Abstract
Context
Protected areas are a cornerstone of the global strategy for conserving biodiversity, and yet their efficacy in comparison to unprotected areas is rarely tested. In the highly fragmented forests of temperate regions, landscape context and forest history may be more important than protection status for plant species diversity.
Objectives
To determine whether there are differences in plant diversity between protected areas and private lands while controlling for landscape context, forest age, and other important factors.
Methods
We used a database of 156 one-hectare forest plots distributed over 120,000 km2 in the fragmented forests of southern Ontario to test whether protected areas and private forests differed in native species richness, relative abundance of exotic species, and the probability of finding species of conservation concern.
Results
Plots with more forest on the surrounding landscape had higher native species richness, lower abundance of exotic species, and greater probability of supporting at least one species of conservation concern. Young forests tended to have higher abundance of exotics, and were less likely to support species of conservation concern. Surprisingly, privately owned forests had greater native species richness and were more likely to support species of conservation concern once these other factors were accounted for. In addition, there were significant interactions between ownership type, forest history, and landscape context.
Conclusions
Our results highlight the importance of privately owned forests in this region, and the need to consider forest history and landscape context when comparing the efficacy of protected areas versus private land for sustaining biodiversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The designation of protected areas (PAs) is a major strategy for conserving biological diversity, and a key goal of the Convention on Biological Diversity (Gaston et al. 2008; Juffe-Bignoli et al. 2014). However, there have been relatively few rigorous tests of the efficacy of PAs relative to privately owned and/or unprotected lands in maintaining biodiversity (Gaston et al. 2008; Rayner et al. 2014). Rayner et al. (2014) reviewed 539 studies that assessed PA performance, finding that less than 5% directly quantified PA effectiveness for maintaining biodiversity. A meta-analysis of studies providing data on species abundance or richness in protected and unprotected sites found an overall positive effect size for protection, but this effect was not significant for plants (Coetzee et al. 2014).
Part of the problem is the scarcity of biological surveys on private lands (Knight 1999; Hilty and Merenlender 2003; Wilcove et al. 2004). This prevents comparison with PAs, and may lead to underestimation of the importance of private lands for conservation (Wilcove et al. 2004). The general assumption is that unprotected areas tend to have less diversity and more invasion, possibly because of more intensive management or more frequent human disturbance. On the other hand, many non-protected areas may be managed in ways that maintain plant and animal populations. In some cases, private lands have been found to support higher levels of biodiversity than PAs (e.g. Richart and Hewitt 2008; Rayner et al. 2014). It is important to test the efficacy of PAs and private land in maintaining biodiversity in order to better understand how to maintain biodiversity and where to focus conservation money and effort.
PAs can have higher levels of biodiversity for two main reasons (Coetzee et al. 2014). First, they may be managed to maintain diversity, either by minimizing disturbances or by active management such as invasive species removal or prescribed burns. For example, protected forest sites in New England had significantly lower rates of disturbance and higher aboveground biomass than nearby private forests (Zhang et al. 2010). Second, PAs may have been designated in areas that already had higher abundance or diversity of target organisms. High diversity is not due to protection from disturbance or any special management, but a result of geographic, climatic, or landscape conditions that favour it. For example, plant species richness may be positively correlated with the total amount of natural habitat found within a certain distance of a focal patch (e.g. Piessens et al. 2004). Small, isolated patches experience greater extirpation rates because they are too far from seed sources for recolonization (e.g. Grashof-Bokdam and Geertsema 1998). PAs may have higher plant diversity simply because they tend to be designated in areas with high amounts of natural habitat on the surrounding landscape. In addition, the history of disturbance at a particular site can influence its current level of biodiversity (e.g. Graae and Sunde 2000; McDonald et al. 2008; Brown and Boutin 2009). In this case, PAs may not have significantly higher diversity than private sites once these effects of landscape context and history are taken into account.
Temperate deciduous forests contain substantial plant diversity, but they have been severely reduced in size due to clearance by humans for lumber, agriculture, and urban land use (Reich and Frelich 2002). Many studies have examined the effects of habitat loss, fragmentation, eutrophication, fire suppression, and the introduction of exotic species on plant diversity and composition in temperate deciduous forests (e.g. Wilcove et al. 1986; Whigham 2004; Verheyen et al. 2012). Temperate forest systems are overrepresented in studies of long-term, local-scale change in plant diversity compared to tropical forests (Vellend et al. 2013). However, there have been surprisingly few tests comparing plant diversity in PAs versus private lands in temperate forests. In their global meta-analysis, Coetzee et al. (2014) compiled over 100 studies that compared plant diversity inside and outside PAs, but only two were in temperate regions, both from grasslands in Europe.
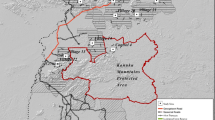
In this study we test differences in native plant species richness, exotic plant abundance, and the occurrence of plant species of conservation concern between protected and private forests in 156 plots scattered over approximately 120,000 km2 in southern Ontario, Canada (Fig. 1). Forests in this region are highly fragmented due to massive forest clearance since European settlement in the 1800 s (Larson et al. 1999). However, in some areas forest has regenerated on former agricultural sites. Our plots vary in the extent of forest on the surrounding landscape and in age, with some sites having regenerated since the mid-1950s. We use this extensive dataset to test whether ownership type (protected or private) affects current plant diversity after landscape context, forest age, and other potentially important factors are accounted for. If PAs in our study region are being successfully managed to maximize native plant diversity, we predicted that forest plots within PAs would have significantly higher native plant species richness, lower relative abundance of exotic species, and higher probability of containing species of conservation concern, even after controlling for landscape context and other factors. Alternatively, if the main determinants of plant diversity in this region are landscape context and history, regardless of protection status, we predicted that ownership type would not be a significant predictor of plant biodiversity once these factors were accounted for.
Methods
Southern Ontario is part of the mixedwood plains ecozone, the smallest ecozone in Ontario (Crins et al. 2009). The region makes up less than 1% of Canada’s total land area, yet it is home to roughly 35% of Canada’s human population (Ontario Biodiversity Council 2010). Forest cover in the region was approximately 90% prior to European settlement, but now ranges from 7 to 40% (Larson et al. 1999). Most of the remaining forest is privately owned. Southern Ontario is one of Canada’s biodiversity hotspots, and one of the three most important regions in Canada for conservation of endangered species (Kerr and Deguise 2004; Warman et al. 2004).
We surveyed 156 one-hectare forest plots in 2014 and 2015 (May–August of each year) (Fig. 1) as part of a study testing the efficacy of species distribution models (SDMs) for predicting suitable habitat for rare plant species of the region (McCune 2016). We chose potential plot locations randomly from a pool of sites predicted to be suitable for one or more species of conservation concern based on SDMs built for each species. The final selection of plots depended on obtaining landowner permission. We sampled as many plots as we could over the two seasons. We thoroughly searched each plot and recorded every species of vascular plant present. Each survey took 3–10 person h; our surveys were exhaustive to ensure the best chance of detecting rare species if present. We estimated the abundance of each species using a coarse categorical scale of 1 (very rare, two or fewer individuals)–5 (dominates the plot). We lumped taxa at the genus level if species could not be reliably distinguished. We define species of conservation concern as those vascular plant species listed as S1 (extremely rare with 1–5 known populations in Ontario), S2 (very rare with 6–20 known populations) or S3 (rare to uncommon with 21–80 known populations) by the Natural Heritage Information Centre of Ontario (Oldham and Brinker 2009).
The original purpose of the surveys was not to test for differences in plant diversity between PAs and privately owned land. However, this large observational study provides a spatially extensive, uniformly collected and detailed dataset with which to model the effect of land ownership while accounting for landscape context and other variables. One hundred and ten plots were located within privately owned forests, while forty-six were located within PAs. PAs included conservation areas owned by Conservation Authorities, county forests owned by municipalities, and nature preserves owned by non-profit organizations.
We gathered information on the characteristics of each surveyed plot to account for other factors known to affect plant diversity. We measured the total area of forested habitat within a 500 m radius of each plot based on the wooded layer of the Southern Ontario Land Resource Information System in ArcGIS (SOLRIS; Smyth 2008). A distance of 500 m is considered the maximum dispersal distance for temperate woodland understory plants, and the amount of forest within this distance has been shown to be a good representation of isolation (Petit et al. 2004; McCune and Vellend 2015; see also Fahrig 2013). We then measured the total area of forested habitat within 500 m of each plot in 1954 using aerial photographs digitized and georectified in ArcGIS. We used these historical aerial photos to determine whether the majority of each plot was located in closed canopy forest in 1954 (old forest), or whether the plot consisted entirely or mainly of non-forest habitat (crop or pasture land) in 1954 that had since regenerated (young forest).
Patch area and the distance to the nearest forest edge may also affect forest diversity (Pellissier et al. 2013; Kimberley et al. 2014), so we also measured these for each plot based on the SOLRIS wooded layer. However, we found that both of these measures were quite strongly correlated with total area of forested habitat. We therefore decided to include only the latter as the response variable representing landscape context, to minimize collinearity amongst predictors. However, we note that any significant effect of total area of forested habitat could also potentially be ascribed to these correlated metrics.
More complex topography may provide more niches and therefore increase plant diversity (Small and McCarthy 2005; Moeslund et al. 2013). We calculated an index of topographic complexity for each plot using 5 m contour lines in ArcGIS. First we determined the minimum sinuosity for all contours within each plot, where sinuosity is the length of a line divided by the shortest distance between the two endpoints. We took the inverse of this measure, such that plots with an inverse sinuosity near one had a very high variation in aspect, whereas plots with an inverse sinuosity of zero had no variation in aspect. Second, we measured the total length of all contour lines within the plot, and divided by the maximum total length found in any plot to give an index of steepness, with the steepest plot having a steepness of one. We added the sinuosity index and the steepness index to give a total for topographic variation within each plot. We determined the latitude of each plot to account for the well-known north–south gradient in species richness. Finally, we noted the calendar day on which each plot was surveyed in order to account for potential changes in species richness as the season progressed (e.g. spring ephemerals, late-season species).
The response variables for our analyses include native plant species richness, relative abundance of exotic plants (summed abundance of all exotic species divided by summed abundance of all species) and the presence or absence of at least one species of conservation concern. The explanatory variables included latitude, day of survey, amount of forest within 500 m (present and past), forest age, topographic complexity, and land ownership type. All variables were minimally inter-correlated (r ≤ 0.42, variance inflation factors ≤1.65) except for the amount of forest within 500 m in the present and in the past (r = 0.80). We used Spearman’s rank correlation tests to check which of these two variables was most strongly correlated with each response variable, and in all cases forest area in 1954 had a stronger correlation. Therefore, we used only past forest area in all our analyses (see Online Appendix S1 in Supplementary Materials). We standardized all continuous predictor variables by scaling them by their mean and standard deviation prior to the analyses.
Our analytical approach involved three steps. Because our study is observational, we used regression modelling to test for an effect of land ownership while accounting for the other potentially important factors. First, we aimed to find a reasonable model for each of our response variables, based on our candidate predictors and potential interactions between them. We built three models, one for native species richness, one for the relative abundance of exotics and one for the presence or absence of species of conservation concern. Models for native species richness and relative abundance of exotics met the assumptions for a linear model. To model presence or absence of species of conservation concern we used a logistic regression (generalized linear model with a binomial link function). For each response variable, we began with all the explanatory variables and all pairwise interactions between them. We then used backward stepwise model selection (using the stepAIC function in R) to determine the single variables and pairwise interactions in the ‘best’ model based on Akaike’s Information Criterion (AIC). We checked model residuals for spatial autocorrelation using spline correlograms, and if spatial autocorrelation was detected we incorporated a spatial autocovariate into the model (Dormann 2007; Bardos et al. 2015). We are aware that some authors consider stepwise model selection to be an inferior model selection tool. However, others have shown that it performs similarly to more complicated all-subset model selection methods (Murtaugh 2009), and our goal was simply to find an adequate model upon which to condition further tests (Banner and Higgs 2017). If land ownership did not appear in the selected model, we would conclude that land ownership was not likely an important factor for the response variable in question. For models of native species richness and the relative abundance of exotics, we report the adjusted R2 as a measure of the model’s goodness-of-fit. For the logistic model, we report AUC (area under the receiver-operating curve; Swets 1988) as a measure of the model’s classification accuracy.
Next, we tested the importance of each factor or interaction in the selected reasonable model while accounting for all other factors using the ‘drop1’ test in R, which performs marginal fitting of terms by comparing the full model to a model excluding the factor in question. We were primarily interested in land ownership, but we performed this test for all factors and interactions in the selected model. We report the results of F-tests or χ2 tests and the AIC values for each model comparison.
Finally, in order to visualize the size and direction of the influence of important factors or interactions on the response variables, while holding all other factors constant, we created partial regression plots. All analyses were carried out in R (R Foundation for Statistical Computing, Vienna, Austria), using the ‘ncf’ package for producing correlograms and the ‘spdep’ package for computing spatial autocovariates. We used the ‘visreg’ package in R (Breheney and Burchett 2013) to create partial regression plots.
Results
Forty-five percent of privately owned plots and 28% of PA plots were in young forest. The median amount of forest within 500 m of the plot in 1954 was 19.2 ha for privately owned plots and 41.0 ha for PA plots. In total, we recorded 705 vascular plant species (551 forbs/grasses/ferns, 80 shrubs and 74 trees). Total species richness per plot ranged from 30 to 176 with a mean of 90 species. Plots on private and protected land both had an average of 72.5 native species. Nonetheless, once all the other important predictors were accounted for, land ownership was a significant predictor of native species richness, with private sites tending to have higher native species richness (predicted values: 71.2 species for protected sites, 77.2 species for private sites, all other factors held at their median; Table 1; Fig. 2). Native species richness declined at higher latitudes, and increased with increasing forest coverage within 500 m in 1954 (Table 1). There were also two significant interactions (Table 1; Fig. S4 in Supplementary Materials).
The relative abundance of exotics ranged from 1.3% to 43.6% with a mean of 15.3%. Plots on private land had an average exotic relative abundance of 16.0% while plots on protected land averaged 13.5%. However, there was an interaction between ownership type and forest age, whereby private sites had higher relative abundance of exotics than protected sites only in old forests (Table 2; Fig. 3). In young forests, protected sites had higher abundance of exotic plants than private sites (Fig. 3). The relative abundance of exotic species declined with increasing forest area within 500 m and survey date, and increased with increasing topographic complexity, all else being equal (Table 2; Fig. S5 in Supplementary Materials).
Forty-two of 156 plots (27%) contained at least one plant species of conservation concern. Eleven of 46 plots within PAs (23.9%) contained at least one of these species, compared to 31 of 110 plots on private land (28.2%). However, the model for presence/absence of species of conservation concern included significant interactions between ownership type and forest age, as well as ownership type and the area of forest within 500 m (Table 3; Fig. 4). The likelihood of finding at least one species of conservation concern increased with the amount of forest on the surrounding landscape, but rare plants were seldom found on protected lands where the forest is young (Fig. 4). Only one of 13 plots on protected land in young forests contained a rare plant (7.7%), compared to 11 of 50 young forest plots on private land (22%). Plots with greater than 40 hectares of forest within 500 m in 1954 on protected land and in old forest had a very high predicted likelihood of species of conservation concern being present (Fig. 4). In plots with less than 40 ha of forest cover within 500 m, plots on privately owned sites had a higher likelihood of supporting a species of conservation concern than protected sites. There were also interactions between ownership type and latitude and ownership type and topographic variation (Fig. S6 in Supplementary Materials).
Partial regression plot based on the best model for presence/absence of at least one species of conservation concern showing the effect of forest area within 500 m in 1954, conditioned by land ownership type and forest age, with all other factors held constant at their median. Open circles are the raw data. Shaded areas are confidence intervals based on leave one out cross-validation repeated 1000 times
Discussion
The efficacy of PAs and privately owned land in conserving terrestrial biodiversity cannot be accurately compared without accounting for landscape context and history. Our data show that landscape context and forest age are important predictors of plant diversity in the fragmented forests of southern Ontario. Nonetheless, land ownership has an effect even when forest age and landscape context are accounted for, in particular via important interactions between land ownership type and these predictors. The effect of ownership type was in the opposite direction than expected, with privately-owned sites tending to have higher native species richness and a higher likelihood of supporting a species of conservation concern than sites within PAs, all else being equal.
The total amount of forest within 500 m was a significant predictor for all three response variables. Plots with more forest on the surrounding landscape tended to have higher native species richness, lower relative abundance of exotic species, and a higher likelihood of supporting at least one species of conservation concern. This is consistent with studies from Great Britain (Petit et al. 2004), British Columbia (McCune and Vellend 2015), and Massachusetts (McDonald et al. 2008). Sites with more forest on the surrounding landscape benefit from greater forest connectivity and greater distance from agricultural or residential sources of disturbance, and it is difficult to disentangle these two effects. Whatever the mechanism, native forest plant diversity tends to be higher in places with more forest on the surrounding landscape.
The age of a forest is also an important predictor of plant diversity. Sites in forests that had regenerated on former agricultural fields or pastures since the mid-1950s tended to have a higher abundance of exotic species, and lower likelihood of supporting a species of conservation concern. The legacy of past agricultural land use on regenerating forests can affect plant community diversity and composition for decades or even longer (Matlack 1994; Dupouey et al. 2002; Flinn and Vellend 2005; McDonald et al. 2008; Brown and Boutin 2009).
The model for presence or absence of at least one species of conservation concern revealed interesting interactions between land ownership and landscape context, and land ownership and forest age (Fig. 4). For privately owned sites, as the amount forest in the surrounding landscape increased, there was a gradual increase in the predicted likelihood of finding a species of conservation concern, regardless of forest age. For protected areas in old forests, there was a distinct threshold around 40 hectares after which the probability of supporting a species of conservation concern was very high. Young, protected forests had a very low probability of hosting a species of conservation concern, regardless of the landscape context. Kimberley et al. (2014) detected a similar effect in deciduous forests in Great Britain, where the average rarity of species in a plant community increased with greater patch area only in older forests. Interestingly, 40 ha is a threshold that has been noted repeatedly in the literature. McCune (2016) found that two of eight rare forest plant species never occurred in areas with less than 40 ha of forest within 500 m of a site. Similarly, Digiovinazzo et al. (2010) found that the number of forest indicator species in northern Italian forests reached a plateau at a patch size of 35–40 ha, and Boetsch et al. (2003) found that a rare plant of the southern Blue Ridge Mountains of the US did not occur in patches of suitable habitat less than 40 ha in size.
It is important to note that the species of conservation concern have been designated as such because of their low number of populations within Ontario, but they have different reasons for their rarity and different ecological requirements and life history strategies (e.g. Rabinowitz 1981). For example, Cornus florida (S2) and Castanea dentata (S2) are both trees that were formerly quite common before they were decimated by fungal diseases. Other species, such as the understory herbs Lithospermum latifolium (S3) and Stylophorum diphyllum (S1), may have been much more common before the extreme loss of floodplain forest habitat they favour, whereas the fern Asplenium scolopendrium (S3) may have always been relatively rare due to its specialization on limestone substrates. Thus, different species of conservation concern require different conditions to promote their survival and expansion. However, we did not see any obvious difference between the types of rare species occurring in PAs and private forest, with most species occurring on both land ownership types.
There are a number of potential explanations for the effects of land ownership type we observed. First, privately owned sites may support more native species on average, but these may tend to be “weedy”, disturbance-associated natives, while PAs support more sensitive late-successional species. For example, Maslo et al. (2015) showed that different guilds of rare bird species in New Jersey tended to concentrate in forests of different ownership, with early-successional species relying more heavily on privately owned forests. To test this, we modeled the average coefficient of conservatism of each of our plots in the same way as we modeled native species richness. The coefficient of conservatism ranges from 0 to 10, and is assigned to each species by experts based on habitat fidelity. Species tolerant of a wide range of conditions including highly disturbed areas have a coefficient of conservatism of 0–3, while species found only in a very narrow range of late-successional conditions receive a 9 or 10, with intermediate species in between (Oldham et al. 1995; Matthews et al. 2015). Privately owned plots in our study had a mean plot-level average coefficient of conservatism of 4.24, while plots in PAs had a mean of 4.48 (significantly higher, Wilcoxon rank sum test p < 0.05). However, once the other predictors were accounted for, land ownership was not a significant predictor of average coefficient of conservatism (see Appendix S3 in Supplementary Materials). The most important predictors of the average coefficient of conservatism were forest age, area of forest within 500 m, and latitude. Therefore, PAs in our dataset do not harbour significantly more conservative forest species than private sites, all else being equal.
Second, the patterns we observed could reflect differing levels of human visitors in PAs compared to privately owned sites. Both private sites and PAs in our sample experience a wide range of management practices, but we lack data to measure this. For example, some private woodlots undergo frequent selective logging, while others are used only for hunting or nature walks. Similarly, some of the PAs in our study have limited public access, but many are open access and favourite recreational areas for nearby urban populations. In 2000, the 500 conservation areas across Ontario had just over 5.7 million visitors (Baldin et al. 2015). This high level of recreational use could explain the higher abundance of exotic species we observed in young forests within PAs relative to young, privately owned sites, if visitors are maintaining open, disturbed areas and/or helping to disperse seeds of exotic species. This could also lead to lower species richness of native species in highly visited PAs, and lower likelihood of finding species of conservation concern in young protected sites with little forest on the surrounding landscape. Human disturbance via recreation is one of the greatest threats to plant species-at-risk in Canada (McCune et al. 2013).
Finally, the effect of land ownership could be due to correlation with unmeasured abiotic variables. Local abiotic conditions including soil pH, soil nutrient levels, and degree of shading are known to be important determinants of forest composition and diversity (e.g. Small and McCarthy 2005; Kimberley et al. 2014). For example, if forests on privately owned sites in our sample had higher average soil nitrogen availability than forests in PAs, this could be the cause of the greater native species richness in privately owned plots after accounting for landscape context, forest age, latitude and topography. The designation of PAs is often more opportunistic than strategic, and less-fertile lands not desired for agriculture are more easily acquired and protected (Scott et al. 2001; Rayner et al. 2014; Maslo et al. 2015). We did not measure local soil conditions at our sites, and so we cannot determine whether this is the case. However, an important determinant of soil nutrient status in temperate deciduous forests is past land use (former agriculture vs. continuous forest; Flinn and Vellend 2005), which is accounted for in our analysis by our determination of forest age. Future comparisons of PAs and private forests in our region would ideally measure soil nutrient levels and other soil factors that could influence plant diversity. Finally, we note that higher nitrogen levels in privately owned plots could explain higher native plant diversity, but it would not explain why private forests were more likely to contain species of conservation concern, since the vast majority of these species are not associated with high nutrient levels in soil.
Regardless of the causal mechanism, our study provides strong evidence for the importance of privately owned forests in conserving plant diversity on this fragmented landscape. Plots in PAs were more often located in mature forest and tended to be surrounded by more forest on the landscape. However, once these factors were accounted for, privately owned forests had higher native plant diversity, equal average coefficients of conservatism, and a greater likelihood of supporting one or more species of conservation concern. This pattern may also occur in other highly-populated regions, particularly where natural habitat is fragmented, and private landowners use these fragments in ways that have relatively low-impact (e.g. selective harvesting of trees, nature appreciation, or low-intensity grazing of grasslands). Our results highlight the importance of conservation programs, such as landowner incentive programs, that foster and encourage the maintenance of natural vegetation on private land. The designation of PAs is a worthy goal and will remain a cornerstone of the strategy to protect land from conversion to agricultural or residential land use. However, evaluations of PA effectiveness must control for the effects of landscape context and land-use history, or the role of private land in maintaining biodiversity may be underestimated.
References
Baldin E, Deadman P, Eagles PFJ (2015) Visitor statistics for conservation authorities in Ontario: current status and methods. University of Waterloo, Waterloo
Banner KM, Higgs MD (2017) Considerations for assessing model averaging of regression coefficients. Ecol Appl 27:78–93
Bardos DC, Guillera-Arroita G, Wintle BA (2015) Valid automodels for spatially autocorrelated occupancy and abundance data. Methods Ecol Evol 6:1137–1149
Boetsch JR, Van Manen FK, Clark JD (2003) Predicting rare plant occurrence in Great Smoky Mountains National Park, USA. Nat Area J 23:229–237
Breheney P, Burchett W (2013) visreg: visualization of regression models. R package version 2.0-2. http://CRAN.R-project.org/package=visreg
Brown CD, Boutin C (2009) Linking past land use, recent disturbance, and dispersal mechanism to forest composition. Biol Conserv 142:1647–1656
Coetzee BWT, Gaston KJ, Chown SL (2014) Local scale comparisons of biodiversity as a test for global protected area ecological performance: a meta-analysis. PLoS ONE 9:e105824
Crins WJ, Gray PA, Uhlig PWC, Wester MC (2009) The Ecosystems of Ontario, Part I: Ecozones and Ecoregions. Ontario Ministry of Natural Resources, Peterborough
Digiovinazzo P, Ficetola GF, Bottoni L, Andreis C, Padoa-Schioppa E (2010) Ecological thresholds in herb communities for the management of suburban fragmented forests. Forest Ecol Manag 259:343–349
Dormann CF (2007) Effects of incorporating spatial autocorrelation into the analysis of species distribution data. Global Ecol Biogeogr 16:129–138
Dupouey JL, Dambrine E, Laffite JD, Moares C (2002) Irreversible impact of past land use on forest soils and biodiversity. Ecology 83:2978–2984
Fahrig L (2013) Rethinking patch size and isolation effects: the habitat amount hypothesis. J Biogeogr 40:1649–1663
Flinn KM, Vellend M (2005) Recovery of forest plant communities in post-agricultural landscapes. Front Ecol Environ 3:243–250
Gaston KJ, Jackson S, Cantu-Salazar L, Cruz-Pinon G (2008) The ecological performance of protected areas. Annu Rev Ecol Syst 39:93–113
Graae BJ, Sunde PB (2000) The impact of forest continuity and management on forest floor vegetation evaluated by species traits. Ecography 23:720–731
Grashof-Bokdam CJ, Geertsema W (1998) The effect of isolation and history on colonization patterns of plant species in secondary woodland. J Biogeogr 25:837–846
Hilty J, Merenlender AM (2003) Studying biodiversity on private lands. Conserv Biol 17:132–137
Juffe-Bignoli D et al (2014) Protected planet report 2014. UNEP-WCMC. Cambridge, UK
Kerr JT, Deguise I (2004) Habitat loss and the limits to endangered species recovery. Ecol Lett 7:1163–1169
Kimberley A, Blackburn GA, Whyatt JD, Smart SM (2014) Traits of plant communities in fragmented forests: the relative influence of habitat spatial configuration and local abiotic conditions. J Ecol 102:632–640
Knight RL (1999) Private lands: the neglected geography. Conserv Biol 13:223–224
Larson BM, Riley JL, Snell EA, Godschalk HG (1999) The Woodland heritage of Southern Ontario. Federation of Ontario Naturalists, Don Mills
Maslo B, Lockwood JL, Leu K (2015) Land ownership patterns associated with declining forest birds: targeting the right policy and management for the right birds. Environ Conserv 42:216–226
Matlack GR (1994) Plant-species migration in a mixed-history forest landscape in eastern North-America. Ecology 75:1491–1502
Matthews JW, Spyreas G, Long CM (2015) A null model test of Floristic Quality Assessment: are plant species’ coefficients of conservatism valid? Ecol Indic 52:1–7
McCune JL (2016) Species distribution models predict rare species occurrences despite significant effects of landscape context. J Appl Ecol. doi:10.1111/1365-2664.12702
McCune JL, Vellend M (2015) Using plant traits to predict the sensitivity of colonizations and extirpations to landscape context. Oecologia 178:511–524
McCune JL, Harrower WL, Avery-Gomm S, Brogan JM, Csergö A-M, Davidson LNK, Garani A, Halpin LR, Lipsen LPJ, Lee C, Nelson JC, Prugh LR, Stinson CM, Whitney CK, Whitton J (2013) Threats to Canadian species at risk: an analysis of finalized recovery strategies. Biol Conserv 166:254–265
McDonald RI, Motzkin G, Foster DR (2008) Assessing the influence of historical factors, contemporary processes, and environmental conditions on the distribution of invasive species. J Torrey Bot Soc 135:260–271
Moeslund JE, Arge L, Bocher PK, Dalgaard T, Svenning JC (2013) Topography as a driver of local terrestrial vascular plant diversity patterns. Nord J Bot 31:129–144
Murtaugh PA (2009) Performance of several variable-selection methods applied to real ecological data. Ecol Letts 12:1061–1068
Oldham MJ, Bakowsky WD, Sutherland DA (1995) Floristic Quality Assessment System for Southern Ontario. Natural Heritage Information System, Ontario Ministry of Natural Resources, Peterborough
Oldham MJ, Brinker SR (2009) Rare Vascular Plants of Ontario, 4th edn. Natural Heritage Information Centre, Ontario Ministry of Natural Resources, Peterborough
Ontario Biodiversity Council (2010) State of Ontario’s biodiversity 2010. A report of the Ontario Biodiversity Council, Peterborough
Pellissier V, Berges L, Nedeltcheva T, Schmitt M-C, Avon C, Cluzeau C, Dupouey J-L (2013) Understorey plant species show long-range spatial patterns in forest patches according to distance-to-edge. J Veg Sci 24:9–14
Petit S, Griffiths L, Smart SM, Smith GM, Stuart RC, Wright SM (2004) Effects of area and isolation of woodland patches on herbaceous plant species richness across Great Britain. Landscape Ecol 19:463–471
Piessens K, Honnay O, Nackaerts K, Hermy M (2004) Plant species richness and composition of heathland relics in north-western Belgium: evidence for a rescue-effect? J Biogeogr 31:1683–1692
Rabinowitz D (1981) Seven forms of rarity. In: Synge H (ed) The biological aspects of rare plant conservation. Wiley, New York, pp 205–217
Rayner L, Lindenmayer DB, Wood JT, Gibbons P, Manning AD (2014) Are protected areas maintaining bird diversity? Ecography 37:43–53
Reich P, Frelich L (2002) Temperate deciduous forests. In: Mooney H, Canadell J (eds) Encyclopedia of global environmental change, vol 2., The earth system: biological and ecological dimensions of global environmental changeWiley, Chichester, pp 565–569
Richart M, Hewitt N (2008) Forest remnants in the Long Point region, Southern Ontario: tree species diversity and size structure. Landscape Urban Plan 86:25–37
Scott JM, Davis FW, McGhie RG, Wright RG, Groves C, Estes J (2001) Nature reserves: do they capture the full range of America’s biological diversity? Ecol Appl 11:999–1007
Small CJ, McCarthy BC (2005) Relationship of understory diversity to soil nitrogen topographic variation, and stand age in an eastern oak forest, USA. Forest Ecol Manag 217:229–243
Smyth I (2008) SOLRIS: Southern Ontario Land Resource Information System. Ontario Ministry of Natural Resources, Peterborough
Swets JA (1988) Measuring the accuracy of disgnostic systems. Science 240:1285–1293
Vellend M, Baeten L, Myers-Smith IH, Elmendorf SC, Beausejour R, Brown CD, De Frenne P, Verheyen K, Wipf S (2013) Global meta-analysis reveals no net change in local-scale plant biodiversity over time. PNAS 110:19456–19459
Verheyen K, Baeten L, De Frenne P, Bernhardt-Romermann M, Brunet J, Cornelis J, Decocq G, Dierschke H, Eriksson O, Hedl R, Heinken T, Hermy M, Hommel P, Kirby K, Naaf T, Peterken G, Petrik P, Pfadenhauer J, Van Calster H, Walther G, Wulf M, Verstraeten G (2012) Driving factors behind the eutrophication signal in understorey plant communities of deciduous temperate forests. J Ecol 100:352–365
Warman LD, Forsyth DM, Sinclair ARE, Freemark K, Moore HD, Barrett TW, Pressey RL, White D (2004) Species distributions, surrogacy, and important conservation regions in Canada. Ecol Lett 7:374–379
Whigham DF (2004) Ecology of woodland herbs in temperate deciduous forests. Annu Rev Ecol Evol Sys 35:583–621
Wilcove DS, Bean MJ, Long B, Snape WJ, Beehler BM, Eisenberg J (2004) The private side of conservation. Front Ecol Environ 2:326–331
Wilcove DS, McLellan CH, Dobson AP (1986) Habitat fragmentation in the temperate zone. In: Soule ME (ed) Conservation Biology: The science of scarcity and diversity. Sinauer Associates, Sunderland, pp 237–256
Zhang D, Heath LS, Ducey MJ, Butler B (2010) Relationships between major ownerships, forest aboveground biomass distributions, and landscape dynamics in the New England region of USA. Environ Manag 45:377–386
Acknowledgements
Thanks to A. Lammers, K. Tisshaw and M. Strub for assistance in the field. M. Oldham and C. Lacroix advised on species identifications. Permission to access land was granted by: Ausable Bayfield Conservation Authority, Bruce Trail Association, Conservation Halton, Grand River Conservation Authority, Hamilton Conservation Authority, Hamilton Naturalists Club, Lanark County Forests, Long Point Basin Land Trust, Ontario Heritage Foundation, Niagara Peninsula Conservation Authority, Upper Thames River Conservation Authority, and 102 private landowners. This project was undertaken with the financial support of the Government of Canada through the federal Department of the Environment. Funding was also provided by the Liber Ero fellowship program and the Ontario Species at Risk Stewardship Fund. The views expressed in this paper are those of the authors and do not necessarily represent those of the funding bodies.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
McCune, J.L., Van Natto, A. & MacDougall, A.S. The efficacy of protected areas and private land for plant conservation in a fragmented landscape. Landscape Ecol 32, 871–882 (2017). https://doi.org/10.1007/s10980-017-0491-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-017-0491-1