Abstract
Human land-use practices have dramatically altered the composition and configuration of native habitats throughout many ecosystems. Within heterogeneous landscapes generalist predators often thrive, causing cascading effects on local biological communities, yet there are few data to suggest how attributes of fragmentation influence local population dynamics of these species. We monitored 25 raccoon (Procyon lotor) populations from 2004 to 2009 in a fragmented agricultural landscape to evaluate the influence of local and landscape habitat attributes on spatial and temporal variation in demography. Our results indicate that agricultural ecosystems support increased densities of raccoons relative to many other rural landscapes, but that spatial and temporal variation in demography exists that is driven by non-agricultural habitat attributes rather than the availability of crops. At the landscape scale, both density and population stability were positively associated with the size and contiguity of forest patches, while at the local scale density was positively correlated with plant diversity and the density of tree cavities. In addition, populations occupying forest patches with greater levels of plant diversity and stable water resources exhibited less temporal variability than populations with limited plant species complexity or water availability. The proportion of populations comprised of females was most strongly influenced by the availability of tree cavities and soft mast. Despite the abundance of mesopredators in heterogeneous landscapes, our results indicate that all patches do not contribute equally to the regional abundance and persistence of these species. Thus, a clear understanding of how landscape attributes contribute to variation in demography is critical to the optimization of management strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, anthropogenic activities such as the clearing of land for agricultural production and grazing cattle have dramatically altered the spatial arrangement and composition of native habitat patches. As a result, understanding the consequences of habitat destruction and fragmentation for wildlife populations is an intrinsic concern of wildlife professionals. Habitat fragmentation threatens the stability and persistence of local populations due to increased genetic, demographic, or environmental stochasticity (Wiens 1976; Gilpin and Hanski 1991; Andrén 1994). Moreover, fragmentation ultimately leads to reductions in the total amount of native habitat available, as well as to changes in the quality of remaining habitats through increased patch isolation, and deleterious edge effects (Yahner 1988).
Habitat fragmentation can alter the spatial structure of vertebrate populations, as the abundance and distribution of wildlife populations often are influenced by the spatial arrangement and composition of habitats within landscapes (Saunders et al. 1991; Kozakiewicz et al. 1999). More importantly, habitat fragmentation can alter the spatial arrangement of critical resources within a landscape, potentially resulting in disparate demographic patterns (e.g., sex ratio, abundance, etc.) for species among habitat patches. However, the degree to which fragmentation alters the demography of wildlife populations should vary as a function of the behavioural plasticity of individual species (Bender et al. 1998; Nupp and Swihart 2000). Generalist species and those with an affinity to edges, often are less affected by habitat fragmentation than are interior species or habitat specialists (Nupp and Swihart 1996; Bender et al. 1998; Hokit and Branch 2003).
Although habitat fragmentation occurs in many contexts throughout the world, the Midwestern United States serves as a renowned example of this type of landscape disturbance, where remnant native habitats persist as a mosaic of small patches within a matrix dominated by agricultural crops and anthropogenic features (Moore and Swihart 2005). Within the agricultural ecosystems of the Midwestern U.S., generalist species with broad habitat tolerances and good mobility often thrive, presumably, at least in part, because of their ability to adapt to the displacement of native food resources by utilizing corn and other crops (Oehler and Litvaitis 1996; Beasley et al. 2007a; Beasley and Rhodes 2008).
Raccoons (Procyon lotor) undoubtedly are among the most plastic of mammals in terms of their ability to modify their ecology and behaviour relative to local environmental conditions, exhibiting substantive variance in nearly every aspect of their ecology across their range (Gehrt 2003). Raccoons are abundant in agricultural ecosystems, where they occur in densities that generally exceed those of raccoons occupying less disturbed landscapes (Beasley and Rhodes 2008). However, despite their general adaptability and behavioural plasticity, previous research suggests that habitat fragmentation can lead to spatial variation in raccoon movement behaviour (Beasley and Rhodes 2010) and levels of kin structure (Dharmarajan et al. 2009) among local populations, presumably in response to variation in local patch quality; although the effects of forest fragmentation on the ecology of raccoon populations are poorly understood. Consequently, demographic parameters of raccoon populations also are likely to vary among habitat patches in heterogeneous landscapes due to local variation in habitat attributes.
For raccoons or other predator species, spatial and temporal variability in population size can have significant consequences on the abundance and persistence of prey species, as well as on the transmission dynamics of infectious diseases. For example, in years of high raccoon abundance, survival of local ground-nesting bird or small mammal populations may be drastically reduced, either through direct predation, or through an increased prevalence of infectious diseases (Robinson et al. 1995; Donovan et al. 1997; Heske et al. 1999; Page et al. 1999). Moreover, spatial variation in the gender composition of raccoon populations could directly influence patch specific reproductive levels, potentially leading to variation in reproductive potential among local populations for this species in agricultural ecosystems. Given the apparent success of mesopredators in fragmented agricultural environments, as well as the potential cascading effects that these species can have on broader ecological processes (e.g. disease transmission, predation, etc.), elucidating the relationships between habitat attributes and population dynamics of such generalist predators is critical to understanding how these complex ecosystems function.
In this study, we utilized a relatively long-term (6-year) mark-recapture data set for raccoons occupying 25 distinct forest patches in a highly fragmented agricultural ecosystem to evaluate the influence of local and landscape habitat attributes on raccoon demography (density, gender ratio, age composition), as well as on the temporal stability of each of these demographic metrics among forest patches. Our objective in this research was to test the hypotheses that (1) the demographic parameters of local raccoon populations exhibit significant levels of spatial and temporal variation, and that (2) habitat attributes associated with fragmentation (e.g. patch size), and patch quality (e.g. water availability) directly contribute to this variance.
Methods
Study area
Our 1,165 km2 study area was located in the Upper Wabash River Basin (UWB) in north-central Indiana, USA. Approximately 71% of the land area within the UWB was in agricultural use, with corn and soybeans comprising the primary crops. All contiguous forest tracts within the study area were confined to major drainages where frequent flooding or locally steep topography made the land unsuitable for crop production. The remaining native forests (predominantly oak-hickory-maple [Quercus-Carya-Acer]) in the basin were highly fragmented, with the distribution of forest patch sizes dominated by patches <5 ha (75%; Moore and Swihart 2005).
Within this landscape raccoon activity is predominantly concentrated within forested habitat or along forest-agricultural interfaces, with little activity occurring within the agricultural matrix (Beasley et al. 2007b; Beasley and Rhodes 2010). Thus, we selected 25 distinct forest patches from our general study area to examine spatial and temporal variation in raccoon demographic characteristics. Forest patches were selected in an effort to represent the distribution of local and landscape level patch characteristics present within the landscape. Based on the spatial distribution of patches sampled throughout our study area (average Euclidean distance between the centre of trapped patches was >16 km) and small home range sizes of raccoons in the UWB (\( \bar{X} \) = 73 ha; Beasley et al. 2007a), we considered each patch to represent a “local population” of raccoons.
Raccoon trapping
Raccoon trapping was conducted in each of the 25 patches annually (late March-early June) from 2004 to 2009 prior to the emersion of young from den sites. We captured raccoons using box live traps (Tomahawk Live Trap Co., Tomahawk, Wisconsin, USA) baited with commercial cat food. Traps were placed in a grid (50-m spacing) within forest patches and pre-baited for 1–3 nights. Following the pre-baiting period traps were opened and maintained for 10 consecutive nights. The total number of traps per grid varied with patch size, with a maximum of 30 traps placed in any single forest patch (range 4–30, \( \bar{X} \) = 19.4). We immobilized captured raccoons with Telazol (Fort Dodge Animal Health, Fort Dodge, IA, USA) at a dosage of five mg/kg of estimated body mass.
Sex was determined for all newly captured raccoons which were subsequently ear-tagged (Monel #3, National Band and Tag Company, Newport, Kentucky, USA), weighed, and aged via tooth-wear (Grau et al. 1970). Raccoons were classified as juveniles (1 year), yearlings (2 year), or adults (≥3 year). In addition, we collected tissue samples (ear biopsy) from each captured individual for genetic analyses. Tagged raccoons captured in subsequent years were immobilized and processed as new individuals (with the exception of tagging and tissue collection) to obtain updated demographic information. All trapping and handling methods conformed to the American Society of Mammalogists guidelines (Gannon et al. 2007) as well as Purdue University Animal Care and Use Committee policies under Protocol 01-079.
Landscape-scale characteristics
To quantify landscape level habitat attributes associated with each forest patch and its local raccoon population, we used a GIS database developed from 1998 U.S. Geological survey digital orthophotos (DOQs) of 1-m resolution. Habitats were delineated into 8 land use classes: forest, shrubland, wooded corridors, grassland, agriculture, water, developed, and roads. Specific details of habitat delineations are provided in Retamosa et al. (2008). In addition, we incorporated two additional layers into our GIS, the National Hydrography Dataset and the National Wetlands Inventory, to more precisely define water availability.
We used ArcMap 9.2 to estimate landscape-level attributes associated with trapped forest patches. We estimated the size, as well as total area (ha) of forest, agriculture, developed, wetland (including ponds/lakes), and stream (length) habitats within a 92 ha buffer of the centroid of each trapped patch. A 92 ha buffer was chosen to account for the movement behaviour of raccoons in our landscape; it represents an area equal to the average home range size (95% fixed kernel) of male raccoons, which maintain larger home ranges than females, in our study area (Beasley et al. 2007a).
To obtain annual crop specific data for all agricultural fields proximal to forest patches trapped for raccoons, we used remote sensing cropland data layers (CDLs; 30-m resolution) for Indiana, collected by the U.S Department of Agriculture-National Agriculture Statistics Service (USDA-NASS) from 2003 to 2008 (USDA-NASS 2004–2009). Because trapping occurred during the spring prior to the development of crops, crop types were assigned to fields based on CDL’s for crops planted during the previous year. We classified all crop fields (corn, soybeans, or wheat) within a 92 ha buffer (see above) of the centroid of forest patches trapped for raccoons each year and estimated the proportion of each buffer comprised of corn.
To evaluate the influence of weather on interannual variation in raccoon demographics, we obtained daily precipitation and temperature data for our study area from the National Oceanic Atmospheric Administration’s National Climatic Data Centre for 2003–2009. From these data we calculated average minimum winter temperatures (Dec–Feb) and cumulative annual precipitation for the period prior to trapping each year (March through February).
Fine-scale patch characteristics
To estimate the potential number of den sites available in each forest patch, we conducted raccoon den surveys prior to leaf emergence during March 2009. For each forest patch we surveyed the entire patch or a maximum area of 20 ha centred on the trapping grid, which was an area equivalent to the average core area of male raccoons in our study area (Beasley et al. 2007a). Consequently, den site estimates represent a complete census of the area surveyed. Prior to conducting den surveys we performed double blind surveys to ensure den counts were consistent among all field personnel.
Forest patches were partitioned into grid sections and systematically searched for potential den sites. We recorded the den type (tree cavity, ground den, brush pile, hollow log) for all observed dens and marked them with chalk to avoid double counting. Tree cavities were visually examined using binoculars to ensure they were sufficiently deep to house raccoons, not positioned to hold water, and only tree cavities with openings estimated to be >8 cm in diameter were counted (Robb et al. 1996). Due to the large number of patches surveyed it was logistically impossible to visually inspect the inside of all tree cavities for their potential utility as a den site. Thus, the number of estimated den sites likely is an overestimate of the true number of available dens as some cavities may not have been suitable for use by raccoons (i.e., held water, not deep enough, etc.). However, there is no reason to suspect that systematic bias existed among forest patches in the proportion of putative dens that were inhospitable. We estimated den tree density and overall density of den sites within each forest patch by dividing the number of dens observed by the total area surveyed.
During the summer of 2008 we conducted vegetation surveys in each of the 25 patches sampled for raccoons to characterize the fine-scale vegetative composition of each patch. Surveys were conducted in nested plots (range 4–10 plots/patch) representing ~5% of the area of each forest patch. Nested plots consisted of one circular 0.081 ha overstory plot (trees >25.6 cm dbh), one circular 0.0405 ha understory plot (trees and shrubs <25.6 cm dbh), and three, 3 m2 herbaceous groundcover plots. Species in each level were characterized in terms of their density and frequency, as well as their percent coverage in the case of herbaceous plants. We calculated basal area for overstory trees to produce basal area densities (BAD) for each taxon and a total BAD for each patch. Within understory plots we counted the number of understory tree or shrub stems as a measure of understory stand density. In addition, within the understory we also estimated the density of soft mast producing species (including Acer spp.) Overall species diversity for each patch was calculated using Shannon’s diversity index (Krebs 1999), incorporating overstory, understory, and herbaceous plant compositions.
Raccoon demography
Based on patterns of ear tearing/scarring, we observed numerous instances of presumed tag removal by raccoons. Thus, to maximize the utility of our data set while eliminating any effects of tag loss on demographic parameter estimates, we used genetic data collected for all individuals within our study population to evaluate whether unidentified matching genotypes were present within our sample set, representing individuals that had lost their tags and been reassigned as new individuals on a subsequent trapping occasion. Using data generated from 14 highly polymorphic microsatellites developed in our lab (See Fike et al. 2007; Dharmarajan et al. 2009 for details), we employed CERVUS 3.0 (Kalinowski et al. 2007) to identify individuals with matching multilocus genotypes. The probability of identity (i.e., probability of finding two identical genotypes in our populations) was 1.02 × 10−16 for our suite of loci. Matching multilocus genotypes with ≤2 mismatches were re-evaluated to confirm allele assignments. Moreover, individuals with matching multilocus genotypes were further evaluated to ensure that compatibility among demographic parameters (i.e., gender, age, weight, location) existed.
We computed raccoon abundance estimates using the Huggins closed capture-recapture modelling procedure (Huggins 1989) in Program MARK (White and Burnham 1999). We chose Huggins models because this approach allows the incorporation of covariates (e.g., gender, age) into models. Moreover, the Huggins estimator excludes population estimates from the likelihood function, allowing initial efforts to be centred on obtaining parsimonious estimates of capture (p) and recapture (c) probabilities for the combined data set, which then can be used to generate more accurate estimates of N for subsets of the data (e.g., local populations; White 2005).
For many forest patches we captured too few individuals (average number captured/patch = 8) to reliably estimate patch specific detection probabilities using only data from a single year. To overcome problems associated with low numbers of individuals per patch, we modelled the combined data from all 25 patches during each year to obtain parsimonious models of the p and c parameters for the combined data set, but obtained patch specific estimates of N by treating each patch as a disparate attribute group in MARK (White 2005). We developed separate models for each of the 6 years to minimize violations of the closure assumption. By constraining the p and c parameters to be constant among patches during each year it is assumed that all populations are closed during the entire sampling period (≤2.5 months). Although it is possible that violations of the closure assumption occurred during our sampling period which could thus bias estimates of population size using Huggins models, any violations likely were minimal as trapping occurred prior to the availability of young (young were constrained to natal dens during our sampling period), spring mortality rates are low for raccoons ≥1 year of age, and dispersal primarily occurs during the fall (Gehrt 2003). Both gender and age of raccoons were considered in the models as covariates. Model fit was evaluated using a bias-corrected version of Akaike’s information criterion (AICc) and we used model averaging to determine final population sizes for all models deviating ≤4 AICc units from one another (Burnham and Anderson 2002).
Based on MARK estimated population sizes, we calculated year specific density estimates for each patch by overlaying a buffer encompassing an area equal to the average raccoon’s home range size in our study area (73 ha; Beasley et al. 2007a) centred on the centroid of each trapping grid. This buffer was assigned to approximate the effective area of our trapping grid by accounting for raccoon movements.
In addition to density, we also calculated annual as well as overall average adult gender (female:male) and age bias (adult: juvenile + yearling) for each patch. Because a number of patch/year combinations contained missing age or gender cohorts (e.g., no males), to avoid problems associated with dividing by zero we estimated patch-specific gender and age bias as (N a − N b )/NT, where a represents the number of females or adults, and b the number of males or juveniles + yearlings. This equation results in gender and age bias estimates ranging from −1 to 1, with values of −1 indicating male or juvenile + yearling biased populations and 1 representing populations comprised exclusively of females or adults. The number of juveniles and yearlings were combined for the estimation of population age structure because both of these age classes have higher probabilities of dispersal than adults (Gehrt 2003).
Spatial and temporal analyses of raccoon demography
To test whether significant temporal (years) or spatial (forest patches) variation existed in overall raccoon population density, adult gender bias, or age bias for our study area (all 25 patches combined) over the course of the study, we used Analysis of Variance (ANOVA) incorporating both year and patch as main effects. Because patch specific raccoon demographic variables represent a single value for each year (i.e., no variance), these models lacked the degrees of freedom necessary to evaluate the interaction of year and patch in the models; therefore, we limit our interpretation of these findings to the main effects (see “Results” section). In addition, when specific demographic metrics were found to vary across years, we evaluated whether interannual variation in rainfall or average minimum winter temperatures contributed significantly to temporal variance in those metrics using univariate ANOVA.
Local and landscape effects on raccoon demography
Pearson correlation tests revealed that several of our landscape attributes or local-scale habitat variables were highly correlated. For example, the availability of streams and Shannon’s diversity index both were highly correlated with forest patch size, and the amount of forest within a home range buffer of trapped patches. Many of these variables also contained redundant information (e.g. total den site density and tree cavity density); thus, to pare down the number of habitat variables (N = 25) we eliminated redundant variables by selecting only those of greatest interest based upon previous studies of raccoon ecology (N = 12 culled; Table S1). Subsequently, for instances in which ≥2 variables of interest were correlated at P ≤ 0.05, we retained the variable with the strongest correlation with each dependent variable of interest. After removing correlated and redundant variables, 13 variables were retained for subsequent analyses (Table 1).
We evaluated the influence of local and landscape habitat attributes on raccoon demography using linear mixed models with repeated measures (PROC MIXED, SAS ver. 9.1, SAS Institute, Cary, N.C.). We developed separate models for raccoon density, adult gender bias, and age bias, with habitat variables incorporated into each model as fixed effects, patch as a random effect, and year as the repeated measure. For each dependent variable we developed a global model incorporating all habitat variables. Using the global model we first investigated the covariance structure of this model for each dependent variable by evaluating model fit for each of four common covariance structures (compound symmetry, unstructured, autoregressive, and heterogeneous autoregressive) using AICc (Burnham and Anderson 2002). Based on these analyses an autoregressive covariance structure was found to most closely fit our data for each dependent variable and was used for all subsequent analyses. Using the residuals from each global model, we examined normal probability plots and Kolmogorov–Smirnov goodness of fit statistics to confirm that our data conformed to the assumptions of normality and homoscedasticity. For each dependent variable, we then developed a series of models incorporating all combinations of habitat variables and ranked candidate models relative to the global model using AICc. In addition, we also calculated Akaike weights (w i ) for all models within two AICc units of the top model to assist in model selection.
We also were interested in modelling the influence of habitat characteristics on the number of adult male and female raccoons present within patches. Using the same repeated measures mixed models approach described above, we developed a suite of models incorporating all combinations of habitat variables as independent variables and the number of male or female raccoons captured each year as dependent variables and ranked competing models using AICc. Previous research has suggested that den trees may be a potentially limiting resource for raccoons (Endres and Smith 1993). Therefore, we used univariate regressions to further explore the nature of the relationship between den tree density (as opposed to overall den site density) within habitat patches and the following variables (each averaged across the 6 years of our study): number of females captured, number of males captured, and gender bias (1 patch was an outlier due to its size (0.5 ha) and thus was removed from these analyses).
In addition to our spatial analyses, we evaluated temporal variation in raccoon demography by estimating the coefficient of variation (CV = SD/\( \bar{X} \)) associated with estimates of raccoon density calculated over the 6-year study period. We then developed a series of best subsets multiple regression models, incorporating all combinations of habitat variables, to evaluate the influence of habitat characteristics on interannual variance (as measured by CV) in raccoon density, and ranked competing models using AICc and w i . All statistical analyses were performed using SAS (SAS ver. 9.1).
Finally, because many of our habitat variables were correlated we were unable to explore the relationship between several variables of interest and spatial or temporal variance in raccoon demography in our previous analyses. Therefore, we also regressed patch specific estimates of mean raccoon density, mean adult gender ratio, the average number of females captured, and density CV against habitat variables excluded from our previous analyses associated with forest fragmentation (e.g. patch size), and additional attributes thought to be important to raccoons based on previous research on this species (e.g. water availability) using univariate linear regression (Figs. S1-S3).
Results
Raccoon trapping and demography
A total of 852 unique raccoons were captured 2,214 times during the 6 years of this study. The within year recapture rate for the global population (all patches combined) was 43%. A total of 236 raccoons were captured in >1 year and 81 individuals were captured in ≥3 years. The number of raccoons captured within a single 10-day trapping period was highly variable among forest patches, ranging from 0 to 22 (\( \bar{X} \) = 8.03, SD = 4.51). Based upon our genetic analyses, 124 matching genotypes were present within our data set representing 43 unique individuals that had lost their tags between years and been retagged as new individuals (several individuals were tagged >2 times); no instances of misread tags occurred and no individuals lost both tags within a trapping round. Interestingly, of the 43 individuals that were retagged during this study, 41 (95%) were males. This exemplifies the value of combining genetic and demographic techniques in mark-recapture studies, as failing to account for a gender bias in tag loss could result in inaccurate gender-specific estimates of demography (e.g. survival, density).
Based on our MARK analyses, local raccoon population estimates ranged from 0 to 29 (\( \bar{X} \) = 10.17, SD = 5.64) across the 6 year sampling period. Density estimates derived from these abundance estimates ranged from 0 to 40 raccoons/km2 (\( \bar{X} \) = 13.93, SD = 7.73). Modified adult gender bias (see “Methods” section) averaged across the 6 years of our study also was highly variable among patches, ranging from −1 to 0.48 (\( \bar{X} \) = − 0.21, SD = 0.34). Similarly, average modified age bias (see “Methods” section) ranged from −1 to 0.73 (\( \bar{X} \) = 0.30, SD = 0.21) among forest patches.
Spatial and temporal analyses of raccoon demography
Neither overall raccoon density nor adult gender bias differed over the 6 years of our study for the global population (F = 2.01, P = 0.08; F = 0.46, P = 0.80, respectively). However, significant differences among forest patches were observed for each of these demographic measures (density: F = 5.71, P < 0.0001, adult gender bias: F = 2.28, P = 0.002). Tukey post-hoc tests adjusted for multiple comparisons revealed that several patches differed significantly from one another in density as well as in adult gender bias (See Tables S2 and S3 for pairwise comparisons of all populations).
In contrast, no differences were observed in raccoon age bias among forest patches (F = 1.27, P = 0.20), but differences were observed in age bias among years for the global population (F = 2.71, P = 0.02); although a Tukey post-hoc comparisons test failed to identify differences among specific years. Because the age bias of raccoon populations was found to differ among years, we evaluated whether variation in weather parameters among years explained a significant portion of this variance. From this analysis, raccoon age bias (adult: juvenile + yearling) within habitat patches was found to be positively associated with the annual amount of precipitation during the previous year (F = 7.0, P = 0.009).
Local and landscape effects on raccoon demography
Analyses of the influence of habitat attributes on spatial variation in raccoon density produced four highly competitive models retaining 3–5 habitat variables (ΔAICc < 4; Table 2). Our analysis of raccoon adult gender bias produced a single best-fit model, in which the variables TREECAV and PROPCORN were retained (β = 0.28, F = 8.321,96, P = 0.005; β = 0.50, F = 3.501,96, P = 0.065, respectively). Similarly, a single best-fit model was identified for raccoon age bias, in which only overall plant species diversity (DIV) was retained (β = 0.43, F = 5.671,123, P = 0.02).
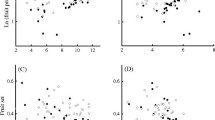
Analyses of the influence of habitat characteristics on the number of adult females captured within patches produced two competing models (ΔAICc = 1.5), with the best-fit model containing the variables FORBUFF, TREECAV, and GRASS, all of which were significant in the model (β = 0.05, F = 9.421,125, P = 0.003; β = 1.22, F = 13.821,125, P < 0.001; β = −7.0, F = 7.011,125, P = 0.009, respectively). For determining the influence of habitat characteristics on the number of adult males captured within patches, a single best-fit model was produced in which FORBUFF (β = 0.07, F = 40.961,124, P < 0.001) and GRASS (β = −3.07, F = 2.951,124, P = 0.088) were retained. Among the univariate analyses performed, den tree density was significantly positively correlated with the average number of females captured (F = 7.491,23, P = 0.01) and the average raccoon gender bias (F = 13.851,23, P = 0.001) within local populations (Fig. 1). In contrast, no relationship was observed between den tree density and the number of males captured within a patch (F = 0.401,23, P = 0.53; Fig. 1)
The examination of temporal variation (CV across years) in raccoon density produced 11 competitive models (ΔAICc < 2; Table 3). The variable FORBUFF appeared in all 11 models, and explained a significant proportion of the variance in raccoon density CV in each model (α = 0.05). The variables SOFTMAST (4 models), FORBS (2 models), BAD (2 models), TREECAV (2 models), WETLAND (1 model), URBBUFF (1 model), and SHRUB (1 model) were present in at least 1 model, although none of these variables explained a significant proportion of the variance in density CV in any model.
Discussion
Raccoons arguably are one of the most plastic mammals in terms of their ability to adapt to and exploit nearly all terrestrial ecosystems throughout their range (Fritzell 1978; Prange et al. 2004; Beasley et al. 2007a). Despite this plasticity, our results suggest that even in landscapes in which raccoons appear to flourish, this apparent productivity reflects considerable spatial and temporal variance that is directly tied to local and landscape habitat attributes. Accordingly, our results imply that all patches do not contribute equally to the regional abundance and persistence of raccoon populations in fragmented landscapes, and that a fraction of patches likely contribute most (probably as a source of emigrants) to overall population abundance. Given the patterns of variation in demographic parameters we observed for raccoons, interpatch variation in demography likely is magnified for species that are sensitive to the high levels of spatial and temporal variation in resource distribution that are typical of fragmented agricultural landscapes, and may explain the limited success of such species in these landscapes (Nupp and Swihart 2000; Rushton et al. 2000).
The importance of forested habitat to wildlife inhabiting human-altered landscapes has been well established in the scientific literature (e.g. Chamberlain et al. 2003; Henner et al. 2004; Beasley et al. 2007b). However, the magnitude to which underlying fine-scale attributes of forest habitat contribute to patch-specific variation in population dynamics has received considerably less attention. At the landscape scale, both raccoon density and overall population stability were positively associated with the size and contiguity of forest patches. Similarly, Beasley and Rhodes (2010) observed that home range size in raccoons is inversely related to both forest patch size and patch isolation in fragmented agricultural landscapes, presumably due to the higher quality of large, contiguous patches. Within intensively farmed landscapes, contiguous forest tracts primarily are confined to river drainages where the local topography makes land unsuitable for agricultural production. Consequently, these larger forest tracts likely have increased water availability, as well as more diverse plant communities than small, isolated patches due to a greater variance in microhabitat conditions. Indeed, both the overall length of streams and Shannon’s diversity index were highly correlated with forest patch size and the amount of forest within a home range buffer surrounding target patches in our study.
Within fragmented landscapes, large, contiguous patches also undoubtedly facilitate dispersal, either through increased detection probabilities of patches, or by facilitating movement within the landscape (Gustafson and Gardner 1996; Zollner and Lima 1999; Selonen and Hanski 2004). Thus, local populations within contiguous habitat patches likely are buffered from stochasticity in resource availability and survival to a greater degree than small, isolated populations due to the increased availability of dispersers. Alternatively, contiguous patches may simply facilitate high rates of reproductive success and juvenile survival due to the presence of streams and increased prevalence of non-agricultural food resources. Although the underlying mechanism driving this observed variance in population stability was unclear, our data suggest that emergent properties associated with fragmented agricultural landscapes contribute to spatial and temporal variation in raccoon density.
In addition to landscape-level factors associated with fragmentation, our results indicate that variability in habitat quality also contributes to spatial and temporal variation in density. At the local scale, raccoon density was negatively associated with the proportion of the forest floor comprised of grass, but positively correlated with plant diversity and the density of tree cavities. These findings are biologically intuitive as increased plant diversity likely corresponds to a greater diversity of food resources, higher densities of tree cavities should result in greater reproductive output, and patches with higher proportions of grass likely have reduced densities of overstory and understory trees. Indeed, grass availability was negatively correlated with both overstory basal area (P < 0.001) and the density of understory trees (P = 0.004).
Similarly, the temporal stability of raccoon populations was positively correlated with a number of fine-scale habitat attributes associated with patch quality. Populations occupying forest patches with greater plant diversity and stable water resources (streams) exhibited less temporal variability than populations occupying patches with limited plant species complexity or water availability. Water has been identified as a critical resource to raccoons (Gehrt and Fritzell 1998; Beasley and Rhodes 2010); thus, the positive relationship between population stability and water availability is not surprising. Similarly, greater plant diversity likely minimizes interannual variation in food availability. Interestingly, we failed to detect a response in raccoon density to changes in the availability of corn, despite the importance of corn in the diet of raccoons inhabiting agricultural ecosystems (Rivest and Bergeron 1981; Beasley and Rhodes 2008). This suggests that large quantities of corn are not necessary to maintain high densities of raccoons and that local variation in non-agricultural resources may have a greater influence on raccoon population sizes and temporal stability than the availability of corn.
Among mammals, female site fidelity often is tied to the distribution of critical resources as female reproductive success is linked to their ability to exploit these resources, whereas male reproductive success is driven by their ability to find and mate with females (Wrangham 1980; Rowell 1988; Clutton-Brock 1989). Accordingly, in addition to variation in density among local populations, we also observed substantive spatial variance in raccoon gender bias, with the proportion of local populations comprised of females most strongly influenced by local habitat attributes associated with patch quality. In particular, female biased populations were positively associated with the availability of tree cavities and soft mast species (e.g., Prunus serotina, Acer spp., Vitis spp., Rubus spp.) within local patches.
Although raccoons exhibit a large degree of plasticity in terms of den site selection (e.g. tree cavities, brush piles, rock outcroppings, etc.), females preferentially select tree cavities to rear young, presumably due to the increased protection against predation afforded by these structures (Rabinowitz and Pelton 1986; Endres and Smith 1993). The strong relationship observed between female abundance and den tree density suggests that the availability of den trees is critical to the overall reproductive success of a habitat patch, and ultimately patch-specific variation in den tree availability may lead to reproductive variance among forest patches throughout highly fragmented landscapes. Indeed, recent genetic analyses have revealed high levels of variance in kin structure among local raccoon populations, although the influence of den tree availability on this pattern has yet to be evaluated (Dharmarajan et al. 2009).
In contrast to the patterns observed for raccoon density and gender composition, the age bias of local raccoon populations did not differ among patches within the landscape; although in general, adult-biased populations were associated with increased plant diversity. However, we did detect significant interannual variation in the age bias of the global population, with adult-biased populations positively associated with the total rainfall during the previous year. Interannual variation in the age structure of a population suggests that survival of specific age classes may vary from year to year. Increased rainfall has the potential to contribute to greater softmast production (Kelly and Sork 2002), as well as increased availability (and likely stability) of water and likely food resources associated with water (e.g., crayfish, amphibians, etc.; Kiesecker et al. 2001). Thus, increased rainfall may have contributed to greater adult survival and increased patch philopatry among adults due to the higher quality of forest patches in terms of food and water availability during years of abundant rainfall, but increased dispersal and consequently mortality among young due to the saturation of patches by adults.
Conclusions
Our results clearly indicate that variance in demographic parameters occurs among local raccoon populations in heterogeneous landscapes, likely as a consequence of the variation in composition and configuration of habitat attributes associated with agricultural ecosystems. Thus, even for a species which exhibits high levels of abundance in discontinuous landscapes, there are habitat patches which presumably are net exporters and importers of individuals. Future research utilizing both genetic and fine scale demographic data will be needed to test this intriguing hypothesis about the spatial dynamics of raccoon demography in fragmented ecosystems.
A final ramification of this research pertains to disease transmission dynamics associated with raccoons and other wildlife species inhabiting agricultural ecosystems, and may provide a foundation for understanding how diseases and parasites are expected to be distributed and spread in these types of environments. Recent research suggests that the prevalence of infectious diseases can vary considerably among local populations of wildlife species in fragmented landscapes, both as a function of population demography (Dharmarajan et al. Unpublished Data) and degree of habitat fragmentation (Page et al. 2001; reviewed by Ostfeld et al. 2005). Given the large number of infectious diseases and parasites associated with raccoons and other generalist species which thrive in anthropogenically modified landscapes (Kazacos and Boyce 1989; Schmidt and Ostfeld 2001; Raizman et al. 2009), a clear understanding of how landscape configuration alters population demography and subsequently disease transmission dynamics is of eminent concern. In particular, as raccoon rabies continues to spread westward across the United States towards the heavily fragmented landscapes of the Midwest, a greater understanding of how landscape attributes contribute to spatial and temporal variance in raccoon demography will greatly enhance our ability to effectively manage this disease.
References
Andrén H (1994) Effects of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat: a review. Oikos 71:355–366
Beasley JC, Rhodes OE Jr (2008) Relationship between raccoon abundance and crop damage. Hum-Wildl Confl 2:36–47
Beasley JC, Rhodes OE Jr (2010) Influence of patch and landscape level attributes on the movement behaviour of raccoons in agriculturally fragmented landscapes. Can J Zool 88:161–169
Beasley JC, DeVault TL, Rhodes OE Jr (2007a) Home range attributes of raccoons in a fragmented agricultural region of northern Indiana. J Wildl Manag 71:844–850
Beasley JC, DeVault TL, Retamosa MI, Rhodes OE Jr (2007b) A hierarchical analysis of habitat selection by raccoons in northern Indiana. J Wildl Manag 71:1125–1133
Bender DJ, Contreras TA, Fahrig L (1998) Habitat loss and population decline: a meta-analysis of the patch size effect. Ecology 79:517–533
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Chamberlain MJ, Conner LM, Leopold BD, Hodges KM (2003) Space use and multi-scale habitat selection of adult raccoons in Central Mississippi. J Wildl Manag 67:334–340
Clutton-Brock TH (1989) Mammalian mating systems. P R Soc Lond B Bio 236:339–372
Dharmarajan G, Beasley JC, Fike JA, Rhodes OE Jr (2009) The population genetic structure of raccoons (Procyon lotor) inhabiting a highly fragmented landscape. Can J Zool 87:814–824
Donovan TM, Jones PJ, Annand EM, Thompson FR III (1997) Variation in local-scale edge effects: mechanisms and landscape context. Ecology 78:2064–2075
Endres KM, Smith WP (1993) Influence of age, sex, season, and availability on den selection by raccoons within the central basin of Tennessee. Am Midl Nat 129:116–131
Fike JA, Drauch AM, Beasley JC, Dharmarajan G, Rhodes OE Jr (2007) Development of fourteen multiplexed microsatellite loci for raccoons (Procyon lotor). Mol Ecol Notes 7:525–527
Fritzell EK (1978) Aspects of raccoon (Procyon lotor) social organization. Can J Zool 56:260–271
Gannon WL, Sikes RS, The Animal Care and Use Committee of the American Society of Mammalogists (2007) Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J Mammal 88:809–823
Gehrt SD (2003) Raccoon. In: Feldhamer GA, Thompson BC, Chapman JA (eds) Wild mammals of North America: biology, management, and conservation. Johns Hopkins University Press, Baltimore, MD, pp 611–634
Gehrt SD, Fritzell EK (1998) Resource distribution, female home range dispersion and male spatial interactions: group structure in a solitary carnivore. Anim Behav 55:1211–1227
Gilpin M, Hanski I (eds) (1991) Metapopulation dynamics: empirical and theoretical investigations. Academic Press, London
Grau GA, Sanderson GC, Rogers JP (1970) Age determination of raccoons. J Wildl Manag 34:364–372
Gustafson EJ, Gardner RH (1996) The effect of landscape heterogeneity on the probability of patch colonization. Ecology 77:94–107
Henner CM, Chamberlain MJ, Leopold BD, Wes Burger L Jr (2004) A multi-resolution assessment of raccoon den selection. J Wildl Manag 68:179–187
Heske EJ, Robinson SK, Brawn JD (1999) Predator activity and predation on songbird nests on forest-field edges in east-central Illinois. Landscape Ecol 14:345–354
Hokit DG, Branch LC (2003) Habitat patch size affects demographics of the Florida scrub lizard (Sceloporus woodi). J Herpetol 37:257–265
Huggins RM (1989) On the statistical analysis of capture-recapture experiments. Biometrika 76:133–140
Kalinowski ST, Taper ML, Marshall TC (2007) Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol 16:1099–1106
Kazacos KR, Boyce WM (1989) Baylisascaris larva migrans. J Am Vet Med Assoc 195:894–903
Kelly D, Sork VL (2002) Mast seeding in perennial plants: why, how, where? Ann Rev Ecol Syst 33:427–447
Kiesecker JM, Blaustein AR, Belden LK (2001) Complex causes of amphibian population declines. Nature 410:681–684
Kozakiewicz M, Gortat T, Kozakiewicz A, Barkowska M (1999) Effects of habitat fragmentation on four rodent species in a Polish farm landscape. Landscape Ecol 14:391–400
Krebs CJ (1999) Ecological methodology, 2nd edn. Addison-Wesley Educational Publishers, New York
Moore JE, Swihart RK (2005) Modelling patch occupancy by forest rodents: incorporating detectability and spatial autocorrelation with hierarchically structured data. J Wildl Manag 69:933–949
Nupp TE, Swihart RK (1996) Effect of forest patch area on population attributes of white-footed mice (Peromyscus leucopus) in fragmented landscapes. Can J Zool 74:467–472
Nupp TE, Swihart RK (2000) Landscape-level correlates of small-mammal assemblages in forest fragments of farmland. J Mammal 81:512–526
Oehler JD, Litvaitis JA (1996) The role of spatial scale in understanding responses of medium- sized carnivores to forest fragmentation. Can J Zool 74:2070–2079
Ostfeld RS, Glass GE, Keesing F (2005) Spatial epidemiology: an emerging (or re-emerging) discipline. Trends Ecol Evol 20:328–336
Page KL, Swihart RK, Kazacos KR (1999) Implications of raccoon latrines in the epizootiology of Baylisascariasis. J Wildlife Dis 35:474–480
Page KL, Swihart RK, Kazacos KR (2001) Changes in transmission of Baylisascaris procyonis to intermediate hosts as a function of spatial scale. Oikos 93:213–220
Prange S, Gehrt SD, Wiggers EP (2004) Influences of anthropogenic resources on raccoon (Procyon lotor) movements and spatial distribution. J Mammal 85:483–490
Rabinowitz AR, Pelton MR (1986) Day-bed use by raccoons. J Mammal 67:766–769
Raizman EA, Dharmarajan G, Beasley JC, Wu CC, Pogranichniy RM, Glickman L, Rhodes OE Jr (2009) Serological survey for select infectious diseases in raccoons (Procyon lotor) in Indiana, USA. J Wildlife Dis 45:531–536
Retamosa MI, Humberg LA, Beasley JC, Rhodes OE Jr (2008) Modelling wildlife damage to crops in northern Indiana: relative influence of landscape composition and configuration. Hum-Wildl Confl 2:225–239
Rivest P, Bergeron JM (1981) Density, food habits, and economic importance of raccoons (Procyon lotor) in Quebec agrosystems. Can J Zool 59:1755–1762
Robb JR, Cramer MS, Parker AR, Urbanek RP (1996) Use of tree cavities by fox squirrels and raccoons in Indiana. J Mammal 77:1017–1027
Robinson SK, Thompson FR III, Donovan TM, Whitehead DR, Faaborg J (1995) Regional forest fragmentation and the nesting success of migratory birds. Science 267:1897–1990
Rowell TE (1988) Beyond the one-male group. Behaviour 104:189–201
Rushton SP, Barreto GW, Cormack RM, Macdonald DW, Fuller R (2000) Modelling the effects of mink and habitat fragmentation on the water vole. J Appl Ecol 37:475–490
Saunders DA, Hobbs RJ, Margules CR (1991) Biological consequences of ecosystem fragmentation: a review. Conserv Biol 5:18–32
Schmidt KA, Ostfeld RS (2001) Biodiversity and the dilution effect in disease ecology. Ecology 82:609–619
Selonen V, Hanski IK (2004) Young flying squirrels (Pteromys volans) dispersing in fragmented forests. Behav Ecol 15:564–571
United States Department of Agriculture (USDA), National Agricultural Statistics Service (NASS) (2004–2009) USDA-NASS’S 1:100,000-scale [2003–2008] cropland data layer. A Crop-Specific Digital Data Layer for Indiana http://www.nass.usda.gov/research/Cropland/SARS1a.htm. Accessed 5 August 2009
White GC (2005) Correcting wildlife counts using detection probabilities. Wildl Res 32:211–216
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird Study Suppl 46:120–138
Wiens JA (1976) Population responses to patchy environments. Ann Rev Ecol Syst 7:81–120
Wrangham R (1980) An ecological model of female-bonded primate groups. Behaviour 75:262–300
Yahner RH (1988) Changes in wildlife communities near edges. Conserv Biol 2:333–339
Zollner PA, Lima SL (1999) Search strategies for landscape level inter-patch movements. Ecology 80:1019–1030
Acknowledgements
This study would not have been possible without the cooperation of numerous landowners who permitted us access to their land. We thank M. Retamosa, J. Fike, and the numerous field technicians for their assistance with this research. We also thank P. Zollner, B. Pijanowski, and two anonymous reviewers for providing comments on drafts of this manuscript. Funding for this research was provided by the Department of Forestry and Natural Resources at Purdue University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Beasley, J.C., Olson, Z.H., Dharmarajan, G. et al. Spatio-temporal variation in the demographic attributes of a generalist mesopredator. Landscape Ecol 26, 937–950 (2011). https://doi.org/10.1007/s10980-011-9619-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-011-9619-x