Abstract
Edge effects are among the most important drivers of species interactions in fragmented habitats, but the impacts of edge effects on multitrophic interactions are largely unknown. In this study we assess edge effects on species interactions within an ant–plant mutualistic system—where ants protect plants against herbivory—to determine whether habitat edges alter the amount of protection ants provide. We focus on a single species of myrmecophytic plant, Solanum americanum, and experimentally manipulate ant access to study plants in large-scale fragmented habitat patches at the Savannah River Site National Environmental Research Park, USA. In this system, S. americanum commonly hosts honeydew-producing aphids that are tended by ants, and grasshoppers are the primary herbivores. We measured edge effects on the per-plant abundance of aphids and protective ants as well as the abundance of grasshoppers in each habitat patch, and we evaluated levels of ant protection against herbivory near and far from habitat edges. We found that ants provided significant protection to plants far from edges, where herbivory pressure was highest, despite the fact that aphids and ants were least abundant on these plants. Conversely, ants did not provide significant protection near edges, where herbivory pressure was lowest and aphids and ants were most abundant. We conclude that a strong edge effect on grasshopper abundance was a key factor determining the amount of protection ants provided against herbivory. Future studies of the impacts of habitat fragmentation on ant–plant mutualisms will benefit from studies of ant behavior in response to herbivory threats, and studies of edge effects on other species interactions may also need to consider how species’ behavioral patterns influence the interactions in question.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Herbivory is one of the greatest challenges to plant survival and reproduction, and plants have evolved a diverse arsenal of defenses against it (Futuyma and Agrawal 2009; Agrawal 2011). Among the most effective defenses are ant-guard systems, whereby plants establish mutualisms with ants that can protect them against herbivores (Beattie and Hughes 2002; Massad et al. 2011). Myrmecophytes, i.e. “ant-plants,” may reward ants directly with domatia or food (e.g., Janzen 1966, 1967, 1969), or indirectly, by hosting insect herbivores that provide ants with sugar-rich honeydew (Reviewed in Way 1963; Rico-Gray and Oliveira 2007); either arrangement can increase plant growth and fitness (Messina 1981; Horvitz and Schemske 1984; Beattie 1985; Vasconcelos 1991; Rico-Gray and Oliveira 2007). Even in indirect cases, when ants protect honeydew-producing herbivores, the damage caused to plants by the honeydew-producers is often outweighed by the ants’ deterrence of other herbivores (Carroll and Janzen 1973; Beattie and Hughes 2002; Oliveira and Del-Claro 2005). Given that ants are among the most abundant and widespread animal groups in the world and are leading predators of insects and small invertebrates (Hölldobler and Wilson 1990), it is not surprising that many plant species depend on ants for protection against herbivory (Oliveira and Freitas 2004 and references therein).
Habitat edges created by fragmentation can alter patterns of herbivory in habitat fragments (e.g., Meiners et al. 2000; Tscharntke et al. 2002; Bach and Kelly 2004; Lopez-Barrera et al. 2006; Valladares et al. 2006), but the influence of edges on herbivory in multitrophic systems, including ant-guard systems, is unknown (Bruna et al. 2005; Wimp et al. 2011). Ecologists have studied edge effects for more than 100 years, yet the vast majority of edge studies are focused on single species or pairwise interactions (Martinson 2009). We do know a good deal about the causes edge effects: Abiotic conditions, e.g., light, moisture, and temperature, near habitat edges often are very different than conditions far from edges, and these differences can determine the availability of resources and the abundance of organisms as a function of distance from the edges (reviewed in Collinge 2009).
Current theory describes edge effects on species interactions largely as additive phenomena, resulting from edge effects on the abundance and “encounter frequencies” of the individual species in question (Fagan et al. 1999; Ries et al. 2004). Studies of ant–plant mutualism show that levels of ant protection against herbivory can vary strongly with the abundance of honeydew-producing insects, ants, and other herbivores (Beattie 1985; Cushman and Addicott 1991; Schupp and Feener 1991; Bronstein 1994; Stadler and Dixon 2005; Rico-Gray and Oliveira 2007), and edges are also known to alter each of these factors (Carvalho and Vasconcelos 1999; Braschler et al. 2003; Tscharntke and Brandl 2004; Debuse et al. 2007; Laurance et al. 2011; Orrock et al. 2011). This evidence, taken together, suggests that edge effects on the distribution and “encounter frequencies” of honeydew-producing insects, ants, and other herbivores may determine levels of herbivory and the amount of protection ants provide plants in fragmented habitats.
In this study, we assess the impacts of edge effects on levels of herbivory in an ant–plant mutualistic system. Our study site is a landscape-scale fragmentation experiment that is explicitly designed to test for edge effects on plants and plant-animal interactions (as described in “Study system” section below). We focus on a single species of myrmecophytic plant, Solanum americanum (Solanaceae), which attracts a variety of ants in our study system by hosting honeydew-producing aphids (hemiptera), and we focus on herbivory by grasshoppers (orthoptera), which are the primary consumers of S. americanum in the study area. Our objectives are twofold: (1) We test for edge effects on the per-plant abundance of aphids and protective ants as well as the abundance of grasshoppers in the experimentally fragmented habitat patches that comprise our study system; and (2) We evaluate whether changes in the abundances of these interacting species alter levels of ant-guard protection against herbivory near and far from habitat edges. This second question addresses a key issue for myrmecophytic plants. As habitats around the world are increasingly fragmented, ant-plants may be increasingly exposed to novel gradients in habitat quality, levels of herbivory, and ant-guard protection against herbivory.
Study system
We conducted this study in eight experimentally fragmented landscape replicates (blocks) at the Savannah River Site (SRS) National Environmental Research Park, USA. The landscapes were created in 1999 and 2000 by clear-cutting habitat patches from mature loblolly pine (Pinus taeda) plantation forest. The patches in each landscape are early-successional longleaf pine (Pinus palustris) savannah; they have had hardwoods removed and have been burned four times since 1999 to promote savannah restoration, while the mature forest matrix of each landscape remains intact. Each landscape is ~50 ha, including forested matrix area, and consists of five habitat patches of approximately equal area (~1 ha) (Fig. 1a). These experimental landscapes present an excellent system in which to study edge effects on plants and plant–animal interactions, for three reasons. First, because the patches are early-successional savannah habitat and the matrix is densely planted mature forest, the edge boundaries between the patches and the matrix are ecologically distinct. Second, the geometry of the patches permits explicit tests of the impacts of edge effects; each patch either is a rectangle that is 100 × 137 m or has a square central region that is 100 × 100 m, so study plants can be located on transects that bisect the right angle corners of each patch, spanning from the corners to the center regions of the patch, and the nearest patch edges will be equidistant from each plant on each transect (See “Study design” section below and Fig. 1b). Third, because there are five habitat patches per landscape and eight landscape replicates, edge studies in this system are conducted at a scale large enough to detect landscape effects.
a Map of eight replicated landscape blocks at the SRS, with insert showing the patch configuration of one landscape. Each landscape consists of five open-savannah habitat patches that either are rectangular (100 × 137 m) or have a square central region (100 × 100 m), shown in white. All habitat patches are surrounded by mature pine forest, shown in black. (Also shown, in gray, are two other features of the experimental landscapes that we do not consider in this study: a corridor of open-savannah habitat that connects two patches in each landscape, and “wings” of savannah habitat that extend from the square central regions of some of the patches. We disregard these landscape features because they are designed to test corridor and patch-shape effects, not edge effects.) b Diagram of one experimental patch showing locations of all pairs of study plants (treatment and control), and grasshopper surveys.  Locations of pairs of plants, comprising two transects per patch.
Locations of pairs of plants, comprising two transects per patch.  10 m grasshopper survey transects
10 m grasshopper survey transects
Focal species and edge effects that are already known
Solanum americanum is a fast growing annual herbaceous plant that can thrive in the early-successional habitat patches at SRS. Although it is not found in the landscapes except where planted, it is common in the region. Like many other solanaceous plants, S. americanum is a frequent host to honeydew-producing aphids (Semtner et al. 1998; Frechette et al. 2010; Pompon et al. 2011). In a previous study at SRS, presence-absence surveys found aphids on 37 % of all S. americanum plants in the experimental habitat patches (N = 201/541); ants also were frequent visitors to these same plants (Evans and Turley, unpublished data).
Ants are the most abundant ground-dwelling arthropods in the landscapes at SRS, with seven genera numerically dominant: Crematogaster, Dorymyrmex, Formica, Myrmica, Pheidole, Pogonomyrmex, and Solenopsis (Orrock et al. 2011). Among these genera, Dorymyrmex, Formica, Myrmica, Pogonomyrmex, and Solenopsis typically avoid edges and are most abundant in the center “core” area of the habitat patches; Crematogaster is most abundant in the patches near forest edges (Orrock et al. 2011). With the exception of Pogonomyrmex, ants in each of these genera consume aphid honeydew and are facultative plant mutualists that at least occasionally defend plants against insect herbivores (Hölldobler and Wilson 1990; Stechmann et al. 1996; Hubner 2000; Rico-Gray and Oliveira 2007; Warriner et al. 2008; Shibao et al. 2009; Gibb and Johansson 2010; Styrsky and Eubanks 2010). Crematogaster, Formica, and Solenopsis ants, in particular, will aggressively defend plants to protect sources of hemipteran honeydew (Kaplan and Eubanks 2005; Rico-Gray and Oliveira 2007; Styrsky and Eubanks 2010).
Grasshoppers are common consumers of S. americanum in the southeastern United States, and they are the primary consumers of S. americanum in the experimental patches at SRS (based on 4,000 + person-hours in the field and 300 h of video observations). We have observed seven grasshopper species from five genera consuming S. americanum in the patches: Melanoplus impudicus, Melanoplus sanguinipes, Melanoplus scudderi, Pardalophora phoenicoptera, Psinidia fenestralis, Schistocerca americana, and Trimerotropis sp. (Evans et al. 2012). Grasshopper abundance in the patches is positively correlated with temperature, and there is a strong edge effect on temperature, increasing with distance from the edges (Evans et al. 2012). Thus grasshoppers are typically less abundant near patch edges and more abundant in the center of the patches.
Because little is known about how edge effects influence multitrophic species interactions (Martinson 2009), we can only make tentative predictions about how edge effects may influence levels of herbivory and ant-guard protection against herbivory in this system. Given the edge effect on grasshopper abundance, we may expect herbivory to follow a similar pattern—lowest near patch edges and highest in the center of the patches. However, the relative abundance and “encounter frequencies” of grasshoppers and protective ants near and far from edges may cause ant-guard protection to increase from the edges to the center of the patches, or it may decrease, depending on which ant genera protect S. americanum and whether there is an edge effect on the abundance of aphids that may influence the abundance of protective ants (following Fagan et al. 1999; Ries et al. 2004). When we began this study we did not know the answer to these questions, so we designed the study to tease apart the relevant factors and evaluate each in turn.
Methods
Study design and data collection
The plants used for this study originated from four source populations ranging between Miami, Florida and Aiken, South Carolina. Seeds were collected from the source populations and then germinated in a greenhouse prior to planting in the field. In late June 2009, we planted seedlings (~10 cm tall) along two transects in each habitat patch. Each transect consisted of three planting locations at discrete distances—1, 20, and 37 m—from patch edges (Fig. 1b). We used a paired planting design on all transects, with two seedlings spaced ~1 m apart at each planting location. To control for plant provenance effects, the pair of seedlings at each planting location came from the same source population, and pairs of seedlings from the source populations were randomly interspersed among the three planting locations on each transect. We also randomly selected the two corners in each patch (out of four possible corners) where transects were located (Fig. 1b). Thus we used 240 pairs of plants: 1 pair at each planting location × 3 locations per transect × 2 transects per patch × 5 patches per landscape × 8 landscapes. All plants were watered at the time of planting and subsequently 3–4 times per week, receiving approximately the same amount of water each time. Watering continued until herbivory measurements were taken during the last week of August.
With this paired planting design, we conducted an exclusion experiment to determine which ants, if any, protect plants against grasshopper herbivory and whether edge effects alter the amount of protection ants provide. We used Pyganic insecticide spray (1.4 % pyrethrin) to target aphids and ants for this experiment, for two reasons: (1) Pyganic kills aphids and ants on contact and then loses toxicity after a few hours of exposure to air (Casida and Quistad 1995); (2) because Pyganic quickly degrades in the environment, it would not deter grasshoppers most of the time. We sprayed one plant in each pair during each treatment; the other plant was a control. “sprayed” and “control” plants were randomly designated in each pair, and control plants were sprayed with water. We treated all plants every 3–4 days for 8 weeks between 5 July and 26 August. All plants in every landscape were sprayed on the same day for each treatment, between 9:00 AM and 6:00 PM. During the treatments, we frequently observed that Pyganic killed both aphids and ants, and we often observed grasshoppers on both control and sprayed plants. In between treatments, after the insecticide degraded and the sprayed plants were no longer toxic, it was also possible for ants to safely visit the sprayed plants. But we designed the treatments to remove ants’ motivation for doing so—by removing aphids, which are less mobile and thus less able to recolonize sprayed plants.
We counted the number of aphids and ants on all plants five times (weekly) between 28 July and 25 August. During each census, surveyors spent 60 s observing each plant, without touching the plant, to count ants and identify them to genus. After counting ants, surveyors then handled the plant’s leaves as necessary to count aphids. Surveys were conducted between 8:30 AM and 6:00 PM. All plants in each landscape were surveyed on the same day; all landscapes were surveyed within a 4-day period each week. Due to logistical constraints, aphid surveys during the first week were only for presence or absence on each plant, although we did count all of the ants on each plant during the first week. All surveys during the last 4 weeks included full counts of aphids and ants on each plant.
We also conducted visual surveys for grasshoppers in each patch five times between mid-June and late August. Surveys were conducted between 10:00 AM and 6:00 PM along the same transects in the patches where study plants were located—at 1, 20 and 37 m from patch edges (Fig. 1b). Starting at each pair of study plants, surveyors walked 10 m in a direction parallel to one of the patch edges, then returned to the starting point (without counting) and walked 10 m parallel to the other nearby patch edge. While walking the transects, surveyors counted all grasshoppers observed regardless of species and regardless of whether grasshoppers remained stationary, jumped or flew away. Most grasshoppers that jumped or flew moved <15 m, and surveyors avoided counting them more than once. All patches in a landscape were surveyed on the same day; the landscapes were surveyed in a random rotation during each survey period.
In late August, after 8 weeks of spray treatments, we counted all of the leaves on every plant, and we counted the total number of leaves per plant that had leaf area missing due to damage by insect herbivores. We also recorded throughout the experiment whether plants died or were chewed off at the main stem by herbivores. These “clipped” plants usually died; when they survived they produced few leaves. We removed from all analyses any pair of plants in which either plant died or was clipped, with 196 out of 240 pairs of plants remaining.
Statistical analyses
Our first analysis was designed to test the impact of selective aphid and ant removal (via spray treatments with insecticide) on herbivory. We conducted this test to confirm that removing aphids and ants (some of which are presumed to protect plants) would result in increased herbivory on the treated plants. For the purposes of this analysis, and for all subsequent analyses of herbivory, we calculated herbivory as a binomial response variable: the proportion of damaged leaves per plant. We used a generalized linear mixed effects (GLME) model that assessed whether sprayed plants had higher levels of leaf damage than the control plants with which they were paired. All plants (control and sprayed) were included in this analysis, and treatment (control or sprayed) was the only fixed effect. Landscape (1–8), planting location (1–196), and individual plant identification numbers (1–392) were nested random effects. With “planting location” as a blocking factor, significance values assigned to the treatment effect are based on comparisons between control and sprayed plants at each location.
Our next analysis identified which ant genera, if any, provided the most protection to S. americanum against herbivory. We used a model simplification routine based on Akaike’s information criterion (following Crawley 2007) to identify the ant genera whose removal from the study plants (via the insecticide treatment) best explained differences in leaf damage among pairs of sprayed and control plants (See Table 1). Our maximal GLME model included all of the ants that we observed on study plants divided into five groups of ant genera: Crematogaster, Dorymyrmex, Pheidole, Solenopsis, and “other.” Crematogaster, Dorymyrmex, Pheidole, and Solenopsis comprised 89 % of all ants detected on plants; the “other” category mostly included ants that could not be identified in the field. Fixed effects in the maximal model were: treatment (control or sprayed), the mean number of ants per plant for each ant genus (averaged across all ant survey periods), two-way interactions between treatment and the mean number of ants per plant for each genus, and three-way interactions between treatment and the mean numbers of ants per plant for pairs of ant genera that we observed co-occurring on the plants. These three-way interactions account for covariance among ant genera in their response to the treatment. (We never observed more than two ant genera on a plant at a time, but we did observe pairs of ant genera co-occurring on plants 5 % of the time. Thus we included three-way interactions between treatment and the most common pairs that we observed; to avoid excessively complicating the model, we excluded three pairwise combinations that we observed only one time each.) The maximal model also included landscape (1–8), planting location (1–196), and plant identification numbers (1–392) as nested random effects. Starting with the maximal model, we removed ant groups and interaction terms in stepwise fashion, removing the least significant predictor with each step. Whenever an interaction term was the least significant, we removed it alone; whenever an ant group was the least significant, we removed it along with any associated interaction. We continued this routine until we found a “best set” of models, which included only the most protective ants.
After determining which ant genera provided the most protection against herbivory, we also assessed whether the difference in the number of aphids on treated and control plants significantly influenced the difference in the number of protective ants. We conducted this test simply to evaluate whether the relative abundance of aphids explains the relative abundance of protective ants. Only pairs of plants where we observed protective ants (on either control or sprayed plants) were included in this analysis (=114/196 total pairs). We used a GLME model in which the number of protective ants on each plant was the response variable, with a Poisson distribution; and we assessed the influence of the interaction between the treatment effect and the number of aphids at each pair of plants. Fixed effects were: the number of aphids on each plant, treatment (control or sprayed), and their interaction. The survey period during which both aphids and ants were counted (1–4) was a random effect; landscape (1–8) and planting location (1–114) were nested random effects.
To test for edge effects on focal groups of insects, we analyzed the number of aphids per control plant, the number of protective ants per control plant, and the number of grasshoppers at each planting location. For each variable (aphids, protective ants, and grasshoppers), we used a separate GLME model with a Poisson distribution; distance to the nearest edges (1, 20, or 37 m) was a fixed effect; survey period was a random effect (for aphids: 1–4; for ants: 1–5; for grasshoppers: 1–5); and landscape (1–8) and patch identification number (1–40) were nested random effects.
Finally, we evaluated whether ant-guard protection against herbivory varied with distance from the edges by comparing the amount of leaf damage on sprayed and control plants at each distance location (1, 20, and 37 m). Within each distance category, treatment was a fixed effect; landscape (1–8), planting location (1–196) and plant identification numbers (1–392) were nested random effects.
All analyses were fit using the statistical program R (R Development Core Team 2010) and the lme4 package (Bates and Sarkar 2010). We assessed the significance of particular factors, e.g., “treatment” or “distance to edges,” with likelihood ratio tests (following Crawley 2007). This maximum likelihood approach assigns significance values by comparing full models to null models that are exactly the same except that the factor of interest is removed.
When testing for edge effects on the abundance of aphids, protective ants, and grasshoppers, we assessed differences in the numbers of these insects at each distance (1, 20, and 37 m) using the multcomp package (Hothorn 2010) and the Westfall resampling procedure for assigning P-values (following Westfall 1997; Bretz et al. 2010). This procedure is appropriate for models with a Poisson distribution and is based on a bootstrapping method that can produce reliable estimates with zero-inflated data (Westfall and Troendle 2008; Bretz et al. 2010). There is a relatively large portion of zeros in the datasets we used to evaluate edge effects on aphids and protective ants; only 77 % of the control plants hosted aphids (N = 150/196 plants), and only 52 % of the control plants hosted protective ants (N = 102/196 plants).
Results
When aphids and ants were removed from study plants (via the insecticide treatment), herbivory increased significantly (LRT, χ2 = 16.79, df = 1, P < 0.01). Considering all plant pairs together, the odds of leaf damage for sprayed plants were 53 % higher than for control plants. The model simplification routine we used to determine which ant genera provided the most protection against leaf damage identified a combination of two ant genera, pooled together: Crematogaster and Solenopsis (See Table 1). And the difference in the number of aphids on control vs. sprayed plants also significantly predicted the difference in the number of Crematogaster and Solenopsis ants (LRT, χ2 = 13.62, df = 1, P < 0.01). For every 10.0 fewer aphids on sprayed plants (relative to control plants), the number of Crematogaster and Solenopsis ants declined by 20 %.
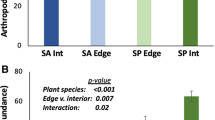
We found strong edge effects on the abundance of aphids, Crematogaster and Solenopsis ants, and grasshoppers. Distance from habitat edges significantly influenced the number of aphids per control plant (LRT, χ2 = 826.66, df = 2, P < 0.01, Fig. 2a), the number of Crematogaster ants per control plant (LRT, χ2 = 125.17, df = 2, P < 0.01, Fig. 2b), the number of Solenopsis ants per control plant (LRT, χ2 = 121.62, df = 2, P < 0.01, Fig. 2c), the number of Crematogaster and Solenopsis ants pooled together (because they are both protective) per control plant (LRT, χ2 = 229.35, df = 2, P < 0.01, Fig. 2d), and grasshopper abundance (LRT, χ2 = 1320.7, df = 2, P < 0.01, Fig. 2e). Aphids were more abundant at 1 m from the edges than at 20 m (z = 8.72, P < 0.01) or 37 m (z = 26.79, P < 0.01), and aphids at 20 m also were more abundant than at 37 m (z = 19.34, P < 0.01). Similarly, Crematogaster ants were more abundant at 1 m than at 20 m (z = 6.68, P < 0.01) or 37 m (z = 8.40, P < 0.01), and they also were more abundant at 20 m than at 37 m (z = 3.64, P < 0.01); Solenopsis ants were more abundant at 1 m than at 20 m (z = 6.40, P < 0.01) or 37 m (z = 9.57, P < 0.01), and they were more abundant at 20 m than at 37 m (z = 4.36, P < 0.01); and Crematogaster and Solenopsis ants pooled together were more abundant at 1 m than at 20 m (z = 8.91, P < 0.01) or 37 m (z = 12.75, P < 0.01), and they were more abundant at 20 m than at 37 m (z = 5.69, P < 0.01). Grasshoppers were less abundant at 1 m than at 20 m (z = 26.34, P < 0.01) or 37 m (z = 30.15, P < 0.01), and grasshoppers also were less abundant at 20 m than at 37 m (z = 5.49, P < 0.01).
Distributions, by distance from patch edges, of aphids, protective ants, grasshoppers, and levels of ant-guard protection against herbivory. a–f are as follows: a the number of aphids per control and sprayed plant, b the number Crematogaster ants per control and sprayed plant, c the number of Solenopsis ants per control and sprayed plant, d the number of Crematogaster and Solenopsis ants pooled together (since both genera protect plants against herbivory), e the number of grasshoppers detected per survey location (two survey transects per location, six locations per patch), and f levels of ant-guard protection against herbivory. The amount of protection at each distance from the edges is shown as a comparison between levels of herbivory on control versus sprayed plants. Graphs show means ± 1 SE for all 40 patches grouped together
Finally, the amount of protection that Crematogaster and Solenopsis ants provided S. americanum against herbivory was greater toward the center of the patches than it was near the edges. The difference in leaf damage between sprayed and control plants at 1 m from patch edges was not significant (LRT, χ2 = 2.45, df = 1, P = 0.12), but the difference in leaf damage was significant 20 m from the edges (LRT, χ2 = 10.53, df = 1, P < 0.01) and 37 m from the edges (LRT, χ2 = 3.81, df = 1, P = 0.05, Fig. 2f). At 20 m from the edges, the odds of leaf damage on sprayed plants, compared to control plants, were 69 % higher; at 37 m from the edges, the odds of leaf damage were 37 % higher.
Discussion
Our results suggest two main conclusions. First, edge effects on herbivore abundance can strongly influence levels of herbivory and the amount of protection ants provide to ant-plants in fragmented habitats, but these interactions are not simply a product of the relative abundance of the interacting species. Second, researchers will likely need to consider the protective behavior of ants, in addition to their abundance, to evaluate how edge effects may alter levels of ant-guard protection. To develop these conclusions, we consider each result in turn.
We found strong edge effects on each focal group of insects in the experimental patches. As expected, grasshopper abundance increased with distance from the edges; this result is consistent with previous studies showing that grasshopper abundance is positively correlated with local habitat temperature, and there is a strong edge gradient on temperature in the patches, increasing with distance from the edges (Evans et al. 2012). The per-plant abundance of Crematogaster ants decreased with distance from the edges, and this is also consistent with a previous study in the same experimental system (Orrock et al. 2011). Similarly, the per-plant abundance of Solenopsis ants decreased with distance from the edges, but this result was surprising because Orrock et al. (2011) found the opposite edge gradient on Solenopsis abundance—increasing with distance from the edges. The discrepancy may stem from the fact that Orrock and colleagues used pitfall traps to sample ants, which would effectively separate their abundance in the patches from their interactions with any particular plant species. As our study shows, the number of Crematogaster and Solenopsis ants on S. americanum is correlated with the number of aphids, which were most abundant close to the edges. Of course, this begs the question why aphids were more abundant close to the edges. We can only speculate, but it is possible that Crematogaster ants would collect aphids and deposit them on S. americanum plants near the edges in order to have ready access to aphid honeydew.
Ultimately, the amount of herbivory on S. americanum was determined by the interactions among these focal groups of insects. Our first three statistical analyses demonstrate the key connections: (1) the spray treatment resulted in dramatically increased herbivory on sprayed plants; (2) the effectiveness of the treatment at reducing Crematogaster and Solenopsis ant abundance contributed to this increase in herbivory; and (3) the effectiveness of the treatment at reducing aphid abundance was a significant factor reducing the number of Crematogaster and Solenopsis ants. Thus Crematogaster and Solenopsis ants protected plants against grasshopper herbivory, and they engaged in this ant-guard behavior largely because aphids were present.
Perhaps the most important landscape factor determining the amount of protection ants provided to S. americanum was the strong edge effect on grasshopper abundance. The difference in levels of herbivory between control and sprayed plants was only significant where grasshoppers were most abundant, at 20 and 37 m from the edges; the difference in herbivory was not significant where grasshoppers were least abundant, 1 m from the edges. This is despite the fact that the per-plant abundance of aphids and protective ants all went the other direction—lowest far from the edges and highest near the edges. It is only where herbivory pressure was highest—far from the edges—that ant-guard protection made a significant difference. This dynamic also likely explains why levels of herbivory on the control plants were very similar at each distance location; as herbivory pressure increased farther from the edges, ant-guard protection also increased.
The edge effects on aphid and ant abundance are more difficult to reconcile with this pattern of ant-guard protection. At first glance, it seems contradictory that ants should provide more protection far from edges, where they were least abundant on the study plants. But it may be explained by considering two aspects of ant-guard behavior. First, ants’ protection of plants against herbivory is inherently defensive; it only arises in response to threats initiated by herbivores. Second, ants are highly mobile and responsive to environmental cues, so the number of ants on a plant at any given time may increase if ants in the surrounding habitat recruit to the plant to protect it against herbivory (Agrawal and Rutter 1998). In this respect, ant-guard protection of S. americanum may be an inducible plant defense. Both Crematogaster and Solenopsis ants are known to recruit congeners in response to threats against plant mutualists (Fiala and Maschwitz 1990; Lapola et al. 2003; Bruna et al. 2004; Ness and Bronstein 2004; Kaplan and Eubanks 2005). Our analyses show that the number of ants per plant will influence levels of protection, but to understand how edge effects alter the amount of protection ants provide, we may also need to consider how ants behave in response to herbivory threats.
Perhaps levels of protection against herbivory were only significant far from the edges because it is in these locations that ants would most readily recruit to plants in response to the increased herbivory pressure from grasshoppers. We detected relatively few protective ants per plant at all three distances from habitat edges (0.4–1.5 ants per plant), so even just a few ants recruiting from the habitat surrounding a threatened plant would substantially increase the number of ant defenders. Solenopsis ants, in particular, are found to be more abundant far from edges in the patches when they are sampled separately from their interactions with any particular plant species (Orrock et al. 2011). The fact that we detected more Solenopsis ants per S. americanum plant close to the edges (because this is where the aphids were concentrated) indicates that the number of Solenopsis ants on the plants is a function of their investment in the plants, not simply determined by their abundance in the patches. While we did not attempt, in this study, to quantify ant behavior, future studies could measure ant-guard defense of their host plants by directly observing how the ants’ abundance and the amount of time they spend on the plants may vary in the presence and absence of threatening herbivores.
In conclusion, our study suggests that edge effects on herbivory pressure will be the primary landscape factor determining the encounter frequencies between herbivores, plants, and protective ants as well as the levels of ant-guard protection against herbivory in edgy habitats. These dynamic species interactions, furthermore, may largely be a product of the inducible and reactive nature of ants’ defensive behavior. The abundance of honeydew-producing herbivores and protective ants is clearly important to the level of protection that ants will provide, but the number of ants per plant, as an inducible defense response, may also be highly variable. In a broader context, our results suggest that it may be difficult to identify general patterns of edge effects on species interactions unless researchers go beyond the simple accounting of species abundance and also consider which species’ behavioral patterns influence particular types of interactions.
References
Agrawal AA (2011) Current trends in the evolutionary ecology of plant defence. Funct Ecol 25:420–432
Agrawal AA, Rutter MT (1998) Dynamic anti-herbivore defense in ant-plants: the role of induced responses. Oikos 83:227–236
Bach CE, Kelly D (2004) Effects of forest edges on herbivory in a New Zealand mistletoe, Alepis flavida. N Z J Ecol 28:195–205
Bates D, Sarkar D (2010) lme4: Linear Mixed-Effects Models Using S4 Classes. R package version 0.999375-33. http://cran.r-project.org/web/packages/lme4/index.html. Accessed 17 July 2013
Beattie AJ (1985) The evolutionary ecology of ant–plant mutualisms. Cambridge University Press, Cambridge
Beattie AJ, Hughes L (2002) Ant–plant interactions. In: Herrera CM, Pellmyr O (eds) Plant–animal interactions: an evolutionary approach. Blackwell Science, Oxford, pp 211–235
Braschler B, Lampel G, Baur B (2003) Experimental small-scale grassland fragmentation alters aphid population dynamics. Oikos 100:581–591
Bretz F, Hothorn T, Westfall P (2010) Multiple Comparisons Using R. CRC Press, New York
Bronstein JL (1994) Conditional outcomes in mutualistic interactions. Trends Ecol Evol 9:214–217
Bruna EM, Lapola DM, Vasconcelos HL (2004) Interspecific variation in the defensive responses of obligate plant–ants: experimental tests and consequences for herbivory. Oecologia 138:558–565
Bruna EM, Vasconcelos HL, Heredia S (2005) The effect of habitat fragmentation on communities of mutualists: amazonian ants and their host plants. Biol Conserv 124:209–216
Carroll CR, Janzen DH (1973) Ecology of foraging ants. Annu Rev Ecol Evol Syst 4:231–257
Carvalho KS, Vasconcelos HL (1999) Forest fragmentation in central amazonia and its effects on litter-dwelling ants. Biol Conserv 91:151–157
Casida JE, Quistad GB (eds) (1995) Pyrethrum flowers: production, chemistry, toxicology, and uses. Oxford University Press, Oxford
Collinge SK (2009) Ecology of fragmented landscapes. Johns Hopkins University Press, Baltimore
Crawley MJ (2007) The R book. Wiley and Sons, Chichester
Cushman JH, Addicott JF (1991) Conditional interactions in ant–plant-herbivore mutualisms. In: Huxley CR, Cutler DF (eds) Ant–plant interactions. Oxford University Press, Oxford, pp 92–103
Debuse VJ, King J, House APN (2007) Effect of fragmentation, habitat loss and within-patch habitat characteristics on ant assemblages in semi-arid woodlands of eastern Australia. Landscape Ecol 22:731–745
Evans DM, Turley NE, Levey DJ, Tewksbury JJ (2012) Habitat patch shape, not corridors, determines herbivory and fruit production of an annual plant. Ecology 93:1016–1025
Fagan WE, Cantrell RS, Cosner C (1999) How habitat edges change species interactions. American Naturalist 153:165–182
Fiala B, Maschwitz U (1990) Studies on the South East-Asian ant–plant association crematogaster: Borneensis macaranga—adaptations of the ant partner. Insectes Soc 37:212–231
Frechette B, Bejan M, Lucas E, Giordanengo P, Vincent C (2010) Resistance of wild Solanum accessions to aphids and other potato pests in Quebec field conditions. J Insect Sci 10:16
Futuyma DJ, Agrawal AA (2009) Macroevolution and the biological diversity of plants and herbivores. Proc Natl Acad Sci USA 106:18054–18061
Gibb H, Johansson T (2010) Forest succession and harvesting of hemipteran honeydew by boreal ants. Ann Zool Fenn 47:99–110
Hölldobler B, Wilson EO (1990) The ants. Belknap Press of Harvard University Press, Cambridge
Horvitz CC, Schemske DW (1984) Effects of ants and an ant-tended herbivore on seed production of a Neotropical herb. Ecology 65:1369–1378
Hothorn TF, Bretz P, Westfall RM, Heiberger, Schutzenmeister A (2010) multcomp: Simultaneous Inference for General Linear Hypotheses. R package version 1.1-7. http://cran.r-project.org/web/packages/multcomp/index.html. Accessed 17 July 2013
Hubner G (2000) Differential interactions between an aphid endohyperparasitoid and three honeydew-collecting ant species: a field study of Alloxysta brevis (Thomson) (Hymenoptera:Alloxystidae). J Insect Behav 13:771–784
Janzen DH (1966) Coevolution of mutualism between ants and acacias in Central America. Evolution 20:249–275
Janzen DH (1967) Interaction of the bull’s-horn acacia (Acacia cornigera L.) with an ant inhabitant (Pseudomyrmex ferruginea F. Smith) in eastern Mexico. Univ Kans Sci Bull 47:315–558
Janzen DH (1969) Allelopathy by myrmecophytes: ant azteca as an allelopathic agent of Cecropia. Ecology 50:147–153
Johnson JB, Omland KS (2004) Model selection in ecology and evolution. Trends Ecol Evol 19:101–108
Kaplan I, Eubanks MD (2005) Aphids alter the community-wide impact of fire ants. Ecology 86:1640–1649
Lapola DM, Bruna EM, Vasconcelos HL (2003) Contrasting responses to induction cues by ants inhabiting Maieta guianensis (Melastomataceae). Biotropica 35:295–300
Laurance WF, Camargo JLC, Luizao RCC, Laurance SG, Pimm SL, Bruna EM, Stouffer PC, Williamson GB, Benitez-Malvido J, Vasconcelos HL, Van Houtan KS, Zartman CE, Boyle SA, Didham RK, Andrade A, Lovejoy TE (2011) The fate of Amazonian forest fragments: a 32-year investigation. Biol Conserv 144:56–67
Lopez-Barrera F, Manson RH, Gonzalez-Espinosa M, Newton AC (2006) Effects of the type of montane forest edge on oak seedling establishment along forest-edge-exterior gradients. For Ecol Manag 225:234–244
Martinson H (2009) Interactions lost and gained: a review of how trophic interactions are impacted by the fragmentation of habitats. Doctoral Dissertation, University of Maryland, College Park
Massad TJ, Fincher RM, Smilanich AM, Dyer L (2011) A quantitative evaluation of major plant defense hypotheses, nature versus nurture, and chemistry versus ants. Arthropod Plant Interact 5:125–139
Meiners SJ, Handel SN, Pickett STA (2000) Tree seedling establishment under insect herbivory: edge effects and interannual variation. Plant Ecol 151:161–170
Messina FJ (1981) Plant protection as a consequence of an ant-membracid mutualism: interactions on goldenrod (Solidago sp.). Ecology 62:1433–1440
Ness JH, Bronstein IL (2004) The effects of invasive ants on prospective ant mutualists. Biol Invasions 6:445–461
Oliveira PS, Del-Claro K (2005) Multitrophic interactions in a Neotropical savannah: ant-hemipteran systems, associated insect herbivores, and a host plant. In: Burslem DFRP, Pinard MA, Hartley SE (eds) Biotic interactions in the tropics. Cambridge University Press, Cambridge
Oliveira PS, Freitas AVL (2004) Ant-plant-herbivore interactions in the neotropical cerrado savanna. Naturwissenschaften 91:557–570
Orrock JL, Curler GR, Danielson BJ, Coyle DR (2011) Large-scale experimental landscapes reveal distinctive effects of patch geometry and connectivity on arthropod communities. Landscape Ecol 26:1361–1372
Pompon J, Li XQ, Pelletier Y (2011) Resistance level to an aphid potato pest varies between genotypes from the same solanum accession. J Econ Entomol 104:1075–1079
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rico-Gray V, Oliveira PS (2007) The ecology and evolution of ant–plant interactions. University of Chicago Press, Chicago
Ries L, Fletcher RJ, Battin J, Sisk TD (2004) Ecological responses to habitat edges: mechanisms, models, and variability explained. Annu Rev Ecol Evol Syst 35:491–522
Schupp EW, Feener DH (1991) Ant-defended plants in a Panamanian forest. In: Huxley CR, Cutler DF (eds) Ant–plant interactions. Oxford University Press, Oxford
Semtner PJ, Tilson WM, Dara SK (1998) Performance of the tobacco aphid (Homoptera:Aphididae) on various host plants. J Entomol Sci 33:180–195
Shibao H, Morimoto M, Okumura Y, Shimada M (2009) Fitness costs and benefits of ant attendance and soldier production for the social aphid Pseudoregma bambucicola (Homoptera:Aphididae:Hormaphidinae). Sociobiology 54:673–698
Stadler B, Dixon AFG (2005) Ecology and evolution of aphid–ant interactions. Annual Review Of Ecology Evolution And Systematics, pp 345–372
Stechmann DH, Volkl W, Stary P (1996) Ant-attendance as a critical factor in the biological control of the banana aphid Pentalonia nigronervosa Coq (Homptera:Aphididae) in Oceania. J Appl Entomol Zeitschrift Fur Angewandte Entomologie 120:119–123
Styrsky JD, Eubanks MD (2010) A facultative mutualism between aphids and an invasive ant increases plant reproduction. Ecol Entomol 35:190–199
Tscharntke T, Brandl R (2004) Plant–insect interactions in fragmented landscapes. Annu Rev Entomol 49:405–430
Tscharntke T, Steffan-Dewenter I, Kruess A, Thies C (2002) Characteristics of insect populations on habitat fragments: a mini review. Ecol Res 17:229–239
Valladares G, Salvo A, Cagnolo L (2006) Habitat fragmentation effects on trophic processes of insect–plant food webs. Conserv Biol 20:212–217
Vasconcelos HL (1991) Mutualism between Maieta guianensis Aubl, a myrmecophytic melastome, and one of its ant inhabitants: ant protection against insect herbivores. Oecologia 87:295–298
Warriner RA, Gold RE, Austin JW (2008) Ecology of Dorymyrmex flavus (Hymenoptera:Formicidae) in Central Texas, including aspects of competition with Solenopsis invicta Buren. Sociobiology 52:229–250
Way MJ (1963) Mutualism between ants and honeydew-producing homoptera. Ann Rev Entomol 8:307–344
Westfall PH (1997) Multiple testing of general contrasts using logical constraints and correlations. J Am Stat Assoc 92:299–306
Westfall PH, Troendle JF (2008) Multiple testing with minimal assumptions. Biometrical J 50:745–755
Wimp GM, Murphy SM, Lewis D, Ries L (2011) Do edge responses cascade up or down a multi-trophic food web? Ecol Lett 14:863–870
Acknowledgments
We thank the USDA Forest Service-Savannah River for creating and maintaining our experimental landscapes; N. Haddad, D. Levey and L. Brudvig for their roles in designing and implementing the landscape experiment; E. Damschen, N. Haddad, D. Levey, and J. Orrock for their roles in maintaining the landscape experiment; the Corridor Project team for fruitful discussions that led to many ideas in this paper; L. Fenner, R. Malinen, and E. Smith for field assistance; J. Hille Ris Lambers and J. Lawler for reviewing the manuscript; and two anonymous reviewers for suggestions that also improved the manuscript. This research was supported by the National Science Foundation (DEB-0613701), by the University of Washington, and by funds provided to the Department of Agriculture, Forest Service, Savannah River, under Interagency Agreement DE-AI09-00SR22188 with the Department of Energy, Aiken, SC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Evans, D.M., Turley, N.E. & Tewksbury, J.J. Habitat edge effects alter ant-guard protection against herbivory. Landscape Ecol 28, 1743–1754 (2013). https://doi.org/10.1007/s10980-013-9917-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-013-9917-6