Abstract
Context
Regime shifts are well known for driving penetrating ecological change, yet we do not recognise the consequences of these shifts much beyond species diversity and productivity. Sound represents a multidimensional space that carries decision-making information needed for some dispersing species to locate resources and evaluate their quantity and quality.
Objectives
Here we assessed the effect of regime shifts on marine soundscapes, which we propose has the potential function of strengthening the positive or negative feedbacks that mediate ecosystem shifts.
Methods
We tested whether biologically relevant cues are altered by regime shifts in kelp forests and seagrass systems and how specific such shifted soundscapes are to the type of driver; i.e. local pollution (eutrophication) vs. global change (ocean acidification).
Results
Here, we not only provide the first evidence for regime-shifted soundscapes, but also reveal that the modified cues of shifted ecosystems are similar regardless of spatial scale and type of environmental driver. Importantly, biological sounds can act as functional cues for orientation by dispersing larvae, and observed shifts in soundscape loudness may alter this function.
Conclusions
These results open the question as to whether shifted soundscapes provide a functional role in mediating the positive or negative feedbacks that govern the arrival of species associated with driving change or stasis in ecosystem state.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surprisingly large shifts towards a simplification of species composition and ecosystem function, often known as regime shifts, occur within human-dominated landscapes of the terrestrial (Walker 1993), freshwater (Carpenter et al. 1999) and marine realms (Bellwood et al. 2004). The consequences of simplification of species diversity are often well known, generally resulting in less productivity than comparable assemblages with more species (Cardinale et al. 2006; Stachowicz et al. 2007). Yet, there remains less appreciation of change among other ecosystem properties that may yield and maintain such large shifts. Indeed, the question of how regime shifts affect other key ecosystem functions remains relatively understudied. One such function is the biological sounds that ecosystems produce (soundscapes, sensu Krause 1987) as potential orientational cues for dispersing taxa that replenish populations (Simpson et al. 2005; Vermeij et al. 2010; Lillis et al. 2015; Stanley et al. 2015) and as biological cues to evaluate habitat quantity and quality (Piercy et al. 2014).
In human-dominated landscapes, the positive and negative feedbacks that hasten or maintain an ecosystem in a particular state are partly a function of the ability for recovery of inhabitants that comprised the previous state (Scheffer and Carpenter 2003). We propose that soundscapes could act as positive or negative feedback by mediating the arrival of species associated with alternate ecosystem states. A soundscape is a mix of biological, geophysical and anthropogenic sounds that reflect various landscape features and ecosystem processes (Pijanowski et al. 2011). Soundscape ecology remains a relatively young field of research undergoing rapid progress (Servick 2014), with particular value for applied ecology, including the study of regime shifts. The quality of individual signals produced by soniferous animals is affected by stress levels (Buchanan et al. 2003), habitat condition (Laiolo et al. 2008) and climate (Botero et al. 2009; Narins and Meenderink 2014). Hence, ecosystems produce distinct acoustic signatures associated with their biodiversity (Sueur et al. 2008) and their ecological condition such as coral cover (Piercy et al. 2014; Tucker et al. 2014).
Sound represents a multidimensional space that carries decision-making information needed for locating resources (Farina et al. 2011). This information informs both resident and dispersing species about the composition of the local species community, the amount of available resources (such as, food, territory and partners), and the presence of potential predators (Slabbekoorn and Bouton 2008; Farina et al. 2011; Simpson et al. 2011; Parmentier et al. 2015). Sound is one of the most reliable directional cues in the ocean because it propagates to long distances with little attenuation and in a predictable fashion. In the marine realm, a variety of marine larval fish species respond to specific soundscapes in a species-specific manner to mediate two possible settlement strategies. Some species orient towards a habitat by being selectively attracted to its soundscape while others select habitats by avoidance of non-preferred soundscapes (Simpson et al. 2011; Parmentier et al. 2015). From the perspective of a dispersing propagule, the spatial heterogeneity of soundscape can therefore be used as a qualitative and directional guide towards the most appropriate adulthood habitat.
We hypothesized that ecosystems, which shift in state, regardless of the identity and scale of driver, produce distinct soundscapes that reflect their particular state. If regime-shifted soundscapes exist, such a finding would support the proposition that sound may well represent a key function that changes with regime shifts. In this study we focus on regime shifts in kelp forests and seagrass beds as model systems for assessing the consequences of locally and globally driven change. Locally driven shifts were assessed in systems in which nutrient pollution drives shifts from a highly diverse and productive state to a less diverse and less productive state (Neverauskas 1987; Connell et al. 2008; Gorman et al. 2009). Globally driven shifts, such as resulting from ocean acidification, were assessed in systems in which natural CO2 vents mimic future change due to increasing human greenhouse gas emissions (Nagelkerken et al. 2016).
Materials and methods
Study sites
Eutrophication is the main driver of ecosystem regime shifts in marine systems on the metropolitan coast of Adelaide (South Australia) (Connell et al. 2008; Gorman et al. 2009). Rocky reefs in this region are typically dominated by canopy forming kelp forests consisting of Eklonia radiata (Connell and Irving 2008). However, excessive nutrient input into this naturally oligotrophic system has driven a shift from kelp to algal turf domination over the last 40 years (Connell et al. 2008). Kelp forests are highly productive carbon storage systems (Copertino et al. 2005) and create a complex three dimensional habitat with their blades and convoluted holdfasts. Snapping shrimps (family Alpheidae), which are the most ubiquitous and noisy soniferous animal in coastal systems (Johnson et al. 1947a; Knowlton and Moulton 1963), commonly inhabit the holdfasts of these canopy-forming algae (Thiel and Vásquez 2000). A previous study that investigated the crypto fauna assemblages living in E. radiata holdfasts in southern Australia found snapping shrimps present in 26 % of the holdfasts collected (62/240) (Connell unpublished data, see Fowler-Walker et al. 2005 for experimental design).
We identified an area between Horseshoe reef and Port Noarlunga (South Australia) where kelp forests and turf dominated areas are present in close proximity and with comparable abiotic factors (Connell et al. 2008) (depth between 1.5 and 9 m depending on site and tide, sedimentary rocks). In this area we selected three kelp forests (continuous canopy patch for a minimum diameter of ~20 m punctuated by small gaps of 1–2 m) and three algal turf dominated (uninterrupted turf forming algae for a minimum diameter of ~20 m punctuated by small kelp remnants) sampling stations where we made audio recordings (Table S1). Recordings at each control and degraded site were replicated three times during different months (Table S2).
Seagrass meadows (Posidonia spp. and Amphibolis spp.) extensively cover sandy substrate in the Gulf St. Vincent (South Australia). However, excessive eutrophication caused by sewage discharge resulted in large areas of seagrass meadows to be overgrown by epiphytes and ultimately die causing the system to shift to a bare sandy substratum (Neverauskas 1987; Walker and McComb 1992). Based on the most recent (year 2013) seagrass cover maps and aerial images (Hart 2013) we identified an area in front of Semaphore beach where extensive patches of seagrass have receded due to eutrophication (sandy patch minimum diameter: 500 m). Surrounding areas with the same water depth (12–14 m), distance to shore and sediment type (Jason Tanner personal communication) were covered by seagrass meadows that we used as controls. We selected three seagrass and three sand sampling stations for our audio recordings (Table S1). Recordings at each control and degraded site were replicated two times (Table S2).
Natural CO2 vents
Three natural CO2 vents in temperate waters–Vulcano Island and Ischia Island (Italy) and White Island (New Zealand) (Table S1)—were selected to assess the effect of ocean acidification on marine soundscapes, resulting from end-of-century levels of anthropogenic CO2 as forecasted under a business-as-usual emission scenario (Meinshausen et al. 2011) (Table S2). Natural CO2 vents, unaffected by elevated temperatures (Table S3), are present in the northern part of Levante Bay at Vulcano Island (Italy). A pH gradient was present with values that range from extreme (>2000 µatm pCO2) to roughly ambient levels along a 700-m stretch of rocky coast along the northern side of Levante Bay (Boatta et al. 2013; Nagelkerken et al. 2016; Rossi et al. 2016a). We selected an elevated CO2 and a control area (Table S3) along this gradient for our sound recordings, which consisted of three temporal replicates for each area (Table S2). Similarly, at the island of Ischia (Italy), shallow CO2 vents are present around Castello Aragonese where sound recordings were done at two well characterized sites based on previous studies (Hall-Spencer et al. 2008; Kerrison et al. 2011) (Table S1). White Island is a volcanic island located in the Bay of Plenty, North Island of New Zealand. CO2 vents are located along the north-east rocky coast of the island (Brinkman and Smith 2015; Nagelkerken et al. 2016; Rossi et al. 2016a). We selected an elevated CO2 and a control site for the sound recordings, which consisted of two temporal replicates for each site (Table S2). Details of the sampling design are shown in Table S2 and the characterization methods of the carbonate chemistry (as shown in Table S3) are described in the Supplementary methods.
Soundscape recordings and analysis
The most common source of marine biological sounds are from snapping shrimps snaps, sea urchins rasping and fish vocalizations all of which are known to peak at dusk during the so called “dusk chorus” and remain active until dawn (Staaterman et al. 2014). For this reason we kept the sampling time constant across all recordings (i.e. from 1 h before to 1 h after sunset). This sampling regime does not aim to comprehensively characterize the soundscapes of these habitats but rather to answer specific questions related to the effects of regime shifts. To enable comparison among sites with different water depths we positioned the hydrophone (held by hand from a craft) at one meter elevation above the substratum in all recordings with the exception of White Island where the same recording apparatus described above was enclosed in a waterproofed barrel fixed to an anchored buoy for overnight recordings (Rossi et al. 2016a). Here the hydrophone was deployed at 5 meters depth over a rocky seafloor whose sea surface ranged between 8 and 12 m depending on site and tide. The recordings were made with a Hydrophone (HiTech HTI-96-MIN with inbuilt preamplifier, manufacturer-calibrated sensitivity −164.1 dB re 1 V/μPa−1; frequency range 0.02–30 kHz; calibrated by manufacturers; High Tech Inc., Gulfport, USA) and a calibrated audio recorder (PCM-M10, 48 kHz sampling rate, Sony Inc., Tokyo). All recordings were high pass filtered at 300 Hz in order to remove the noise of breaking waves (present in White Island). Recordings of at least 7–8 min were obtained in the field in order to allow for unwanted sounds, such as passing boats, to be excluded from the analyses. For consistency, samples of 5-min duration were used in the analysis for all sites. Recordings were acquired at a sampling rate of 96 kHz and 32 bit. Power spectra and sound levels (RMS) were calculated with the package PAMguide (Merchant et al. 2015) in MATLAB® (Natick, MA, USA) and calibrated in SASLab Pro (Avisoft bioacoustics) using a reference signal at full (1 kHz) scale and the hydrophone sensitivity. Power spectra were calculated using the Hann window (length 1 s and 50 % overlap). The frequency of snapping shrimp snaps was measured on band-pass filtered recordings (2.5–30 kHz) using the pulse train analysis function in the software SAS lab pro (Avisoft bioacoustics), where an artificially generated full scale snap was inserted at the beginning of each recording in order to allow valid comparisons between recordings with different noise levels. Sound pressure level (SPL) and frequency of snaps were averaged across multiple days of sampling for each site. Additional details on the experimental design are shown in Table S2. The waveforms and spectrograms of the recordings were visually and aurally inspected in Audition CS6 (Adobe, San Jose, USA) for other sources of biological sound. Boat noise and artefacts were deleted if present.
Fish and invertebrate hearing range
Sound in water is characterized by two components: sound pressure and particle displacement. All fish are able to detect particle displacement with their otoliths and some are sensitive to both particle displacement and sound pressure due to a series of anatomical specializations such as gas chambers and connections between the swim bladder and the inner ear (Popper and Fay 2011). It is worth noting that some fish species can detect sound pressure even if not equipped with such anatomical specializations, suggesting that our morpho-functional understanding of fish hearing is still incomplete (Popper and Fay 2011). The same is true for invertebrates where often, despite showing behavioural and physiological evidence of hearing ability, we cannot associate this sensitivity to any obvious anatomical specialization (Montgomery et al. 2006). Studies suggest that the sound pressure sensitivity in fish ranges widely from a few Hertz to 1, 5 kHz or even higher in some species (Montgomery et al. 2006; Popper and Fay 2011). In order to capture this wide range of sensitivities we calculated the sound pressure levels in our recordings in three overlapping frequency bands (<1, <3, <5 kHz).
Statistical analysis
The sound pressure level in the hearing range of fish (<1, <3 and <5 kHz), full bandwidth (<10 kHz) and the frequency of snapping shrimp snaps were averaged across days of sampling and then tested for the factor habitat condition with one-way ANOVAs using spatial replication (n = 3 sampling stations per treatment for each of the rocky reef and seagrass system) (Table S2). For ocean acidification, the three natural CO2 vents served as spatial replicates. In order to allow multiple comparisons (3) the Bonferroni correction was applied and α adjusted from 0.05 to 0.017. The interaction between the factor stressor (nutrient/CO2) and habitat condition (control/degraded) was tested with a two-way ANOVA on data standardized by habitat. Each habitat group (kelp, seagrass and mixed) was standardized to its mean before the computation. The link between full bandwidth sound pressure level and frequency of snapping shrimp snaps was analysed with a simple regression.
Results
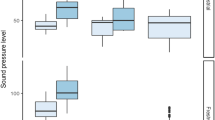
Regime shifts triggered by nutrient pollution and ocean acidification correspond to reduced levels of sound pressure in kelp-dominated and mixed macroalgae-seagrass habitats (nutrient pollution on kelp forests, ANOVA, F(1,4) = 39.3, p = 0.003, ocean acidification on macroalgae-seagrass, ANOVA, F(1,4) = 24.7, p = 0.008) (Fig. 1a). Likewise, the rate of snapping shrimp snaps decreased following the same pattern suggesting that snapping shrimp sound is a key component of biological sound in both nutrient driven change to kelp forests (ANOVA, F(1,4) = 150.2, p < 0.001) and ocean acidification driven change in macroalgae-seagrass habitats (ANOVA, F(1,4) = 32, p = 0.005) (Fig. 1b). Despite the low levels of biogenic noise that characterized seagrass beds, nutrient pollution caused a reduction in the rate of snapping shrimp snaps and a marginal reduction in level of full bandwidth sound pressure (snaps, ANOVA, F(1,4) = 103.1, p < 0.001, full bandwidth sound pressure level, ANOVA, F(1,4) = 8.34, p = 0.045, α = 0.017 with Bonferroni correction). The reduction in number of snaps and full bandwidth sound pressure level was independent of the type of stressor when habitat specific differences were standardized (ANOVA, snap frequency: condition × stressor interaction, F(1,14) = 0.55, p = 0.46, sound pressure level: F(1,14) = 0.55, p = 0.46). Regression analysis incorporating all recordings across habitat types, seasons, and locations showed that the variation in rate of snapping shrimp snaps significantly explained 84 % of the variation (F(1,41) = 271.8, p < 0.001) in full bandwidth sound pressure level (Fig. S1).
Responses of soundscapes to regime shifts a full bandwidth root mean square sound pressure level (>300 Hz, <10 kHz) ± SE. b Mean rate of snapping shrimp snaps counted per minute ± SE. Error bars represent variability due to spatial replication (N = 3 per treatment). Stars indicate significant differences between bar under the brackets
The sound pressure level was also analysed for three frequency bands relevant to fish hearing: 1, <3 and <5 kHz. Sound levels were reduced in the frequency range <5 kHz due to regime shifts triggered by nutrient pollution and ocean acidification on rocky and mixed rocky-seagrass bottoms (kelp forest, ANOVA, F(1,4) = 35, p < 0.004, ocean acidification, ANOVA, F(1,4) = 76.7, p < 0.001) but not on seagrass beds (Fig. 2). In the two lower frequency ranges, however, the sound levels did not decrease significantly due to habitat degradation. Power spectral density plots for nutrient enriched habitats are included in the supplementary materials (Fig. S2, while those for CO2 enrichment are published elsewhere (Rossi et al. 2016a).
Discussion
We propose a new concept; that regime shifts, regardless of their driver, produce distinct soundscapes. We found that although each ecosystem was associated with distinct soundscapes, the shift from diverse and structurally complex macroalgae like kelp, to low lying turfs (Connell et al. 2008) or from seagrass to sand (Bryars et al. 2011), was associated with a reduction in biological sound. Importantly, although these ecosystem shifts are caused by resource enhancement, the driving mechanisms are different; i.e. local nitrogen enrichment (seagrass, Bryars et al. 2011; kelp, Gorman et al. 2009) versus CO2 enrichment at natural vents (kelp, Connell et al. 2013; kelp and seagrass, Nagelkerken et al. 2016). It was striking that regardless of the driver of regime shift (eutrophication or ocean acidification) the magnitude of changes in sound was remarkably similar. It is possible that despite the diversity in drivers of regime shifts, they not only result in similar ecological outcomes of reduced species diversity and productivity, but also reduced soundscapes and the orientational cues they offer. Common to regime shifts is a reduction in resources (Cardinale et al. 2006; Stachowicz et al. 2007), such as food and habitat to inhabiting species, which dispersing animals may be able to detect. As underwater coastal sounds in both temperate and tropical areas are largely biological (Johnson et al. 1947b), the degradation of the habitat is reflected as an impoverished soundscape. This also suggests that soundscapes are a promising, rapid and cost effective monitoring tool for ecosystem condition (Piercy et al. 2014; Tucker et al. 2014).
Soundscapes were dominated by snapping shrimp sound. Despite being more intense on rocky reefs than soft sediment, snapping shrimp crackle was not only ubiquitous, but it also dominated the soundscapes of all types of habitat and their shifts to lower diversity and complexity. This predominance is evidenced by the correlation (84 %) between the rate of snapping shrimp snaps and the total loudness of all seascape sounds. Kelp forests are not only more productive than turf dominated reefs (Copertino et al. 2005), but they also create habitat for cryptic animals like snapping shrimps with their convoluted holdfasts (Fowler-Walker et al. 2005). Similarly, soft sediment covered by seagrass has a much higher productivity compared to bare sand and creates habitat and shelter for a number of organisms (Hemminga and Duarte 2000). Although the quantification of the density of shrimps is difficult due to the highly cryptic nature of these animals, their snapping rate and intensity (i.e. within their main frequency band) are commonly used as an index of their abundance (Kennedy et al. 2010; Lillis et al. 2014; Piercy et al. 2014; Staaterman et al. 2014; Nedelec et al. 2015). In both kelp and seagrass ecosystems, a decrease in snapping reflected the regime-shifts that result in a simplification of habitat complexity and loss of habitat (i.e. kelp holdfasts).
The ecological implications of impoverished biological soundscapes may depend on the extent to which a species relies on sound for navigation. Time of day may also play a role as the effects found in this study are likely to be less marked during daytime when snapping shrimps are less active. Many marine vertebrates (Simpson et al. 2005; Montgomery et al. 2006; Huijbers et al. 2012; Parmentier et al. 2015) and invertebrates (Vermeij et al. 2010; Lillis et al. 2015; Stanley et al. 2015) can use sound as directional cue towards settlement habitats or away from undesired habitats. Based on the reduction of soundscapes measured, we suggest that those organisms that rely more heavily on higher frequency sound (i.e. snapping shrimp sounds >500 Hz) are likely to be more penalized by shifted soundscapes. However, this effect remains hard to quantify because of our incomplete knowledge about sound pressure hearing sensitivities in fish and invertebrate larvae and the complexity of sound propagation in shallow waters.
Models suggest that ocean acidification levels expected by the end of the century will significantly decrease sound absorption in the ocean (Ilyina et al. 2010), potentially compensating for the loss in biogenic sound caused by ocean acidification. (Ilyina et al. 2010) predict that sound absorption will be attenuated at <10 kHz and will be most pronounced in the lower part of this range. However, by considering all important energy loss mechanisms the effect of pH on sound absorption is negligible in shallow waters and at the distances likely to be relevant for larval orientation (<10 km) because geometric spreading accounts for most of the sound loss from the source (Au and Hastings 2008; Reeder and Chiu 2010).
We propose that if regime shifts produce distinct soundscapes that reflect their particular state, sound may represent a functional change of regime shifts. Shifted soundscapes may act as one of the mechanisms that control resistance or resilience of ecosystems, notably where sound mediates the supply of keystone species. For example, shifted soundscapes could hamper the recovery of ecosystems by directly attracting a lower number of engineer species’ propagules such as coral and oyster larvae (Vermeij et al. 2010; Lillis et al. 2015). Analogously, chemical cues from coral or seaweeds reinforce the persistence of coral or seaweed dominated alternative states respectively by acting as attractor or repellent for dispersing coral and fish larvae (Dixson et al. 2014).
Another example is that of simplification of soundscapes could lead to reduced supply of herbivores that resist ecosystem shifts (e.g. Ghedini et al. 2015), and that would act as negative feedbacks to stabilise the shifted state characterised by low herbivory (i.e. resistance to positive change: Connell and Ghedini 2015). Shifted soundscapes could also reduce the settlement of keystone predators, such as lobsters (Ling et al. 2009; Stanley et al. 2015), which are crucial to limit catastrophic overgrazing by sea urchins and maintain the system in its kelp-dominated state. Either way, we propose that if shifted soundscapes exist as a functional ecosystem trait, then the possibility that they function as positive or negative feedbacks to dispersing taxa that govern regime shifts also exists.
Dispersing propagules may be able to compensate for lowered soundscape value by using alternative cues such as olfaction and vision (Dixson et al. 2010; Ferrari et al. 2012). However, these cues have a limited reach relative to sound which is one of the cues that propagate the furthest and most predictably with distance from the source (Montgomery et al. 2006; Simpson et al. 2013). Furthermore, the value of these cues is not immune to habitat degradation and is likely to similarly decrease (Dixson et al. 2014). This will be further exacerbated in the future, where regardless of the type of cue used, ocean acidification will disrupt propagule orientation by causing ineffective processing of all these sensory cues (Munday et al. 2009; Dixson et al. 2010; Ferrari et al. 2012; Rossi et al. 2015, 2016b).
In conclusion, the simplification of habitats within human-dominated landscapes has similarly profound effects on their soundscapes. The specific consequences remain elusive, but this concept is likely to attract considerable attention given its potential to mediate a diversity of key ecological processes and become a new cost effective monitoring tool. Importantly, our study reveals that regime shifts within human-dominated landscapes impoverish natural soundscapes, tending towards ‘sounds of silence’.
References
Au WW, Hastings MC (2008) Principles of marine bioacoustics. Springer, New York
Bellwood DR, Hughes TP, Folke C, Nyström M (2004) Confronting the coral reef crisis. Nature 429(6994):827–833
Boatta F, D’Alessandro W, Gagliano A, Liotta M, Milazzo M, Rodolfo-Metalpa R, Hall-Spencer JM, Parello F (2013) Geochemical survey of Levante Bay, Vulcano Island (Italy), a natural laboratory for the study of ocean acidification. Mar Pollut Bull 73(2):485–494
Botero CA, Boogert NJ, Vehrencamp SL, Lovette IJ (2009) Climatic patterns predict the elaboration of song displays in mockingbirds. Curr Biol 19(13):1151–1155
Brinkman T, Smith A (2015) Effect of climate change on crustose coralline algae at a temperate vent site, White Island New Zealand. Mar Freshw Res 66(4):360–370
Bryars S, Collings G, Miller D (2011) Nutrient exposure causes epiphytic changes and coincident declines in two temperate Australian seagrasses. Mar Ecol Prog Ser 441:89–103
Buchanan KL, Spencer K, Goldsmith A, Catchpole C (2003) Song as an honest signal of past developmental stress in the European starling (Sturnus vulgaris). Proc R Soc Lond B 270(1520):1149–1156
Cardinale BJ, Srivastava DS, Duffy JE, Wright JP, Downing AL, Sankaran M, Jouseau C (2006) Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 443(7114):989–992
Carpenter SR, Ludwig D, Brock WA (1999) Management of eutrophication for lakes subject to potentially irreversible change. Ecol Appl 9(3):751–771
Connell SD, Ghedini G (2015) Resisting regime-shifts: the stabilising effect of compensatory processes. Trends Ecol Evol 30(9):513–515
Connell SD, Irving AD (2008) Integrating ecology with biogeography using landscape characteristics: a case study of subtidal habitat across continental Australia. J Biogeogr 35(9):1608–1621
Connell SD, Kroeker KJ, Fabricius KE, Kline DI, Russell BD (2013) The other ocean acidification problem: CO2 as a resource among competitors for ecosystem dominance. R Soc Philos Trans Biol Sci 368(1627):20120442
Connell S, Russell B, Turner D, Shepherd SA, Kildea T, Miller D, Airoldi L, Cheshire A (2008) Recovering a lost baseline: missing kelp forests from a metropolitan coast. Mar Ecol-Prog Ser 360:63–72
Copertino M, Connell SD, Cheshire A (2005) The prevalence and production of turf-forming algae on a temperate subtidal coast. Phycologia 44(3):241–248
Dixson DL, Abrego D, Hay ME (2014) Chemically mediated behavior of recruiting corals and fishes: a tipping point that may limit reef recovery. Science 345(6199):892–897
Dixson DL, Munday PL, Jones GP (2010) Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol Lett 13(1):68–75
Farina A, Lattanzi E, Malavasi R, Pieretti N, Piccioli L (2011) Avian soundscapes and cognitive landscapes: theory, application and ecological perspectives. Landscape Ecol 26(9):1257–1267
Ferrari MC, McCormick MI, Munday PL, Meekan MG, Dixson DL, Lonnstedt O, Chivers DP (2012) Effects of ocean acidification on visual risk assessment in coral reef fishes. Funct Ecol 26(3):553–558
Fowler-Walker MJ, Connell SD, Gillanders BM (2005) To what extent do geographic and associated environmental variables correlate with kelp morphology across temperate Australia? Mar Freshw Res 56(6):877–887
Ghedini G, Russell BD, Connell SD (2015) Trophic compensation reinforces resistance: herbivory absorbs the increasing effects of multiple disturbances. Ecol Lett 18(2):182–187
Gorman D, Russell BD, Connell SD (2009) Land-to-sea connectivity: linking human-derived terrestrial subsidies to subtidal habitat change on open rocky coasts. Ecol Appl 19(5):1114–1126
Hall-Spencer JM, Rodolfo-Metalpa R, Martin S, Ransome E, Fine M, Turner SM, Rowley SJ, Tedesco D, Buia MC (2008) Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454(7200):96–99
Hart D (2013) Seagrass extent change 2007-13-Adelaide coastal waters. DEWNR Technical note 2013/07. Government of South Australia, through Department of Environment, Water and Natural Resources, Adelaide
Hemminga MA, Duarte CM (2000) Seagrass ecology. Cambridge University Press, Cambridge
Huijbers CM, Nagelkerken I, Lössbroek PAC, Schulten IE, Siegenthaler A, Holderied MW, Simpson SD (2012) A test of the senses: fish select novel habitats by responding to multiple cues. Ecology 93(1):46–55
Ilyina T, Zeebe RE, Brewer PG (2010) Future ocean increasingly transparent to low-frequency sound owing to carbon dioxide emissions. Nat Geosci 3(1):18–22
Johnson MW, Everest FA, Young RW (1947a) The role of snapping shrimp (Crangon and Synalpheus) in the production of underwater noise in the sea. Biol Bull 93(2):122–138
Johnson MW, Everest FA, Young RW (1947b) The role of snapping shrimp (Crangon and Synalpheus) in the production of underwater noise in the sea. Biol Bull 93(2):122–138
Kennedy E, Holderied M, Mair J, Guzman H, Simpson S (2010) Spatial patterns in reef-generated noise relate to habitats and communities: evidence from a Panamanian case study. J Exp Mar Biol Ecol 395(1):85–92
Kerrison P, Hall-Spencer JM, Suggett DJ, Hepburn LJ, Steinke M (2011) Assessment of pH variability at a coastal CO 2 vent for ocean acidification studies. Estuar Coast Shelf Sci 94(2):129–137
Knowlton RE, Moulton JM (1963) Sound production in the snapping shrimps Alpheus (Crangon) and Synalpheus. Biol Bull 125(2):311–331
Krause B (1987) Bioacoustics, habitat ambience in ecological balance. Whole Earth Rev 57:14–18
Laiolo P, Vögeli M, Serrano D, Tella JL (2008) Song diversity predicts the viability of fragmented bird populations. PLoS One 3(3):e1822
Lillis A, Bohnenstiehl DR, Eggleston DB (2015) Soundscape manipulation enhances larval recruitment of a reef-building mollusk. PeerJ 3:e999
Lillis A, Eggleston DB, Bohnenstiehl D (2014) Estuarine soundscapes: distinct acoustic characteristics of oyster reefs compared to soft-bottom habitats. Mar Ecol Prog Ser 505:1–17
Ling S, Johnson C, Frusher S, Ridgway K (2009) Overfishing reduces resilience of kelp beds to climate-driven catastrophic phase shift. Proc Natl Acad Sci USA 106(52):22341–22345
Meinshausen M, Smith SJ, Calvin K, Daniel JS, Kainuma MLT, Lamarque JF, Matsumoto K, Montzka SA, Raper SCB, Riahi K, Thomson A, Velders GJM, van Vuuren DPP (2011) The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Clim Change 109(1–2):213–241
Merchant ND, Fristrup KM, Johnson MP, Tyack PL, Witt MJ, Blondel P, Parks SE (2015) Measuring acoustic habitats. Methods Ecol Evol 6(3):257–265
Montgomery JC, Jeffs A, Simpson SD, Meekan M, Tindle C (2006) Sound as an orientation cue for the pelagic larvae of reef fishes and decapod crustaceans. Adv Mar Biol 51:143–196
Munday PL, Dixson DL, Donelson JM, Jones GP, Pratchett MS, Devitsina GV, Doving KB (2009) Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc Natl Acad Sci USA 106(6):1848–1852
Nagelkerken I, Russell BD, Gillanders BM, Connell SD (2016) Ocean acidification alters fish populations indirectly through habitat modification. Nat Clim Change 6(1):89–93
Narins PM, Meenderink SW (2014) Climate change and frog calls: long-term correlations along a tropical altitudinal gradient. Proc R Soc Lond B 281(1783):20140401
Nedelec SL, Simpson SD, Holderied M, Radford AN, Lecellier G, Radford C, Lecchini D (2015) Soundscapes and living communities in coral reefs: temporal and spatial variation. Mar Ecol Prog Ser 524:125–135
Neverauskas V (1987) Monitoring seagrass beds around a sewage sludge outfall in South Australia. Mar Pollut Bull 18(4):158–164
Parmentier E, Berten L, Rigo P, Aubrun F, Nedelec SL, Simpson SD, Lecchini D (2015) The influence of various reef sounds on coral-fish larvae behaviour. J Fish Biol 86(5):1507–1518
Piercy JJ, Codling EA, Hill AJ, Smith DJ, Simpson SD (2014) Habitat quality affects sound production and likely distance of detection on coral reefs. Mar Ecol Prog Ser 516:35–47
Pijanowski BC, Farina A, Gage SH, Dumyahn SL, Krause BL (2011) What is soundscape ecology? An introduction and overview of an emerging new science. Landscape Ecol 26(9):1213–1232
Popper AN, Fay RR (2011) Rethinking sound detection by fishes. Hear Res 273(1):25–36
Reeder DB, Chiu C-S (2010) Ocean acidification and its impact on ocean noise: phenomenology and analysis. J Acoust Soc Am 128(3):EL137–EL143
Rossi T, Connell SD, Nagelkerken I (2016a) Silent oceans: ocean acidification impoverishes natural soundscapes by altering sound production of the world’s noisiest marine invertebrate. Proc R Soc Lond B 283(1826):20153046
Rossi T, Nagelkerken I, Pistevos JCA, Connell SD (2016b) Lost at sea: ocean acidification undermines larval fish orientation via altered hearing and marine soundscape modification. Biol Lett 12(1):20150937
Rossi T, Nagelkerken I, Simpson SD, Pistevos JCA, Watson S-A, Merillet L, Fraser P, Munday PL, Connell SD (2015) Ocean acidification boosts larval fish development but reduces the window of opportunity for successful settlement. Proc R Soc Lond B 282(1821):20151954
Scheffer M, Carpenter SR (2003) Catastrophic regime shifts in ecosystems: linking theory to observation. Trends Ecol Evol 18(12):648–656
Servick K (2014) Eavesdropping on ecosystems. Science 343(6173):834–837
Simpson SD, Meekan M, Montgomery J, McCauley R, Jeffs A (2005) Homeward sound. Science 308(5719):221
Simpson SD, Munday PL, Wittenrich ML, Manassa R, Dixson DL, Gagliano M, Yan HY (2011) Ocean acidification erodes crucial auditory behaviour in a marine fish. Biol Lett 7(6):917–920
Simpson SD, Piercy JJ, King J, Codling EA (2013) Modelling larval dispersal and behaviour of coral reef fishes. Ecol Complex 16:68–76
Slabbekoorn H, Bouton N (2008) Soundscape orientation: a new field in need of sound investigation. Anim Behav 76(4):e5–e8
Staaterman E, Paris CB, DeFerrari HA, Mann DA, Rice AN, D’Alessandro EK (2014) Celestial patterns in marine soundscapes. Mar Ecol Prog Ser 508:17–32
Stachowicz JJ, Bruno JF, Duffy JE (2007) Understanding the effects of marine biodiversity on communities and ecosystems. Annu Rev Ecol Evol Syst 38:739–766
Stanley JA, Hesse J, Hinojosa IA, Jeffs AG (2015) Inducers of settlement and moulting in post-larval spiny lobster. Oecologia 178(3):685-697
Sueur J, Pavoine S, Hamerlynck O, Duvail S (2008) Rapid acoustic survey for biodiversity appraisal. PLoS One 3(12):e4065
Thiel M, Vásquez JA (2000) Are kelp holdfasts islands on the ocean floor?—indication for temporarily closed aggregations of peracarid crustaceans. Island, ocean and deep-sea biology. Springer, Dordrecht, pp 45–54
Tucker D, Gage SH, Williamson I, Fuller S (2014) Linking ecological condition and the soundscape in fragmented Australian forests. Landscape Ecol 29(4):745–758
Vermeij MJA, Marhaver KL, Huijbers CM, Nagelkerken I, Simpson SD (2010) Coral larvae move toward reef sounds. PLoS One 5(5):e10660
Walker D, McComb A (1992) Seagrass degradation in Australian coastal waters. Mar Pollut Bull 25(5):191–195
Walker BH (1993) Rangeland ecology: understanding and managing change. Ambio 22:80-87
Acknowledgments
This study was supported by Australian Research Council (ARC) Future Fellowship to I.N. (Grant No. FT120100183) and a grant from the Environment Institute (The University of Adelaide). S.D.C. was supported by an ARC Future Fellowship (Grant No. FT0991953).
Author contributions
All authors contributed to the design of the study, collection of the data, and writing of the article. T.R. analysed the data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rossi, T., Connell, S.D. & Nagelkerken, I. The sounds of silence: regime shifts impoverish marine soundscapes. Landscape Ecol 32, 239–248 (2017). https://doi.org/10.1007/s10980-016-0439-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-016-0439-x