Abstract
Context
Variation in biological communities is used to identify biodiversity responses to anthropogenic drivers, and to guide conservation responses. Often, such data are only available for a limited group of species, with uncertain applicability to unmonitored taxa.
Objective
Using equivalent data on the community structure of two contrasting taxa, we examine spatial co-variation in both communities, and test the extent to which any associations may result from large-scale latitudinal patterns, variation in habitat-type, or other factors.
Methods
Birds and butterflies were surveyed using standard methods across a stratified random sample of 1-km2 squares across the UK. Four measures of community structure were calculated and used to examine their association between the two taxa, before accounting for effects of latitude, habitat-type and observer.
Results
Species richness, diversity and community specialisation were significantly correlated between birds and butterflies, but evenness was not. There were strong latitudinal gradients in bird community specialisation, and butterfly richness and diversity. Habitat diversity significantly affected bird communities, whilst butterfly evenness and specialisation was reduced on farmland and human-related habitats. Covariation in richness and diversity between taxa remained after including effects of latitude and habitat-type.
Conclusions
Surrogacy approaches may be useful when considering fine-scale variation in species richness and diversity to inform site-based conservation and management decisions. However, limited covariance in evenness and specialisation metrics suggest that decisions based on the needs of rare or specialist species may be less relevant to other taxa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Indicators of global biodiversity loss based on long-term monitoring data show that most trends are in decline, despite conservation efforts to reduce this loss (e.g., Ceballos and Ehrlich 2002; Balmford et al. 2003; Butchart et al. 2010; Hoffman et al. 2010). The calculation of these depends upon long-term monitoring data, which are primarily available for a subset of well-monitored taxa. The extent to which these trends can be applied to unmonitored taxa, and therefore have wide generality, is an important uncertainty. Information about spatial and temporal variation in the abundance and community structure of other taxa has to be inferred using surrogacy approaches, assuming that these other taxa have similar patterns of variation to those recorded (Araujo et al. 2004; Magurran 2004).
There is considerable debate about whether this assumption is robust, and the extent to which community structure of different taxa may be inter-correlated (e.g., Pearson and Cassola 1992; Sætersdal et al. 1993; Pearson and Carroll 1998; Ricketts et al. 1999; Vessby et al. 2002; Wolters et al. 2006; Qian and Ricklefs 2008; Eglington et al. 2012). Some suggest that birds, for example, are good indicators of environmental change and of trends in wider biodiversity (Baillie 1991), whilst others argue that each taxon and system must be dealt with on a case by case basis, depending upon species resource requirements and the spatial scales over which organisms acquire those resources (Dormann et al. 2007). For instance, birds are highly mobile and tend to have relatively large territories or home-ranges. They also frequently respond to the spatial physiognomy of landscapes and the structural complexity of vegetation (e.g., Fuller et al. 1997; Hinsley et al. 2009). Conversely, plants occupy more specific niches characterised by microclimate and soil type, which in turn influences the distribution of specialist herbivores, such as many butterflies, which are highly coevolved with their food plants, growth forms and micro-climate (Ehrlich and Raven 1964). In addition to these species differences, environmental context may also influence the degree of surrogacy between groups. In geographical areas where landscapes are simple, as opposed to highly fragmented and complex semi-natural landscapes, the patterns of species richness covariance may also be simpler (Prendergast and Eversham 1997).
Much of the previous work examining cross-taxa relationships in species richness has been carried out at a resolution appropriate to landscapes, regions or countries, and shows that the strength of cross-taxonomic correlations in diversity is greatest at large spatial scales (Grenyer et al. 2006; Eglington et al. 2012). Whilst this is appropriate to examine broad ecological patterns, or to identify landscapes or regions of high biodiversity importance, this is not the correct resolution to understand the causes of biodiversity loss, or to guide conservation intervention, for which a much finer-resolution of assessment is required. However, at fine resolution, data on the community structure of different taxa are rarely collected simultaneously.
This study attempts to fill this gap by examining the relationship between four different measures of community structure (species richness, diversity, evenness and specialisation) in birds and butterflies, two taxa of conservation concern frequently used to identify biodiversity hotspots and monitor trends in biodiversity state. Although both groups are relatively well-monitored, they do provide the contrast between large, highly mobile species, and small, specialised herbivores outlined above. In the UK, bird populations have been monitored using standardised annual surveys since 1960s, with the breeding bird survey (BBS) providing effective annual monitoring data for over 100 bird species since 1994, and now covering a stratified random sample of some 3200 1 km2 squares (Risely et al. 2013). In 2009, volunteers were invited to survey butterflies at existing BBS survey squares as part of the wider countryside butterfly survey (WCBS; Brereton et al. 2011). These data provide a unique opportunity to compare covariance in measures of community structure between birds and butterflies at a 1 km2 spatial scale, but across a national extent, and to suggest the mechanisms which underpin any potential relationships. This is achieved by firstly testing the extent to which any correlations remain after accounting for large-scale latitudinal variation, and secondly, after accounting for variation in land-cover between survey squares. This second analysis also allows the extent to which birds and butterfly communities show common responses to different drivers, to be tested. Thirdly, if measures of bird and butterfly communities covary after accounting for latitude and land-cover, then we use this to indicate that fine-scale covariation can occur in community structure between taxa within land-cover types, for example with respect to habitat quality, that may be more relevant to management.
Methods
Square selection
BBS surveys are conducted annually on a random sample of over 3000 1-km squares, stratified regionally by intensity of coverage by survey volunteers (Risely et al. 2013). BBS volunteers that contribute to the WCBS record breeding birds from these squares during the breeding season (April–July) and then return to the square for additional visits in July and August to survey butterflies. In this paper, we analysed bird and butterfly data from 2009, the first full year of the WCBS. Data were analysed for this single year as we were interested only in spatial variation in these patterns. As subsequent years have been marked by severe winter (2009/2010, 2010/2011) and summer (2012) weather events, incorporating additional temporal variation would have added to the analytical complexities without significantly improving our ability to look at spatial patterns.
Bird data
Two early-morning visits were made to each square by the same observer, typically the first from early April to mid-May and the second from mid-May to late June. During each visit, birds were recorded by sight and sound at each side of two roughly parallel 1-km transects divided into 200 m sections. The perpendicular distance of each non-flying individual from the transect line was recorded in three distance bands (0–25, 25–100, 100 m or more); flying birds were recorded separately. The habitat of each 200 m transect section was classified using a hierarchical coding system (Crick 1992).
Butterfly data
Butterfly counts were conducted on 356 of the 3260 BBS squares surveyed for birds in 2009. Butterflies were recorded along the same two 1-km transects used for the bird survey. All butterflies within a moving box, 2.5 m either side of the transect, 5 m ahead and to a height of 5 m were counted, an area within which detectability is known to be high (Isaac et al. 2011). Volunteers were asked to undertake a minimum of two visits to each square in July and August, with at least 10 days between the two visits. Optional extra visits could also be made from April to September, providing they were at least 10 days apart from another visit. Butterfly surveys were walked between 10:00 and 16:30 and only when weather conditions were suitable for butterfly activity: dry, wind speed less than Beaufort Scale 5, and temperature 13 °C or greater if there is at least 60 % sunshine, or more than 17 °C if overcast (Brereton et al. 2011).
Habitat data
The habitat data collected were summarised into 12 habitat classes using the approach of Newson et al. (2009). A maximum of two different high-level habitat-types could be recorded per transect, for example to differentiate between different habitat-types on either side of the transect route. Thus, a maximum of 20 separate habitat records could be obtained per 1-km square. To standardise this information, these categories were used to calculate the percentage composition of different habitat-types along the transect sections within each square from the sum of the records of each habitat-type (double counting the habitat class from a section comprising only one habitat) divided by double the number of transect sections surveyed.
Measures of community structure
Variation in ecological communities may be measured in a wide-range of different ways (Magurran 2004). We described the structure of both bird and butterfly communities in each 1-km square, using four standard metrics; species richness, Simpson’s measure of evenness (E), reciprocal Simpson’s index (D) and community specialisation index (CSI).
Species richness is simply the total number of species recorded in each visit.
Diversity is a function of both richness and evenness. Reciprocal Simpson’s index (D) provides a good estimate of diversity at relatively small sample sizes and ranks assemblages consistently, taking into account abundance of individuals (i.e., dominance). It therefore captures the variance of species abundance distributions, and was calculated by the following formula:
where nij is the total number of organisms of a species i recorded in square j, and Nj is the total number of organisms of all species in that square.
Evenness measures how different the abundances of species within a community are from one another. Evenness is highest when a community is not dominated by a few highly abundant species or when all species have an equal abundance. Simpson’s evenness (E) is not sensitive to species richness (Magurran 2004) and refers to the observed value of Simpson’s diversity divided by the maximum possible diversity for a landscape i.e., how close in numbers each species in an environment are as follows:
where s is the total number of species recorded in that square. These two Simpson’s indices were used as they are considered to be robust and easy to interpret (Magurran 2004).
The CSI was calculated following the methods of Julliard et al. (2006) and Devictor et al. (2008) to identify the degree to which communities were composed of generalists compared with specialists. Species specialization indices (SSIs) were calculated from the transect-section level data using the coefficient of variation (standard deviation/mean) of species’ density across habitats using data from 2009 to avoid changes in SSI through time (Barnagaud et al. 2011). Only species with greater than 10 observations were included. The CSI was calculated for each square as the average SSI weighted for species abundance (Julliard et al. 2006; Devictor et al. 2008) as follows:
where n is the total number of species recorded, a ij is the abundance of individuals of species i in plot j, and SSI i its specialization index.
Statistical analysis
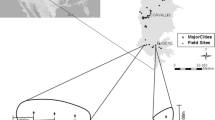
In order to generate comparable estimates of community structure between birds and butterflies, we restricted our analysis to the two butterfly survey visits in July and August, providing data which were directly comparable to the two bird survey visits from April to June. Such data were available for 183 squares for which habitat data were also collected (Fig. 1).
We estimated community metrics for each square in two ways, separately for each of the two visits and then averaged, and derived from the maximum count of the number of individuals recorded for each species across the two visits. Square-level estimates of community structure produced using these two approaches were strongly correlated in all cases (birds: richness, r = 0.97; D, r = 0.95; E, r = 0.93; CSI, r = 0.99. Butterflies: richness, r = 0.96; D, r = 0.82; E, r = 0.65; CSI, r = 0.89). We present the analysis of community structures averaged across the two visits to each square in the paper. Equivalent results were obtained when using maximum counts of individuals recorded across both visits (Supplementary Material 1).
Butterflies were counted within a 5-m wide detection box whilst birds were recorded within 100 m of the transect line. Given that the detectability of birds varies widely within this range (Johnston et al. 2014), whilst that of butterflies should not, we additionally corrected the estimates of bird community structure for variation in detectability using distance sampling (Buckland et al. 2001, see Davey et al. 2012). Equivalent results were obtained when this detectability correction was not applied (Supplementary Material 2).
Using these standardised measures of community structure, we undertook the following tests:
First, we tested whether measures of bird and butterfly community structure (species richness, D, E and CSI) were correlated at the 1-km2 resolution across the full dataset.
Secondly, using latitude as a single measure that correlates strongly with many large-scale environmental variables in Britain, such as temperature and daylength, we tested whether significant correlations may be related to large-scale spatial gradients across Britain (Turner et al. 1987; Evans et al. 2005). We modelled each taxon-specific community measure as a function of the equivalent measure from the other taxon plus latitude using a generalised linear model (GLM) with a normal error distribution.
Thirdly, we tested whether covariation in butterfly and bird community measures was attributable to variation in habitat-type between transects surveyed within each square. We modelled each taxon-specific community structure measure as a function of the equivalent measure for the other taxon, plus latitude and habitat variables. Habitat-type was summarised as the percentage cover of four amalgamated habitat classes: farmland (arable and agricultural grassland), woodland (woodland and scrub), grassland (semi-natural grassland and heathland) and human-related (urban and rural settlement). Habitat diversity (the total number of these broad habitat classes recorded in each square plus wetland, rock and other) was included as an additional predictor variable. Because of likely spatial autocorrelation in habitat variables, we conducted the modelling within a generalised linear mixed model, fitting spherical spatial covariate function and applying the Kenward–Rogers correction for the degrees of freedom (Littell et al. 1996).
Finally, we assumed that any residual covariation between bird and butterfly community measures that remained after accounting for latitude and habitat-type was not related to large-scale drivers or variation in habitat cover, and may therefore be a function of fine-scale variation in habitat-type or quality, as opposed to gross land-cover variation. To confirm that any covariation was not due to systematic variation among observers, the structure of this final model was repeated, but with observer additionally specified as a random effect.
Results
Covariance in bird and butterfly community measures
Three of the four measures of community metrics were correlated between birds and butterflies. A highly significant positive relationship between bird and butterfly species richness accounted for 16.0 % of the variation in the measure, whilst weaker correlations for D and CSI accounted for 8.2 and 2.6 % of the variation between taxa respectively. There was no significant association in measures of E between the two taxa (Fig. 2).
Accounting for latitude
Latitude was significantly correlated with butterfly species richness, D and CSI, suggesting more northerly squares contained fewer butterfly species and had lower overall diversity, but supported a greater proportion of specialists (Table 1b). More northerly squares also contained a significantly greater proportion of bird specialists (Table 1a). Accounting for latitude had little impact on the previously identified relationships between bird and butterfly species richness and D (Table 1), suggesting that observed covariance was not a function of large-scale correlations with unmeasured climatological or other factors. The previously identified correlation in CSI between birds and butterflies became non-significant following the inclusion of latitude, indicating that this largely reflects large-scale gradients in specialisation across the country.
Accounting for habitat
Habitat-type was a significant predictor of spatial variation in the community structure of both birds and butterflies. Habitat diversity was strongly positively correlated with both the species richness and D for birds, and negatively with CSI, although was unrelated to butterfly community structure. Avian CSI was negatively correlated with the cover of all four main habitat-types, particularly farmland and woodland, suggesting it is positively correlated with the rarer habitats. For butterflies, both E and CSI were depressed by high cover of farmland and human-related habitats (Table 2). Within these models, the effects of latitude were negligible due to the inclusion of spatial covariance.
After accounting for habitat-type, the previous statistical associations between bird and butterfly species richness and D remained significant, suggesting that fine-scale covariation in community structure between the two taxa occurred even within broad habitat-types (Table 2). The inclusion of observer as a random effect to account for the potential effects of observer identify on these relationships did not alter these results (Table 3), indicating they are unlikely to be caused by systematic variation in observer quality.
Discussion
There was significant covariance across the UK in bird and butterfly species richness, diversity (D) and community specialisation (CSI), when assessed at the 1-km2 resolution. This supports the results of similar fine-scale analyses from specific systems that link common responses of bird and butterfly communities to a gradient of urbanisation (Blair 1999), native plant cover (Burghardt et al. 2009), tropical habitat degradation (Schulze et al. 2004) and grassland habitat variation (Swengel and Swengel 1999; Su et al. 2004). Importantly, our study extends these associations from context-specific studies to a national scale, and provides some support for the principal of surrogacy operating between contrasting taxa at relatively fine spatial scales.
Our findings contrast somewhat with the inference of previous national-scale analyses from the UK, where there was relatively spatial little overlap in biodiversity hotspots of different taxa. Only 12–17 % of bird and butterfly hotspots coincided, with no correlation in species richness between the two groups at that scale (Prendergast et al. 1993; Franco et al. 2009). However, these previous studies were based upon 10-km2 atlas data, suggesting that the appropriateness of surrogacy approaches may be scale-dependent (Eglington et al. 2012), although methodological differences between the studies may also account for some of this contrast.
We examined some of the potential mechanisms underpinning covariance in community structure. Firstly, we tested for potential latitudinal gradients in the diversity of both birds (Evans et al. 2005; Davey et al. 2012) and butterflies (Turner et al. 1987), for which there is good evidence that they result largely from variation in temperature and energy in Britain (Hillebrand 2004; Clarke and Gaston 2006). Our analysis supported these previous studies for butterflies, but found weaker evidence for latitudinal gradients in bird communities (Table 1), probably because we included relatively few squares in northern Britain compared to these other studies (Fig. 1). Importantly, for the purposes of this study, the observed correlations between bird and butterfly richness and diversity were robust to the inclusion of latitude, indicating that they were not a result of common effects of temperature or energy upon both groups. However, including latitude appeared to account for the relatively weak, but significant, association between birds and butterfly CSI, suggesting that this pattern is only found across large-spatial gradients.
Secondly, habitat-type was a significant predictor of bird community structure. Squares containing a greater number of separate habitats tended to support a greater richness and diversity of birds, as expected from the habitat heterogeneity hypothesis, which states that structurally complex habitats provide more niches and diverse ways of exploiting environmental resources, and thus are associated with greater species diversity (Atauri and de Lucio 2001). Contrary to other studies (Atauri and de Lucio 2001; Menéndez et al. 2007) conducted at larger spatial scales, these findings were not replicated in our analysis of butterfly community structure, where diversity was largely unrelated to habitat-type and diversity. This may be a function of the relatively broad habitat-types used in our analysis, which may be poorer descriptors of the environment for butterflies than they are for birds. Alternatively, the relationship between butterfly community structure and habitat diversity may be scale-dependent. Whilst it is possible that our estimates of butterfly community structure based on two visits are less robust than the estimates derived from a greater intensity of sampling used by Menéndez et al. (2007), this does not appear to be the case. Estimates of community structure from eight squares which received four survey visits were strongly correlated (0.86 < r < 0.96) with the equivalent measures based on the subsample of two visits used in the main analysis, suggesting that our two survey visits produced a reasonable description of butterfly communities. It is also worth emphasising that we have only examined alpha diversity in this study, and do not consider how communities vary along environmental gradients (beta diversity), such as between habitats. Thus, the absence of a relationship between diversity and habitat-type does not necessarily mean that communities are stable across habitats.
Despite contrasting large-scale drivers of spatial variation in richness and diversity in birds, where habitat diversity was the strongest correlate, and butterflies, where latitude was most important, bird and butterfly species richness and diversity covaried significantly, even after accounting for these other drivers, and potential effects of observer variation. The principal of surrogacy was therefore partially supported, demonstrating that fine-scale variation in bird and butterfly communities occurs not as a result of large-scale latitudinal gradients, or variation in land cover, but probably through some common effects of fine-scale variation in habitat quality or unmeasured habitat heterogeneity that similarly affect both bird and butterfly community richness and diversity. A possible candidate for this variation may be fine-scale variation in vegetation structure and heterogeneity within habitat-types (Atauri and de Lucio 2001; Tews et al. 2004; Huang et al. 2014).
In contrast to richness and diversity, estimates of evenness were uncorrelated between birds and butterflies, whilst the correlation with community specialisation became non-significant once the effect of latitude was accounted for. This suggests that the more specialised and rarer species in each taxa do not associate at relatively fine-spatial scales. This is intuitive, given the contrasting ecologies of the two groups. Many specialist butterflies rely on one or a small number of food plants and are likely to also be sensitive to fine-scale variation in other resources, such as nectar availability, whilst bird species tend to be associated with particular habitat-types rather than specific plant resources (Julliard et al. 2006). The scale of specialisation between the two species groups is therefore different. Although the rarest and most specialised butterfly and bird species are not well sampled by BBS squares, which tend to be randomly distributed across the wider countryside rather than concentrated in rare natural and semi-natural habitats where the most specialist species tend to be found, this limitation is unlikely to have significant affected our results, which sampled species proportionally to their occurrence.
There were common effects of habitat upon both E and CSI measures in butterflies, with both strongly negatively related to the cover of farmland and human-related habitat. Effects of habitat upon equivalent measures in birds were weaker, with widespread negative correlations between habitat-type and CSI indicative of the most specialised bird species being associated with rarer habitats, such as wetland. These results strongly suggest that butterfly communities in heavily anthropogenic habitats tend to be dominated by a relatively small number of evenly abundant generalist species, supporting previous results (e.g., Blair 1999; Dallimer et al. 2012; Flick et al. 2012), and the general principle that CSI decreases with land-use intensity (Devictor et al. 2008; Devictor and Robert 2009; Filippi-Codaccioni et al. 2010a, b). The negative correlation between habitat-diversity and avian CSI may result from squares with more heterogeneous habitat cover (indicative of fragmentation) being more likely to favour generalist, rather than specialist species (Dolman et al. 2007).
It was important to test the extent to which associations may result from variation between observers. As both bird and butterfly surveys were conducted by the same individual, it is possible that the best bird recorders were also better butterfly recorders, resulting in a spurious association in species richness. However, our results were robust to the inclusion of observer identity as a random effect, suggesting that this was not the case, and supporting other studies suggesting that observer experience has limited impact on the generation of BBS population trends (Eglington et al. 2010). Thus it appears that although observers may vary in their capabilities, the standardized methodologies for both surveys at least partially ensure that observer effects do not unduly influence the results.
Given the increasing evidence for common large-scale changes in the community structure of both birds and butterflies, it is perhaps not surprising that bird and butterfly communities covaried at the 1-km scale studied. Both taxa have responded to warming in similar ways, although at slightly different rates (Devictor et al. 2012). Large-scape patterns of community homogenisation have also been described in both birds (Devictor et al. 2008; Le Viol et al. 2012) and butterflies (White and Kerr 2007). Although our results can be well-placed in the context of these other studies, ours is one of only a small number of studies to have examined surrogacy in community structure at this relatively fine-scale (see Blair 1999; Dallimer et al. 2012), and to our knowledge, the first that does so across such an extensive geographical area.
To conclude, the fact we found common fine-scale variation in richness, diversity and community specialisation of birds and butterfly communities across the UK, provides support for the use of surrogacy approaches for biodiversity monitoring. Importantly, covariation in richness and diversity was not simply a function of correlations with large-scale drivers such as climate and day-length, as explained by latitude, or even with land-cover type, but instead was likely to have resulted from common responses to variation in habitat-type, and particularly, quality, and may therefore reflect responses to habitat parameters amenable to management. Common patterns of bird and butterfly richness and diversity indicate that, in general, sites of high diversity for well-monitored taxa may be more likely to contain a greater species richness and diversity for other taxa also. We therefore suggest that, with appropriate caveats given the variation associated with these correlations (Fig. 2), surrogacy approaches may be extended from protected area selection and biodiversity hotspot prioritisation to finer-resolution consideration of the drivers of biodiversity change and to inform land management for conservation at the scale of individual sites. Evidence of deteriorating environmental quality, as shown through reductions in species richness or diversity of well-monitoring groups such as birds and butterflies, which are often based upon extensive citizen science monitoring data such as that used in this analysis, are therefore likely to also reflect equivalent changes in other, less-well monitored taxa. However, the lack of covariance between birds and butterflies in measures of evenness, and of community specialisation after accounting for latitude, means that when considering rarer and specialised species in particular, which tend to be those of the highest conservation priority, the surrogacy approach may have limitations. Site selection or conservation management for priority bird species may therefore be unlikely to benefit butterflies of conservation concern; unless population trends in both groups have a common driver (e.g., Wilkinson et al. 2012).
References
Araujo MB, Densham PJ, Williams PH (2004) Representing species in reserves from patterns of assemblage diversity. J Biogeogr 31:1037–1050
Atauri JA, de Lucio JV (2001) The role of landscape structure in species richness of birds, amphibians, reptiles and lepidopterans in Mediterranean landscapes. Landscape Ecol 16:147–159
Baillie SR (1991) Monitoring terrestrial breeding bird populations. In: Goldsmith FB (ed) Monitoring for conservation and ecology. Chapman and Hall, London, p 112
Balmford A, Green R, Jenkins M (2003) Measuring the changing state of nature. TREE 18:326–330
Barnagaud JY, Devictor V, Jiguet F, Archaux F (2011) When species become generalists: on-going large-scale changes in bird habitat specialization. Glob Ecol Biogeogr 20:630–640
Blair RB (1999) Birds and butterflies along an urban gradient: surrogate taxa for assessing biodiversity? Ecol Appl 9:164–170
Brereton TM, Cruickshanks KL, Risely K, Noble DG, Roy DB (2011) Developing and launching a wider countryside butterfly survey across the United Kingdom. J Insect Conserv 15:290. doi:10.1007/s10841-010-9345-8
Buckland ST, Anderson DR, Burnham KP, Laake JL, Borchers DL, Thomas L (2001) Introduction to distance sampling: estimating abundance of biological populations. Oxford University Press, Oxford
Burghardt KT, Tallamy DW, Shriver WG (2009) Impacts of native plants on bird and butterfly biodiversity in suburban landscapes. Conserv Biol 23:219–224
Butchart SHM, Walpole M, Collen B, van Strien A, Scharlemann JPW, Almond REA, Baillie JEM, Bomhard B, Brown C, Bruno J, Carpenter KE, Carr GM, Chanson J, Chenery AM, Csirke J, Davidson NC, Dentener F, Foster M, Galli A, Galloway JN, Genovesi P, Gregory RD, Hockings M, Kapos V, Lamarque J-F, Leverington F, Loh J, McGeoch MA, McRae L, Minasyan A, Morcillo MH, Oldfield TEE, Pauly D, Quader S, Revenga C, Sauer JR, Skolnik B, Spear D, Stanwell-Smith D, Stuart SN, Symes A, Tierney M, Tyrell TD, Vié J-C, Watson R (2010) Global Biodiversity: indicators of recent declines. Science 328:1164–1168
Ceballos G, Ehrlich PR (2002) Mammal population losses and the extinction crisis. Science 296:904–907
Clarke A, Gaston KJ (2006) Climate, energy and diversity. Proc R Soc B 273:2257–2266
Crick HQP (1992) A bird-habitat coding system for use in Britain and Ireland incorporating aspects of land management and human activity. Bird Study 39:1–12
Dallimer M, Rouquette JR, Skinner AMJ, Armsworth PR, Maltby LM, Warren PH, Gaston KJ (2012) Contrasting patterns in species richness of birds, butterflies and plants along riparian corridors in an urban landscape. Divers Distrib 18:742–753
Davey CM, Chamberlain DE, Newson SE, Noble DG, Johnston A (2012) Rise of the generalists: evidence for climate driven homogenization in avian communities. Glob Ecol Biogeogr 21:568–578
Devictor V, Robert A (2009) Measuring community responses to large-scale disturbance in conservation biogeography. Divers Distrib 15:122–130
Devictor V, Julliard R, Clavel J, Jiguet F, Lee A, Couvet D (2008) Functional biotic homogenization of bird communities in disturbed landscapes. Glob Ecol Biogeogr 17:252–261
Devictor V, van Swaay C, Brereton T, Brotons L, Chamberlain D, Heliölä J, Herrando S, Julliard R, Kuussaari M, Lindström Å, Reif J, Roy DB, Schweiger O, Settele J, Stefanescu C, Strien AV, Turnhout CV, Vermouzek Z, WallisDeVries M, Wynhoff I, Jiguet F (2012) Differences in the climatic debts of birds and butterflies at a continental scale. Nat Clim Change 2:121–124
Dolman PM, Hinsley SA, Bellamy PE, Watts K (2007) Woodland birds in patchy landscapes: the evidence base for strategic networks. Ibis 149(S2):146–160
Dormann CF, Schweiger O, Augenstein I, Bailey D, Billeter R, De Blist G, DeFilippi R, Frenzel M, Hendrickx F, Herzog F, Klotz S, Liira J, Maelfait J-P, Schmidt T, Speelmans M, Van Wingerden WKRE, Zobel M (2007) Effects of landscape structure and land-use intensity on similarity of plant and animal communities. Glob Ecol Biogeogr 16:774–787
Eglington SM, Davis SE, Joys AC, Chamberlain DE, Noble DG (2010) The effect of observer experience on English Breeding Bird Survey population trends. Bird Study 57:129–142
Eglington SM, Noble DG, Fuller RJ (2012) A meta-analysis of spatial relationships in species richness across taxa: birds as indicators of wider biodiversity in temperate regions. J Nat Conserv 20:301–309
Ehrlich PR, Raven PH (1964) Butterflies and plants: a study in coevolution. Evol 18:586–608
Evans KL, Greenwood JJD, Gaston KJ (2005) Dissecting the species–energy relationship. Proc R Soc B 272:2155–2163
Filippi-Codaccioni O, Devictor V, Bas Y, Julliard R (2010a) Toward more concern for specialisation and less for species diversity in conserving farmland biodiversity. Biol Conserv 143:1493–1500
Filippi-Codaccioni O, Devictor V, Bas Y, Clobert J, Julliard R (2010b) Specialist response to proportion of arable land and pesticide input in agricultural landscapes. Biol Conserv 143:883–890
Flick T, Feagan S, Fahrig L (2012) Effects of landscape structure on butterfly species richness and abundance in agricultural landscapes in eastern Ontario, Canada. Agric Ecosyst Environ 156:123–133
Franco AMA, Anderson BJ, Roy DB, Gillings S, Fox R, Moilanen A, Thomas CD (2009) Surrogacy and persistence in reserve selection: landscape prioritization for multiple taxa in Britain. J Appl Ecol 46:82–91
Fuller RJ, Trevelyan RJ, Hudson RW (1997) Landscape composition models for breeding bird populations in lowland English farmland over a 20 year period. Ecography 20:295–307
Grenyer R, Orme CDL, Jackson SF, Thomas GH, Davies RG, Davies TJ, Jones KE, Olson VA, Ridgely RS, Rasmussen PC, Ding T-S, Bennett PM, Blackburn TM, Gaston KJ, Gittleman JL, Owens IPF (2006) Global distribution and conservation of rare and threatened vertebrates. Nature 444:93–96
Hillebrand H (2004) On the generality of the latitudinal diversity gradient. Am Nat 163:192–211
Hinsley SA, Hill RA, Fuller RJ, Bellamy PE, Rothery P (2009) Bird species distributions across woodland canopy structure gradients. Comm Ecol 10:99–110
Hoffman M, Hilton-Taylor C, Angulo A, Böhm M, Brooks TM, Butchart SHM, Carpenter KE, Chanson J, Collen B, Cox NA, Darwall WRT, DulvyNK, Harrison LR, Katariya V, Pollock CM, Quader S, Richman NI, Rodrigues ASL, Tognelli MF, Vié J-C, Aguiar JM, Allen DJ, Allen GR, Amori G, Ananjeva NB, Andreone F, Andrew P, Aquino Ortiz AL, Baillie JEM, Baldi R, Bell BD, Biju SD, Bird JP, Black-Decima P, Blanc JJ, Bolaños F, Bolivar WG, Burfield IJ, Burton JA, Capper DR, Castro F, Catullo G, Cavanagh RD, Channing A, Chao NL, Chenery AM, Chiozza F, Clausnitzer V, Collar NJ, Collett LC, Collette BB, Cortez Fernandez CF, Craig MT, Crosby MJ, Cumberlidge N, Cuttelod A, Derocher AE, Diesmos AC, Donaldson JS, Duckworth JW, Dutson G, Dutta SK, Emslie RH, Farjon A, Fowler S, Freyhof J, Garshelis DL, Gerlach J, Gower DJ, Grant TD, Hammerson GA, Harris RB, Heaney LR, Blair Hedges S, Hero J-M, Hughes B, Hussain SA, Icochea MJ, Inger IF, Ishii N, Iskandar DT, Jenkins RKB, Kaneko Y, Kottelat M, Kovacs KM, Kuzmin SL, La Marca E, Lamoreux JF, Lau MWN, Lavilla EO, Leus K, Lewison RL, Lichtenstein G, Livingstone SR, Lukoschek V, Mallon DP, McGowan PJK, McIvor A, Moehlman PD, Molur S, Muñoz Alonso A, Musick JA, Nowell K, Nussbaum1 RA, Olech W, Orlov NL, PapenfussTJ, Parra-Olea G, Perrin WF, Polidoro BA, Pourkazemi M, Racey PA, Ragle JS, Ram M, Rathbun G, Reynolds RP, Rhodin AGJ, Richards SJ, Rodríguez LO, Ron SR, Rondinini C, Rylands AB, Sadovy de Mitcheson Y, Sanciangco JC, Sanders KL, Santos-Barrera G, Schipper J, Self-Sullivan C, Shi Y, Shoemaker A, Short FT, Sillero-Zubiri C, Silvano DL, Smith KG, Smith AT, Snoeks J, Stattersfield AJ, SymesAJ, Taber AB, Talukdar BK, Temple HJ, Timmins T, Tobias JA, Tsytsulina K, Tweddle D, Ubeda C, Valenti CV, van Dijk PP, Veiga LM, Veloso A, Wege DC, Wilkinson M, Williamson EA, Xie F, Young BE, Akçakaya HR, Bennun L, Blackburn TM, Boitani L, Dublin HT, da Fonseca GAB, Gascon C, Lacher Jr. TE, Mace GM, Mainka SA, McNeely JA, Mittermeier RA, Reid GM, Rodriguez RP, Rosenberg AA, Samways MJ, Smart J, Stein BA, Stuart SN (2010) The impact of conservation on the status of the world’s vertebrates. Science 330:1503–1509
Huang Q, Swatantran A, Dubayah R, Goetz SJ (2014) The influence of vegetation height heterogeneity on forest and woodland bird species richness across the United States. PLoS ONE 9:e103236
Isaac NJB, Cruickshanks KL, Weddle AM, Rowcliffe JM, Brereton TM, Dennis RLH, Shuker DM, Thomas CD (2011) Distance sampling and the challenge of monitoring butterfly populations. Methods Ecol Evol 2:585–594
Johnston A, Newson SE, Risely K, Musgrove AJ, Massimino D, Baillie SR, Pearce-Higgins JW (2014) Species traits explain variation in detectability of UK birds. Bird Study 61:340–350
Julliard R, Clavel J, Devictor V, Jiguet J, Couvet D (2006) Spatial segregation of specialists and generalists in bird communities. Ecol Lett 9:1237–1244
Le Viol I, Jiguet F, Brotons L, Herrando S, Lindström A, Pearce-Higgins JW, Reif J, Van Turnhout C, Devictor V (2012) More and more generalists: two decades of changes in the European avifauna. Biol Lett 8:780–782
Littell RC, Milliken GA, Stroup WW, Wolfinger RD (1996) SAS system for mixed models. SAS Institute, Cary
Magurran AE (2004) Measuring biological diversity. Blackwell Publishing, Malden
Menéndez R, González-Megías A, Collingham Y, Fox R, Roy DB, Ohlemüller R, Thomas CD (2007) Direct and indirect effects of climate and habitat factors on butterfly diversity. Ecology 88:605–611
Newson SE, Ockendon N, Joys A, Noble DG, Baillie SR (2009) Comparison of habitat-specific trends in the abundance of breeding birds in the UK. Bird Study 56:233–243
Pearson DL, Carroll SS (1998) Global patterns of species richness: spatial models for conservation planning using bioindicator and precipitation data. Conserv Biol 12:809–821
Pearson DL, Cassola F (1992) World-wide species richness patterns of tiger beetles (Coleoptera: Cicindelidae): indicator taxon for biodiversity and conservation studies. Conserv Biol 6:376–391
Prendergast JR, Eversham BC (1997) Species richness covariance in higher taxa: empirical tests of the biodiversity indicator concept. Ecography 202:210–216
Prendergast JR, Quinn RM, Lawton JH, Eversham BC, Gibbons DW (1993) Rare species, the coincidence of diversity hotspots and conservation strategies. Nature 365:335–337
Qian H, Ricklefs RE (2008) Global concordance in diversity patterns of vascular plants and terrestrial vertebrates. Ecol Lett 11:547–553
Ricketts TH, Dinerstein E, Olson DM, Loucks C (1999) Who’s where in North America? Bioscience 49:369–381
Risely K, Massimino D, Newson SE, Eaton MA, Musgrove AJ, Noble DG, Procter D, Baillie SR (2013) The breeding bird survey, BTO research report 645 2012. British Trust for Ornithology, Thetford
Sætersdal M, Line JM, Birks HJ (1993) How to maximize biological diversity in nature reserve selection: vascular plants and breeding birds in deciduous woodlands, Western Norway. Biol Conserv 66:131–138
Schulze CH, Waltert M, Kessler PJA, Pitopang R, Veddeler D, Mühlenberg M, Gradstein R, Leuschner C, Steffan-Dewenter I, Tscharntke T (2004) Biodiversity indicator groups of tropical land-use systems: comparing plants, birds and insects. Ecol Appl 14:1321–1333
Su JC, Debinksi DM, Jabukbauskas ME, Kindscher K (2004) Beyond species richness: community similarity as a measure of cross-taxon congruence for coarse-filter conservation. Conserv Biol 18:167–173
Swengel SR, Swengel AB (1999) Correlations in abundance of grassland songbirds and prairie butterflies. Biol Conserv 90:1–11
Tews J, Brose U, Grimm V, Tielbörger K, Wichmann MC, Schwager M, Jeltsch F (2004) Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. J Biogeogr 31:79–92
Turner JRG, Gatehouse CM, Corey CA (1987) Does solar energy control organic diversity? Butterflies, moths and the British climate. Oikos 48:195
Vessby K, Soderstrom B, Glimskar A, Svensson B (2002) Species-richness correlations of six different taxa in Swedish semi-natural grasslands. Conserv Biol 16:430–439
White PJT, Kerr JT (2007) Human impacts on environment–diversity relationships: evidence for biotic homogenization from butterfly species richness patterns. Glob Ecol Biogeogr 16:290–299
Wilkinson NI, Wilson JD, Anderson GQA (2012) Agri-environment management for corncrake Crex crex delivers higher species richness and abundance across other taxonomic groups. Agric Ecosyst Environ 155:27–34
Wolters V, Bengtsson J, Zaitsev AS (2006) Relationship among the species richness of different taxa. Ecology 87:1886–1895
Acknowledgments
We would like to thank all those professional and amateur ornithologists who contributed to the collection of field data. The BBS is funded jointly by the British Trust for Ornithology (BTO), the Royal Society for the Protection of Birds (RSPB) and the Joint Nature Conservation Committee (JNCC). The WBCS is a partnership between the Centre for Ecology and Hydrology (CEH), Butterfly Conservation and BTO, and is funded by a consortium of government agencies [Defra, Joint Nature Conservation Council (JNCC), Natural Resources Wales (NRW), Scottish Natural Heritage (SNH), Natural England and the Forestry Commission (FC)].
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Eglington, S.M., Brereton, T.M., Tayleur, C.M. et al. Patterns and causes of covariation in bird and butterfly community structure. Landscape Ecol 30, 1461–1472 (2015). https://doi.org/10.1007/s10980-015-0199-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-015-0199-z