Abstract
Context
Development and survival vary across a species’ geographic range and are also affected by local conditions like urban warming, which may drive changes in biology that magnify or reduce the risks of hazardous organisms to people. Larvae of the pine processionary moth (Thaumetopoea pityocampa Schiff; PPM) are covered with setae (hair-like structures) that cause allergic reactions in warm-blooded vertebrates upon contact with the skin, eyes, or respiratory tract.
Objectives
Our objective was to determine whether PPM larva development, phenology, and survival change with urban warming in ways that affect the risks of this organism to people.
Methods
In Orléans, France, we conducted a field study of PPM larvae across a gradient of urbanization from forests to city center to measure winter survival and the timing and duration of the life stage that poses the greatest risk to people.
Results
Larvae in the city spent more time in the fifth, most dangerous, instar than larvae in the forest. Urban warming indirectly increased larva survival by advancing phenology of urban larvae to a more cold-tolerant life stage prior to the winter cold period. Our results indicate that local urban warming drives changes in larva biology that increase the risks the organisms pose to people.
Conclusions
In recent decades, the PPM has expanded its geographic range to higher latitudes with rising temperatures. Our study highlights that local landscape variation, such as a mosaic of warmer and cooler temperatures in cities, can alter the effects of this type of range expansion for people.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The urban heat island effect makes cities warmer than surrounding natural areas (Oke 1973). This urban warming influences organisms’ body size, phenology, and fitness and contributes to local population extinctions and explosions (Dale and Frank 2014; Diamond et al. 2014; Chown and Duffy 2015; Youngsteadt et al. 2015; Merckx et al. 2018). Organisms that affect human health are of special management concern, and differences in development, behavior, or survival in cities can magnify or reduce their risks to people (Robinet et al. 2012; LaDeau et al. 2015). For example, urban micro-climates can increase or decrease the transmission of mosquito-vectored diseases by altering mosquito survival and biting rates in cities (reviewed in LaDeau et al. 2015). Ragweed (Ambrosia artemisiifolia) grows larger, has bigger flowers, and produces more allergenic pollen in the city due to higher temperatures and CO2 levels (Ziska et al. 2003). Predictions of risk depend on knowing how hazardous organisms respond to urban environments, where high human population densities have the potential to magnify risks.
The pine processionary moth (PPM), Thaumetopoea pityocampa Denis and Schiffermüller (Lepidoptera: Notodontidae) (Fig. 1a, b), is native to forests in the Mediterranean Basin but has invaded higher latitudes and elevations in recent decades as winter temperatures have increased (Battisti et al. 2005). At present, it is commonly found but rarely studied in urban areas (Rossi et al. 2016b) (Fig. 1c). PPM larvae have up to one million urticating, hair-like structures called setae that are dispersed by wind (Petrucco Toffolo et al. 2014) and cause allergic reactions in warm-blooded vertebrates upon contact with the skin, eyes, or respiratory tract (Vega et al. 2011a, b; Moneo et al. 2015) (Fig. 1a). The risk that PPM larvae pose to people changes throughout larva development because seta abundance, density, and allergenicity increase with instar (Rebollo et al. 2002; Moneo et al. 2015). Although PPM larvae bear urticating setae starting in the third instar (Battisti et al. 2017), most skin reactions (94% in one study (Vega et al. 2011b)), occur when they are in the final (fifth) instar, making the duration of this life stage a key predictor of risk (Moneo et al. 2015). Survival rates during cold periods, which increase with instar (Battisti et al. 2005), affect the density of larvae in an area as well as long-term population dynamics.
PPM biology and ecology have received considerable attention in forests (Roques 2015) but not in urban areas. The urban heat island effect may drive changes in PPM establishment, development, and survival rates (Robinet et al. 2012). Development rates (change in life stage per unit time) increase with temperature, reducing the amount of time spent in a life stage (Atkinson 1994; Forster et al. 2011). Winter survival rates also increase with temperature (Bale and Hayward 2010). In this study, we test whether the development, phenology, and winter survival of PPM larvae change across a gradient of urbanization (specifically, a gradient of surface temperatures) in Orléans, north-central France, with special interest in how differences affect the risks of larvae to people. We predict that PPM larvae will develop faster in urban areas than in forests, reducing the amount of time they spend in the dangerous fifth instar and diminishing the risk posed by each larva. However, we also predict, following Battisti et al. (2017), that warmer temperatures and rapid development of larvae in the city will lead to higher survival and larva abundance, and greater risk overall.
Materials and methods
Study system and location
In central France, PPM adults emerge in summer and females lay 100–200 eggs in a single cluster on host tree needles (mainly native or exotic pines, Pinus spp., and cedars, Cedrus spp.) (Masutti and Battisti 1990; Pimentel et al. 2012; Battisti et al. 2015; Rossi et al. 2016b). Larvae develop through five instars during the winter, living gregariously in silk nests that they construct in the tree canopy (Fig. 1c). PPM larvae feed at night, only when nighttime air temperatures are above 0 °C and temperatures the preceding day reached at least 9 °C within the nest (Battisti et al. 2005; Robinet et al. 2007). Larvae have an average supercooling point of − 7 °C, but some can survive to at least − 17 °C when inside their nests (Hoch et al. 2009). Fifth instars descend from trees usually from late winter to mid-spring in a single-file “procession” to locate pupation sites in the soil (Fig. 1b) (Robinet et al. 2015). The exposure risk for humans, pets, and cattle is the highest during this phase (Vega et al. 2011b). Pupae remain in the soil until adults emerge. PPM usually completes its lifecycle in one year, but some pupae may enter prolonged diapause for up to 7 years (Battisti et al. 2015).
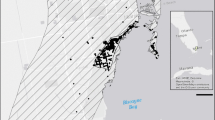
The Orléans, France, metropolitan area (47.90 N, 1.91 E) ranges from forested to densely urbanized (Fig. 2). It is bordered on the northeast by Orléans Forest, the largest state-owned forest in France, and on the south by Sologne, a highly forested region (Fig. 2). PPM host trees are frequently planted in Orléans as urban street and landscape trees (Rossi et al. 2016b). Black pine (Pinus nigra), which we use in this study, is not native to the area but was used for afforestation and is the most common and most attacked PPM host tree in the city (Rossi et al. 2016a, b). In the early 1990s, PPM colonized Orléans as its range expanded northward (Goussard et al. 1999) and is now widespread in the area (Rossi et al. 2016b).
Landsat thermal map of the Orléans region from February 23, 2018. The two major forest regions, Orléans Forest (upper) and Sologne (lower) are marked. The Loire River, marked in gray, flows east to west through central Orléans. The inset shows the gradient of thermal variation of an urban area in more detail. Thermal map pixels are 30 m by 30 m
Larva development and survival across a gradient of urbanization
PPM nests, made of white silk, are easily detectable from the ground (Rousselet et al. 2013) (Fig. 1c). We used a tree inventory (Rossi et al. 2016b) in combination with on-the-ground searching to locate black pine (Pinus nigra) trees with accessible PPM nests in the Orléans metropolitan area in December 2017. An accessible nest was one we could reach with a 15 m pole pruner without entering private property. We also had access to five forest stands along the periphery of the metropolitan area (Fig. 2). To assign a temperature to each accessible nest for site selection purposes, we extracted surface temperatures with interpolation from three Landsat thermal maps, one each from 2015, 2016, and 2017, using ArcMap 10.5 (ESRI 2011) and took the per-pixel average of the three maps (Fig. 2, Table S1). Each map provides a snapshot of surface temperatures on a single day. Though they do not represent the real air temperatures during the study, these maps establish the relative spatial patterns of variation in surface temperature.
To ensure that relatively hot and relatively cold sites were represented throughout the study area, we grouped the available urban trees into five patches mapping roughly onto different districts within the Orléans agglomeration (Fig. 2) and randomly selected four trees from the hotter half and four trees from the colder half of the available trees in each district (n = 40). This site selection method resulted in a gradient of temperatures both within and between districts (Table S2). Trees were separated by at least 200 m. Within each forest stand, we selected two to eight black pine trees with PPM nests on the forest edge (n = 23) (Table S2). Forest stands were separated by at least 200 m and trees within stands were separated by at least 30 m. To obtain the development status of PPM colonies before the winter cold period, from December 13 to 15, 2017, we collected one nest from each of the study trees. After freezing the nests overnight at − 20 °C, we dissected them to count the number of larvae in each instar.
The coldest temperatures recorded during the study period were on February 28, 2018. March 5 was the next date with minimum temperatures above 0 °C at all sites. From March 6 to 9, we collected 52 additional PPM nests to determine mortality following this cold period. Using the same methods as in December, we selected 39 urban nests, with eight nests per district except for one district where we only located seven accessible nests (Fig. 2, Table S3). We also collected 13 nests from four forest stands, two to four nests per stand (Fig. 2, Table S3). Forest nests were rare, and two forest stands had nests separated by a minimum of 10 m out of necessity. The nests were stored in a 3 °C chamber until counting. To record both development stage and survival, we dissected the nests to count the number of alive and dead larvae of each instar (Supplemental Information).
Analyses
We analyzed December and March data separately in R 3.5.1 in RStudio, using package lme4 1.1–18-1 (Bates et al. 2015) to fit all models and reviewing model diagnostics with HLMdiag 0.3.1 (Loy and Hofmann 2015) for linear mixed models and DHARMa 0.2.0 (Hartig 2018) for generalized linear mixed models. We used restricted maximum likelihood estimation to obtain final model coefficients. All random effects were random intercepts.
To establish a temperature gradient for analysis across all sites used in the study, we extracted surface temperatures with interpolation from a Landsat 8 thermal map captured on 23 February, 2018. We ground-truthed these satellite data using minimum daily air temperatures recorded by HOBO pendant data loggers (Onset Computer Corporation, Bourne, MA, USA; model UA-001-08) on trees across the study area (Fig. 2, Figure S1, Supplemental Information). Throughout the paper, we use the surface temperature data, which we call relative temperature, as the measure of the gradient of urbanization.
To test for changes in colony size across the gradient of urbanization, we fitted linear mixed models with colony size as a numeric response, relative temperature as a fixed effect, and group (district for urban sites and forest stand for forest sites) as a random effect. We did not detect spatial autocorrelation in the residuals of the models for December (p = 0.646) or March (p = 0.935) using Moran’s I in ape 5.0 (Paradis et al. 2004).
To compare development stage across the gradient of urbanization, we classified larvae as in or not in the fifth instar (Figure S2). Separately for December and March, we fitted generalized linear mixed effects models with a binomial distribution and logit link function, using instar as a binary response (fifth = 1, not fifth = 0) for each larva, relative temperature as a fixed effect, and colony ID nested in group as a random effect. Following Rhodes et al. (2009), we used a spline correlogram in ncf 1.2-6 (Bjornstad 2018) to test for spatial autocorrelation in the residuals of the models. The 95% pointwise bootstrap confidence intervals included zero across the entire range, and we concluded that spatial autocorrelation was not a problem.
To model survival of larvae following a cold period in March, we used the same type of model as for instar, with survival as a binary response (alive = 1, dead = 0). Both instar and colony size have previously been shown to affect survival (Battisti et al. 2005; Ronnås et al. 2010). We began by fitting a model with a three-way interaction of relative temperature, instar (fifth = 1, not fifth = 0), and colony size as fixed effects and colony ID nested in group as a random effect. We mean-centered relative temperature and colony size to improve model convergence. We removed the three-way interaction (p > 0.05) and, following a subsequent test of all two-way interactions, removed the two interactions containing relative temperature (p > 0.05). Our final model included relative temperature, instar, colony size, and an instar by colony size interaction as fixed effects and colony ID nested in group as a random effect. We used a spline correlogram in ncf to check for spatial autocorrelation in the residuals of the model. The variance inflation factor was less than two, suggesting little collinearity in the data.
Pupation timing
In the Orléans region, pupation processions typically occur in March and April (Battisti et al. 2015). Three urban districts granted us permission to install commercial traps (Fig. 1d, Colacci et al. 2018) on municipally-managed trees (Fig. 2) to monitor pupation phenology. We also received approval to install traps in Orléans forest and in privately-owned forest in Sologne. We used a combination of tree inventories, suggestions from municipal staff, and on-the-ground searching to identify eligible sites with at least two black pine trees with visible PPM nests. From these eligible sites, we selected six forest sites and took stratified random samples of urban sites in relatively hot and cold locations for a total of 15 urban sites (Fig. 2). In late February 2018, we installed 65 traps (collier écopiège®, La Mésange Verte, Bages, France), with two to five traps per site. We changed the bags on the traps weekly in March and April and every two weeks in May and June, ending on June 14. Each time we collected bags, we filtered the soil and counted the number of individuals caught in the trap.
Analyses
The mean procession date for each trap was calculated using a standard weighted mean, with dates converted to integer week (starting with March 2 as Week 1) and larva abundances as weights (Supplemental Information, Moussus et al. 2010). We fit a linear mixed model with mean procession date as the response, relative temperature as a fixed effect, and site as a random effect. Moran’s I did not indicate significant spatial autocorrelation in the residuals of the model (p = 0.164).
PPM larvae pose the greatest risk to humans during the pupation procession period (Martin 2015), so we also considered whether the total range of dates that larvae were collected in each trap and the number of individual weeks with trap catches changed along the gradient of urbanization. The range was calculated using range = week of last catch − week of first catch + 1, where a range of one indicates that all larvae collected in a trap were collected during a single week (Supplemental Information). We used linear mixed models with number of nests per tree and relative temperature as fixed effects, site as a random effect, and log-transformed range or log-transformed number of weeks with trap catches as the response. Using Moran’s I, we did not detect spatial autocorrelation in the residuals of the models for the range or weeks counted (p > 0.20).
Results
The coldest air temperatures we recorded with HOBO data loggers occurred on February 28, 2018, when nighttime minimum temperatures ranged from − 12.07 °C at a forest site to − 8.98 °C at the warmest urban site.
In December, 60 nests had a mean (± sem) colony size of 110.97 (± 7.38) larvae per nest (urban: 124.18 (± 9.19); forest: 88.14 (± 11.00)). In March, 43 nests had a mean (± sem) colony size of 90.16 (± 7.68) larvae per nest (urban: 96.38 (± 9.11); forest: 72.09 (± 13.23)), excluding larvae killed by fungus or natural enemies. Colony size increased with relative temperature in December (χ2 = 3.964, df = 1, p = 0.0465; R2M = 0.081, R2C = 0.318) but not in March (χ2 = 0.951, df = 1, p = 0.329) (Table S4).
When we measured development stage in both December and March, the probability that a larva was in the fifth instar increased with relative temperature (Fig. 3, Table S5). In forests, the majority of larvae were in the fourth instar in both December (98.3% fourth instar) and March (90.0% fourth instar) (Fig. 3). In urban areas, the majority of larvae were in the fifth instar in December (63.1% fifth instar) and March (88.6% fifth instar) (Fig. 3).
Mixed effects logistic regression models predicting the probability that larvae are in the fifth instar across a relative temperature gradient. December data and model are in gray (p < 0.001), and March data and model are in black (p < 0.001). Circles indicate the number of larvae in a nest in the third/fourth (0) or fifth instar (1) and are offset slightly from 0.0 and 1.0 for improved visibility. Top panel shows the number of larvae in the most common instar (L4 = fourth instar, L5 = fifth instar)
We found a significant interaction between colony size and instar in explaining survival after a cold period in March (Table 1). We compared the fitted model for fifth instar larvae (instar = 1) to the fitted model for fourth instar larvae (instar = 0) and found that survival was higher for fifth instars at all but the smallest (< 37 larvae) colony sizes.
When we considered pupation phenology, there was a linear relationship between the mean date of pupation processions and relative temperature, with larvae at warm sites making processions earlier than larvae at cool sites (χ2 = 15.113, df = 1, p < 0.001; R2M = 0.371, R2C = 0.488; Fig. 4). Neither the range of collection dates nor the number of weeks with catches increased with relative temperature, but both increased with the number of nests on the tree (Table 2). Of the 46 traps that caught at least five larvae, 18 had a small number of larvae (< 5) that descended from the tree one to six weeks before the first catch of five or more individuals (Fig. 5). Thirty of 46 traps had their first catch of five or more larvae between April 13 and April 27 (Fig. 5).
Linear mixed model indicating that larvae at warmer sites descend from trees earlier than larvae at colder sites. Each point is the weighted mean procession week for a single trap, with the week number (starting with March 2 = Week 1) weighted by larva abundance. Open circles are traps in forests, and closed circles are traps in urban areas. Line (y = 11.14 − 1.05x) is fit with the fixed effects coefficients of a mixed model. R2M = 0.371, R2c = 0.488
Calendar of pupation procession dates for PPM larvae collected weekly in traps. Horizontal lines indicate the range of dates when larvae were collected. The range begins with the first date that at least five larvae were collected and ends when the last larva was collected. Each black point is the weighted mean procession week for a single trap. Open circles are traps in forests, and closed circles are traps in urban areas. Black points with no horizontal lines indicate that all larvae were collected during the same week. Gray points indicate weeks when a small number of larvae (< 5) descended from the tree prior to processions with larger numbers of larvae. Traps are arranged along the y-axis from warmest (top) to coldest (bottom)
Discussion
We found, unexpectedly, that PPM larvae in warm urban areas spent more time in the fifth, most hazardous, instar than larvae in relatively cool areas. When PPM setae are released by larvae or are blown from nests, they are readily carried by the wind, causing allergic reactions in people who have not directly contacted larvae (Werno and Lamy 1990; Vega et al. 2011b; Petrucco Toffolo et al. 2014). Fifth instars are responsible for most reactions in people because they have significantly more setae than earlier instars and these setae are more potent (Rebollo et al. 2002; Vega et al. 2011b; Moneo et al. 2015). In our study, many larvae at warm urban sites, but almost none at forests sites, had already developed to the fifth instar in December. Three months later in early March, the majority of larvae in relatively cool sites were still in the fourth instar, but there was not a corresponding three-month delay in pupation processions. The extended duration of fifth instars in the city lengthens the window of time that people are exposed to the PPM in its most hazardous life stage.
The concentration of PPM setae in the air is highest near larvae and nests and declines with distance from the infested area (Werno and Lamy 1990). Walking near an infested tree is a key risk factor for people to be contaminated with setae from PPM (Vega et al. 2011b) as well as from the congeneric oak processionary caterpillar (Thaumetopoea processionea) (Maier et al. 2003; Gottschling and Meyer 2006). Most of our urban study trees in Orléans were located in residential areas, public parks, or along sidewalks that have frequent pedestrian traffic. Nests on these urban trees had a higher percentage of fifth instars in December and March and, in December, higher colony sizes than forest trees, resulting in greater risk for airborne contamination for at least three months during the winter. In addition, the human population density in Orléans is 88 to 140 times higher than the population density of surrounding rural communes where we collected forest nests (Tavernier 2016). The combination of high seta abundance and human population densities in cities could magnify the problem of airborne seta dispersal and associated allergenic reactions. Our results are consistent with other studies showing that variation in urban temperatures affects the temporal distribution of airborne allergens. For example, in Detroit, Michigan, USA, peak oak flowering time was strongly correlated with February nighttime temperatures along a gradient of urbanization, and these differences in peak flowering time affected local airborne pollen concentrations (Katz et al. 2019). In Baltimore, Maryland, USA, ragweed pollen production at an urban site started approximately 15 days earlier than at a rural site but ended only 10 days earlier (Ziska et al. 2003).
Direct contact with PPM larvae, though less common than airborne contamination with setae, occurs primarily during pupation processions and results in acute exposure to a high concentration of setae (Martin 2015; Moneo et al. 2015). In our study, the range in procession dates and the number of weeks with trap catches were relatively consistent across the gradient of urbanization. Therefore, the specific timing of pupation processions, which affects duration of the hazardous fifth instar (Battisti et al. 2015), may be more important than the range in procession dates for assessing differences in risk along a gradient of urbanization. The percentages of larvae in the fourth and fifth instars in December and March indicate that, overall, differences in development stage that occurred before the winter were maintained until spring. Then, approximately half of the pupation processions started during a two-week period, suggesting fully developed larvae were waiting in the trees for appropriate pupation conditions such as favorable temperature and light (Salman et al. 2018), which may also have boosted the development of fourth instars in relatively cool areas. During years when autumn temperatures are warm enough for some larvae to complete their development before winter, they may make early processions (Huchon and Demolin 1971; Robinet et al. 2015). This would reduce the amount of time larvae spend in the fifth instar but could result in two separate high-risk procession periods in cities unless early processions occur for all nests in urban areas (Robinet et al. 2015).
PPM is one of the few species whose range expansion has been causally linked to increasing winter temperatures (Battisti et al. 2005; Rosenzweig et al. 2007). Orléans is near the northern end of the PPM range where feeding and survival are sometimes limited by cold (Robinet et al. 2007). The coldest air temperature we observed during our study period was − 12.07 °C at a forest site, which is five degrees warmer than the minimum temperature larvae can survive in their nests (Hoch et al. 2009). We did not expect, and did not find, a direct effect of temperature on survival of fourth and fifth instars at the temperatures we observed. However, PPM cold tolerance increases with development stage (Battisti et al. 2005), and in our study most larvae in warm urban areas, but not in forests, had already reached the fifth instar before the cold period. Urbanization indirectly affected larva survival by advancing larva development prior to the cold period, documenting that cities may increase PPM survival at the northern edge of its range. As organisms’ ranges shift or expand with climate change (Rosenzweig et al. 2007), cities may speed the process by offering thermal refuge in otherwise unsuitable areas (Hart et al. 1986; Parris and Hazell 2005; Robinet et al. 2012; Frank and Just 2020).
Our results indicate that local landscape variation, such as the mosaic of warmer and cooler temperatures in cities, alters the implications of large-scale phenomena like climate change for people. Increasing winter temperatures have caused PPM’s range to expand into areas where millions of people live and where PPM wasn’t present several decades ago (Battisti et al. 2005; Robinet et al. 2012; Roques et al. 2015). By selecting a study city in this expansion range, we were able to detect an interaction between climate change and urban warming, where the effects of the range expansion for people are contingent upon the degree of urban warming. Across a gradient of urbanization, we found that PPM larvae spent more time in the most dangerous life stage in urban areas compared to forests, which resulted in a higher abundance of urticating setae in urban compared to forest nests through most of the winter. This response to temperature affects risk in cities and may also predict higher risk throughout the northern part of the range as climate warms. Nearly all previous research on PPM has been conducted in forests, where management goals often focus on minimizing economic losses from defoliation and reducing direct contact between forestry workers and PPM larvae or contaminated materials (Rossi et al. 2016b). Rossi et al. (2016a, b) called for increased assessment of the costs of continued PPM invasion into new areas, and our results provide evidence that cities, through a combination of urban warming, high population densities, and abundant host plants, are altering the consequences of the expansion at a local scale. The costs to human health of not managing PPM in urban areas are higher than previously realized. Our study highlights that urban areas require special consideration when calculating the risks of dangerous organisms to humans.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
The R code used to analyze data for the study is available from the corresponding author on reasonable request.
References
Atkinson D (1994) Temperature and organism size—A biological law for ectotherms? Adv Ecol Res 25:1–58
Bale JS, Hayward SAL (2010) Insect overwintering in a changing climate. J Exp Biol 213:980–994
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Battisti A, Stastny M, Netherer S, Robinet C, Schopf A, Roques A, Larsson S (2005) Expansion of geographic range in the pine processionary moth caused by increased winter temperatures. Ecol Appl 15:2084–2096
Battisti A, Avcı M, Avtzis DN, Ben Jamaa ML, Berardi L, Berretima W, Branco M, Chakali G, El Alaoui El Fels, Frérot MA, Hódar B, JA Ionescu-Malancus I, Ipekdal K, Larsson S, Manole T, Mendel Z, Meurisse N, Mirchev P, Nemer N, Paiva MR, Pino J, Protasov A, Rahim N, Rousselet J, Santos H, Sauvard D, Schopf A, Simonato M, Yart A, Zamoum M (2015) Natural history of the processionary moths (Thaumetopoea spp.): new insights in relation to climate change. In: Roques A (ed) Processionary moths and climate change: an update. Springer, Dordrecht, pp 15–79
Battisti A, Larsson S, Roques A (2017) Processionary moths and associated urtication risk: Global change–driven effects. Annu Rev Entomol 62:323–342
Bjornstad O (2018) ncf: spatial covariance functions. R Packag version 12-6
Chown SL, Duffy GA (2015) Thermal physiology and urbanization: perspectives on exit, entry and transformation rules. Funct Ecol 29:902–912
Colacci M, Kavallieratos NG, Athanassiou CG, Boukouvala MC, Rumbos CI, Kontodimas DC, Pardo D, Sancho J, Benavent-Fernández E, Gálvez-Settier S, Sciarretta A, Trematerra P (2018) Management of the pine processionary moth, Thaumetopoea pityocampa (Lepidoptera: Thaumetopoeidae), in urban and suburban areas: trials with trunk barrier and adhesive barrier trap devices. J Econ Entomol 111:227–238
Dale AG, Frank SD (2014) Urban warming trumps natural enemy regulation of herbivorous pests. Ecol Appl 24:1596–1607
Diamond SE, Cayton H, Wepprich T, Jenkins CN, Dunn RR, Haddad NM, Ries L (2014) Unexpected phenological responses of butterflies to the interaction of urbanization and geographic temperature. Ecology 95:2613–2621
ESRI (2011) ArcGIS Desktop: Release 10
Forster J, Hirst AG, Woodward G (2011) Growth and development rates have different thermal responses. Am Nat 178:668–678
Frank SD, Just MG (2020) Can cities activate sleeper species and predict future forest pests? A case study of scale insects. Insects 11:142
Gottschling S, Meyer S (2006) An epidemic airborne disease caused by the oak processionary caterpillar. Pediatr Dermatol 23:64–66
Goussard F, Saintonge FX, Geri C, Auger-Rozenberg M-A, Pasquier-Barre F, Rousselet J (1999) Accroissement des risques de dégats de la processionnaire du pin, Thaumetopoea pityocampa Denis & Schiff. en Région Centre, du au réchauffement climatique (Lepidoptera: Thaumetopoeidae). Ann la Société Entomol Fr 35:341–343
Hart ER, Miller FD Jr, Bastian RA (1986) Tree location and winter temperature influence on mimosa webworm populations in a northern urban environment. J Arboric 12:237–240
Hartig F (2018) DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R Packag version 020. https://CRAN.R-project.org/package=DHARMa
Hoch G, Toffolo EP, Netherer S, Battisti A, Schopf A (2009) Survival at low temperature of larvae of the pine processionary moth Thaumetopoea pityocampa from an area of range expansion. Agric For Entomol 11:313–320
Huchon H, Demolin G (1971) La bioécologie de la processionnaire du pin. Dispersion potentielle - Dispersion actuelle Phytoma 225:11–20
Katz DSW, Dzul A, Kendel A, Batterman SA (2019) Effect of intra-urban temperature variation on tree flowering phenology, airborne pollen, and measurement error in epidemiological studies of allergenic pollen. Sci Tot Environ 653:1213–1222
LaDeau SL, Allan BF, Leisnham PT, Levy MZ (2015) The ecological foundations of transmission potential and vector-borne disease in urban landscapes. Funct Ecol 29:889–901
Loy A, Hofmann H (2015) HLMdiag: a suite of diagnostics for hierarchical linear models in R. J Stat Softw 56:1–28
Maier H, Spiegel W, Kinaciyan T, Krehan H, Cabaj A, Schopf A, Hönigsmann H (2003) The oak processionary caterpillar as the cause of an epidemic airborne disease: survey and analysis. Br J Dermatol 149:990–997
Martin J-C (2015) Development of environmentally-friendly strategies in the management of processionary moths. In: Roques A (ed) Processionary moths and climate change: an update. Springer, Dordrecht, pp 411–427
Masutti L, Battisti A (1990) Thaumetopoea pityocampa (Den. & Schiff.) in Italy: bionomics and perspectives of integrated control. J Appl Entomol 110:229–234
Merckx T, Souffreau C, Kaiser A, Baardsen LF, Backeljau T, Bonte D, Brans KI, Cours M, Dahirel M, Debortoli N, De Wolf K, Engelen JMT, Fontaneto D, Gianuca AT, Govaert L, Hendrickx F, Higuti J, Lens L, Martens K, Matheve H, Matthysen E, Piano E, Sablon R, Schön I, Van Doninck K, De Meester L, Van Dyck H (2018) Body-size shifts in aquatic and terrestrial urban communities. Nature 558:113–116
Moneo I, Battisti A, Dufour B, García-Ortiz JC, González-Muñoz M, Moutou F, Paolucci P, Petrucco Toffolo E, Rivière J, Rodríguez-Mahillo A-I, Roques A, Roques A, Vega JM, Vega J (2015) Medical and veterinary impact of the urticating processionary larvae. In: Roques A (ed) Processionary moths and climate change: an update. Springer, Dordrecht, pp 359–410
Moussus J-P, Julliard R, Jiguet F (2010) Featuring 10 phenological estimators using simulated data. Methods Ecol Evol 1:140–150
Oke TR (1973) City size and the urban heat island. Atmos Environ 7:769–779
Oke TR (1982) The energetic basis of the urban heat island. Q J R Meteorol Soc 108:1–24
Paradis E, Claude J, Strimmer K (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290
Parris KM, Hazell DL (2005) Biotic effects of climate change in urban environments: the case of the grey-headed flying-fox (Pteropus poliocephalus) in Melbourne, Australia. Biol Conserv 124:267–276
Perez-Contreras T, Soler JJ, Soler M (2003) Why do pine processionary caterpillars Thaumetopoea pityocampa (Lepidoptera, Thaumetopoeidae) live in large groups? An experimental study. Ann Zool Fennici 40:505–515
Petrucco Toffolo E, Zovi D, Perin C, Paolucci P, Roques A, Battisti A, Horvath H (2014) Size and dispersion of urticating setae in three species of processionary moths. Integr Zool 9:320–327. https://doi.org/10.1111/1749-4877.12031
Pimentel C, Santos M, Ferreira C, Nilsson J-A (2012) Temperature, size, reproductive allocation, and life-history evolution in a gregarious caterpillar. Biol J Linn Soc 105:340–349
R Core Team (2018) R: a language and environment for statistical computing
Rebollo S, Moneo I, Vega JM, Herrera I, Caballero ML (2002) Pine processionary caterpillar allergenicity increases during larval development. Int Arch Allergy Immunol 128:310–314
Rhodes JR, McAlpine CA, Zuur AF, Smith GM, Leno EN (2009) GLMM applied on the spatial distribution of koalas in a fragmented landscape. In: Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM (eds) Mixed effects models and extensions in ecology with R. Springer, New York, pp 469–492
Robinet C, Baier P, Pennerstorfer J, Schopf A, Roques A (2007) Modelling the effects of climate change on the potential feeding activity of Thaumetopoea pityocampa (Den. & Schiff.) (Lep., Notodontidae) in France. Glob Ecol Biogeogr 16:460–471
Robinet C, Imbert CE, Rousselet J, Sauvard D, Garcia J, Goussard F, Roques A (2012) Human-mediated long-distance jumps of the pine processionary moth in Europe. Biol Invasions 14:1557–1569
Robinet C, Laparie M, Rousselet J (2015) Looking beyond the large scale effects of global change: Local phenologies can result in critical heterogeneity in the pine processionary moth. Front Physiol. https://doi.org/10.3389/fphys.2015.00334
Ronnås C, Larsson S, Pitacco A, Battisti A (2010) Effects of colony size on larval performance in a processionary moth. Ecol Entomol 35:436–445
Roques A (2015) Processionary moths and climate change: an update. Springer, Dordrecht
Roques A, Rousselet J, Avci M, Avtzis DN, Basso A, Battisti A, Lahbib Ben Jamaa M, Bensidi A, Berardi L, Berretima W, Branco M, Chakali G, Çota E, Dautbašić M, Delb H, El Alaoui El Fels MA, El Mercht S, El Mokhefi M, Forster B, Garcia J, Georgiev G, Glavendekić MM, Goussard F, Halbig P, Henke L, Hernańdez R, Hódar, JA, Ipekdal K, Jurc M, Klimetzek D, Laparie M, Larsson S, Mateus E, Matošević D, Meier F, Mendel Z, Meurisse N, Mihajlović L, Mirchev P, Nasceski S, Nussbaumer C, Paiva M-R, Papazova I, Pino J, Podlesnik J, Poirot J, Protasov A, Rahim N, Sańchez Peña G, Santos H, Sauvard D, Schopf A, Simonato M, Tsankov, G, Wagenhoff E, Yart A, Zamora R, Zamoum M, Robinet C (2015) Climate warming and past and present distribution of the processionary moths (Thaumetopoea spp.) in Europe, Asia Minor and North Africa. In: Roques A (ed) Processionary moths and climate change: an update. Springer, Dordrecht, pp 81–161
Rosenzweig C, Casassa G, Karoly DJ, Imeson A, Liu C, Menzel A, Rawlins S, Root TL, Seguin B, Tryjanowski P (2007) Assessment of observed changes and responses in natural and managed systems. In: Parry M (ed) Climate change 2007: impacts, adaptation and vulnerability. Contribution of working group II to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, pp 79–131
Rossi J, Garcia J, Roques A, Rousselet J (2016a) Trees outside forests in agricultural landscapes: spatial distribution and impact on habitat connectivity for forest organisms. Landsc Ecol 31:243–254
Rossi J, Imbault V, Lamant T, Rousselet J (2016b) A citywide survey of the pine processionary moth Thaumetopoea pityocampa spatial distribution in Orléans (France). Urban For Urban Green 20:71–80
Rousselet J, Imbert CE, Dekri A, Garcia J, Goussard F, Vincent B, Denux O, Robinet C, Dorkeld F, Roques A, Rossi J-P (2013) Assessing species distribution using google street view: a pilot study with the pine processionary moth. PLoS One 8:1–7
RStudio Team (2015) RStudio: integrated development for R
Salman MHR, Giomi F, Laparie, Lehmann P, Battisti A (2018) Prepupal diapause synchronizes adult emergence in the pine processionary moth Thaumetopoea pityocampa (Lepidoptera: Notodontidae). Agric For Entomol 20:582–588
Sun R, Lü Y, Yang X, Chen L (2019) Understanding the variability of urban heat islands from local background climate and urbanization. J Clean Prod 208:743–752
Tavernier J-L (2016) Populations légales en vigeur à compter du 1er janvier 2017: 45 Loiret. In: Inst. la Stat. des Etudes Econ. https://www.insee.fr/fr/statistiques/fichier/2525755/dep45.pdf%0A
USGS (2016) Landsat 8 data users handbook, Version 2.0. https://www.usgs.gov/land-resources/nli/landsat/landsat-8-data-users-handbook
Vega J, Vega JM, Moneo I (2011a) Skin reactions on exposure to the pine processionary caterpillar (Thaumetopoea pityocampa). Actas Dermosifiliogr 102:658–667
Vega JM, Moneo I, Ortiz JCG, Palla PS, Sanchís ME, Vega J, Gonzalez-Muñoz M, Battisti A, Roques A (2011b) Prevalence of cutaneous reactions to the pine processionary moth (Thaumetopoea pityocampa) in an adult population. Contact Dermatitis 64:220–228
Werno J, Lamy M (1990) Pollution atmosphérique d’origine animale: les poils urticants de la chenille processionnaire du pin (Thaumetopoea pityocampa Schiff.) (Insectes, Lépidoptères). Comptes Rendus l’Académie des Sci Paris 310:325–331
Youngsteadt E, Dale AG, Terando AJ, Dunn RR, Frank SD (2015) Do cities simulate climate change? A comparison of herbivore response to urban and global warming. Glob Chang Biol 21:97–105
Ziska LH, Gebhard DE, Frenz DA, Faulkner S, Singer BD, Straka JG (2003) Cities as harbingers of climate change: common ragweed, urbanization, and public health. J Allergy Clin Immunol 111:290–295
Acknowledgements
We thank Patrick Pineau for assistance with field work, Michael Just for advice on statistical analyses, and Nick Haddad and Michael Reiskind for helpful comments on the manuscript. Landsat data are available from the United States Geological Survey. We are grateful to Orléans Métropole, the town councils of Saint-Jean-de-Braye and Fleury-les-Aubrais, and their employees in charge of urban green spaces for permissions and assistance (especially Hervé Mifsud, Jean-Pierre Orange, Frédéric Viaud and Bernard Chevallier). We also thank the Office National des Forêts—Agence Val de Loire (especially Dominique de Villebonne, Patricia Ricois, and Caroline Samyn) and Pierre de Feraudy (private owner, "Le Bois de la Ferme Neuve").
Funding
This material is based upon research supported by the Chateaubriand Fellowship of the Office for Science & Technology of the Embassy of France in the United States to K.B.; National Science Foundation Graduate Research Fellowship and Graduate Research Opportunities Worldwide awards under Grant No. DGE-1252376 to K.B.; and the PCLIM network funded by the ACCAF program of INRA, France. S.F. was partially funded by Cooperative Agreement No. G15AP00153 from the United States Geological Survey. This manuscript is submitted for publication with the understanding that the United States Government is authorized to reproduce and distribute reprints for governmental purposes. Its contents are solely the responsibility of the authors and do not necessarily represent the views of the National Science Foundation, the Southeast Climate Adaptation Science Center, or the USGS.
Author information
Authors and Affiliations
Contributions
The study was conceived by KB, JR, SF, and AR. Material preparation and data collection were performed by KB and AB. Analysis was performed by KB. The first draft of the manuscript was written by KB and all authors commented on and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no financial or proprietary interests in any material discussed in this article.
Informed consent
All authors have consented to publication of this material.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Backe, K., Rousselet, J., Bernard, A. et al. Human health risks of invasive caterpillars increase with urban warming. Landscape Ecol 36, 1475–1487 (2021). https://doi.org/10.1007/s10980-021-01214-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-021-01214-w