Khuller GK (2001) Therapeutic potential of human neutrophil peptide 1 against experimental tuberculosis. Antimicrob Agents Chemother 45:639–640
Soheili V, Tajani AS, Ghodsi R, Bazzaz BSF (2019) Anti-PqsR compounds as next-generation antibacterial agents against Pseudomonas aeruginosa: a review. Eur J Med Chem 172:26–35
Sonawane A, Santos JC, Mishra BB, Jena P, Progida C, Sorensen OE et al (2011) Cathelicidin is involved in the intracellular killing of mycobacteria in macrophages. Cell Microbiol 13:1601–1617
Swidergall M, Ernst JF (2014) Interplay between Candida albicans and the antimicrobial peptide armory. Eukaryot Cell 13:950–957
Tamamura H, Kuroda M, Masuda M, Otaka A, Funakoshi S, Nakashima H et al (1993) A comparative study of the solution structures of tachyplesin I and a novel anti-HIV synthetic peptide, T22 ([Tyr5,12, Lys7]-polyphemusin II), determined by nuclear magnetic resonance. Biochim Biophys Acta 1163:209–216
Taneja NK, Ganguly T, Bakaletz LO, Nelson KJ, Dubey P, Poole LB et al (2013) d-alanine modification of a protease-susceptible outer membrane component by the Bordetella pertussis dra locus promotes resistance to antimicrobial peptides and polymorphonuclear leukocyte-mediated killing. J Bacteriol 195:5102–5111
Thennarasu S, Tan A, Penumatchu R, Shelburne CE, Heyl DL, Ramamoorthy A (2010) Antimicrobial and membrane disrupting activities of a peptide derived from the human cathelicidin antimicrobial peptide LL37. Biophys J 98:248–257
Theuretzbacher U, Outterson K, Engel A, Karlen A (2020) The global preclinical antibacterial pipeline. Nat Rev Microbiol 18:275–285
Toke O (2005) Antimicrobial peptides: new candidates in the fight against bacterial infections. Biopolymers 80:717–735
Tomasinsig L, Zanetti M (2005) The cathelicidins–structure, function and evolution. Curr Protein Pept Sci 6:23–34
Turner J, Cho Y, Dinh NN, Waring AJ, Lehrer RI (1998) Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother 42:2206–2214
Umerska A, Cassisa V, Bastiat G, Matougui N, Nehme H, Manero F et al (2017) Synergistic interactions between antimicrobial peptides derived from plectasin and lipid nanocapsules containing monolaurin as a cosurfactant against Staphylococcus aureus. Int J Nanomed 12:5687–5699
Vandamme D, Landuyt B, Luyten W, Schoofs L (2012) A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell Immunol 280:22–35
Verma C, Seebah S, Low SM, Zhou L, Liu SP, Li J et al (2007) Defensins: antimicrobial peptides for therapeutic development. Biotechnol J 2:1353–1359
Wade D, Boman A, Wahlin B, Drain CM, Andreu D, Boman HG et al (1990) All-D amino acid-containing channel-forming antibiotic peptides. Proc Natl Acad Sci USA 87:4761–4765
Wang G (2008) Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J Biol Chem 283:32637–32643
Wang G (2014) Human antimicrobial peptides and proteins. Pharmaceuticals (Basel) 7:545–594
Wang L, Hu C, Shao L (2017) The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomed 12:1227–1249
White SH, Wimley WC, Selsted ME (1995) Structure, function, and membrane integration of defensins. Curr Opin Struct Biol 5:521–527
Wimley WC, Hristova K (2011) Antimicrobial peptides: successes, challenges and unanswered questions. J Membr Biol 239:27–34
Winter J, Wenghoefer M (2012) Human defensins: potential tools for clinical applications. Polymers 4:691–709
Worthington RJ, Melander C (2013) Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol 31:177–184
Xhindoli D, Pacor S, Benincasa M, Scocchi M, Gennaro R, Tossi A (2016) The human cathelicidin LL-37—a pore-forming antibacterial peptide and host-cell modulator. Biochim Biophys Acta 1858:546–566
Xiong YQ, Yeaman MR, Bayer AS (1999) In vitro antibacterial activities of platelet microbicidal protein and neutrophil defensin against Staphylococcus aureus are influenced by antibiotics differing in mechanism of action. Antimicrob Agents Chemother 43:1111–1117
Xu T, Levitz SM, Diamond RD, Oppenheim FG (1991) Anticandidal activity of major human salivary histatins. Infect Immun 59:2549–2554
Yang L, Harroun TA, Weiss TM, Ding L, Huang HW (2001) Barrel-stave model or toroidal model? A case study on melittin pores. Biophys J 81:1475–1485
Yeaman MR, Yount NY (2003) Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 55:27–55
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415:389–395
Zhai Y, Wang Y, Rao N, Li J, Li X, Fang T et al (2019) Activation and biological properties of human beta defensin 4 in stem cells derived from human exfoliated deciduous teeth. Front Physiol 10:1304
Zhang L, Falla TJ (2006) Antimicrobial peptides: therapeutic potential. Expert Opin Pharmacother 7:653–663
Zhang J, Feng Y, Teng K, Lin Y, Gao Y, Wang J et al (2014) Type AII lantibiotic bovicin HJ50 with a rare disulfide bond: structure, structure-activity relationships and mode of action. Biochem J 461:497–508
Funding
The study was not funded by any authority.
Author information
Authors and Affiliations
Contributions
BK collaborated in the original idea, concept, design, and writing and drafting the article. SS and NHG contributed with data interpretation, writing, and drafting of the article. BSFB contributed to all stages of the process and mainly participated in drafting the article, writing, and editing the final version to be published. All the authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fazly Bazzaz, B.S., Seyedi, S., Hoseini Goki, N. et al. Human Antimicrobial Peptides: Spectrum, Mode of Action and Resistance Mechanisms. Int J Pept Res Ther 27, 801–816 (2021). https://doi.org/10.1007/s10989-020-10127-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-020-10127-2