Abstract
Three central related issues in ecology are to identify spatial variation of ecological processes, to understand the relative influence of environmental and spatial variables, and to investigate the response of environmental variables at different spatial scales. These issues are particularly important for tropical dry forests, which have been comparatively less studied and are more threatened than other terrestrial ecosystems. This study aims to characterize relationships between community structure and landscape configuration and habitat type (stand age) considering different spatial scales for a tropical dry forest in Yucatan. Species density and above ground biomass were calculated from 276 sampling sites, while land cover classes were obtained from multi-spectral classification of a Spot 5 satellite imagery. Species density and biomass were related to stand age, landscape metrics of patch types (area, edge, shape, similarity and contrast) and principal coordinate of neighbor matrices (PCNM) variables using regression analysis. PCNM analysis was performed to interpret results in terms of spatial scales as well as to decompose variation into spatial, stand age and landscape structure components. Stand age was the most important variable for biomass, whereas landscape structure and spatial dependence had a comparable or even stronger influence on species density than stand age. At the very broad scale (8,000–10,500 m), stand age contributed most to biomass and landscape structure to species density. At the broad scale (2,000–8,000 m), stand age was the most important variable predicting both species density and biomass. Our results shed light on which landscape configurations could enhance plant diversity and above ground biomass.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A central topic of research in ecology concerns the explanation of species distribution (Brown and Lomolino 1998; Gaston 2000; Whittaker et al. 2001). Traditionally, species distribution has been analyzed in terms of gradients of environmental variables (Whittaker 1960). However, species distribution patterns are spatially structured due to intra- and interspecific interactions of individuals, as well as to species responses to environmental factors that are themselves spatially correlated. Thus, identification and explanation of spatial variation is one of the main current issues in ecology (Dale and Fortin 2002). Although different researchers have documented that plant species composition and abundance are related to heterogeneity of soil properties, topography and stage or age of forest succession (Clark et al. 1999; Trejo and Dirzo 2000; Ruiz et al. 2005; Chazdon et al. 2007), there is increasing interest in understanding the relative influence of environmental and spatial drivers of plant community structure and diversity (Jones et al. 2008).
Tropical dry forests (TDF) are the most extensive land cover type in the tropics and one of the most diverse terrestrial ecosystems. More than half of tropical dry forests occur in the Americas, and Mexico contains 38% of the TDF in the continent (Portillo-Quintero and Sánchez-Azofeifa 2010). However, TDF are also one of the most threatened ecosystems in the world as a consequence of human activities (Miles et al. 2006). In particular the TDF of the Yucatan Peninsula have been altered through time not only by natural disturbances such as hurricanes and forest fires but also by human interventions, including subsistence slash and burn agriculture by indigenous Mayan farmers and conversion of forest to grasslands supporting livestock (Lamb et al. 2005), and are poorly represented under protected areas. Deforested areas are often abandoned after a few years, due to soil degradation, weed invasion and changes in socio-economic conditions (Buschbacher 1986, Thomlinson et al. 1996), thus initiating secondary forest succession, which is the mechanism of natural forest recovery. Thus, as old-growth forests throughout the tropics are increasingly reduced, fragmented, and degraded, second-growth forests are increasing in extent and importance for conservation and the provision of resources and ecosystem services (Brown and Lugo 1990; Toledo et al. 1995; Hughes et al. 1999; FAO 2007). Compared to other ecosystems, however, ecological studies in TDF are relatively new, have concentrated in a few sites and have seldom addressed secondary forest succession (Sánchez-Azofeifa et al. 2005; Chazdon et al. 2007; Quesada et al. 2009, but see Lebrija-Trejos et al. 2008). Consequently, information about the ecological drivers of community structure during secondary forest succession is fundamental to fully understand and design effective strategies for conservation and management for these forests that are increasingly reduced and fragmented, largely as a result of land-use changes (Trejo and Dirzo 2000; Sánchez-Azofeifa et al. 2005; Miles et al. 2006).
Most studies on the effects of fragmentation and landscape patterns on plant communities focus on particular patches and on species density (the number of species found in plots of a given area, as a measure of α-diversity), while few studies examine different patch types at the whole landscape level and address effects on attributes of community structure such as abundance or biomass (Hill and Curran 2003; Nascimento and Laurence 2004). The amount of fragmentation that characterizes a given landscape can be described as a function of the size, shape, similarity, contrast of patches and other metrics of the geometry and structure of landscape patterns (Gustafson 1998; McGarigal et al. 2002). Evidence found over the past years suggests that spatial patterns of patches are good predictors of plant species density and abundance in tropical forests (Hernández-Stefanoni and Dupuy 2008). Landscape patterns of forest patches influence plant communities through effects on ecological processes such as pollination, seed dispersal, seed predation and plant competition, and consequently they influence the number and composition of species in a landscape (Laurance et al. 2000; Hill and Curran 2003). Most research examining the effects of landscape configuration on plant community structure and diversity has focused on a single spatial scale (Wu 1999; Boscolo and Metzger 2009). Yet, the degree to which landscape configuration affects the structure and composition of plant communities depends on processes occurring at different spatial scales (Cushman and McGarigal 2004). For example species diversity within a patch is influenced by species ability to obtain and compete for limited resources such as water or nutrients (Tilman 1994). At the landscape level (mosaic of different patches), plant species diversity is influenced by dispersal and the existence of different habitats that allow the co-existence of species with different niche requirements (Bell et al. 2000). Therefore, the assessment of relationships between community attributes and landscape structure should consider scale-dependent ecological processes (Bellier et al. 2007).
Although several studies have also documented that vegetation structure, diversity and composition are strongly influenced by forest succession (Ruiz et al. 2005; Chazdon et al. 2007; Lebrija-Trejos et al. 2008), to our knowledge, the relative importance of landscape structure, stand age and spatial dependence for tropical forest structure and diversity has not been explored. This is in part due to the paucity of chronosequence studies (which characterize vegetation in forest patches of different successional age within a landscape), as well as to insufficient knowledge and documentation of stand age (Groeneveld et al. 2009). Moreover, most chronosequence studies in the tropics have focused on tropical humid forests (see review in Chazdon et al. 2007), while the few studies focusing on TDF have very limited replication (Lebrija-Trejos et al. 2008, but see Ruiz et al. 2005), and fail to address effects of spatial dependence, and landscape structure and configuration.
In this study we relate plant species density and above ground biomass (AGB) with stand age, spatial dependence, and landscape structure in a tropical dry forest. The design and aims of the study address some of the research shortcomings that we have outlined previously. The objectives of this study were threefold: first, to evaluate the relative contribution of landscape structure and stand age to overall variation in plant species density and AGB across the landscape, and to assess which landscape configurations and stages of forest succession could enhance species density and biomass. Second, to quantify the spatial dependence of species density and stand biomass across the landscape, and to evaluate the relative contribution of spatial dependence, landscape structure, and stand age to overall variation in species density and stand biomass. Third, to identify the relationships between response variables (species density and biomass) and explanatory variables (landscape structure and stand age) at multiple spatial scales derived from the sampling scheme of this study. We discuss the implications of our results for land-use and forest management and conservation.
Study area and methods
Study area
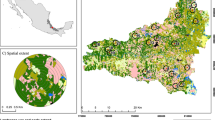
The study was conducted in a landscape mosaic of 22 × 16 km2 located in the Yucatan Peninsula, Mexico (20° 01′7″–20° 09′36″N latitude, 89° 35′59″–89° 23′31″W longitude; Fig. 1). The climate is tropical, warm, with summer rain and a dry season from November to April. Mean annual temperature is ca 26°C, and mean annual precipitation ranges between 1,000 and 1,200 mm, concentrated between June and October. The landscape consists of Cenozoic limestone hills with moderate slope (10–25°) alternating with flat areas, and the elevation ranges from 60 to 160 m.a.s.l. (Flores & Espejel 1994). The soils are predominantly Carstic Renzines, Luvisols, and Nitosols, although Cambisols and Lithosols are also present (Bautista et al. 2003). The landscape is dominated by seasonally dry semideciduous tropical forests of different ages of abandonment after traditional slash-and-burn agriculture. The forest has a relatively low canopy stature (8–13 m) with a few prominent trees attaining 15–18 m in the oldest (60–70 year-old) available stands. Trees and shrubs are the dominant growth forms; vines, epiphytes and herbs are relatively scarce (Carnevali et al. 2003).
Remotely sensed data and imagery processing
A Spot 5 satellite image acquired on January 2005 was geo-referenced to Universal Transverse Mercator Projection (WGS 84) and radiometrically corrected to minimize the effect of atmospheric scattering (Chavez 1996). A false color composite image was created from bands 2 (red), 3 (near infrared), and 4 (mid infrared). This composite image was used as a spatial reference framework for selecting suitable training sites. At least 10 training sites were selected for each of the following land-cover types: (1) 3–8 year-old secondary forest; (2) 9–15 year-old secondary forest; (3) >15 year-old secondary forest on flat areas; (4) >15 year-old secondary forest on hills; (5) agricultural fields; (6) urban areas and roads. The training areas and the false color image were used to perform a supervised classification of the Spot 5 bands using the maximum likelihood algorithm (Idrisi Kilimanjaro V14.1, 2004). The overall accuracy and Cohen’s Kappa statistic were used to assess the accuracy of the map (Jensen 2000; Campbell 1987).
Species density and biomass data
Field data were recorded from a hierarchical plant survey conducted during the rainy season of 2008 and 2009. First, 23 landscapes units of 1 km2 were selected encompassing the whole range of forest fragmentation; within each landscape unit, 12 sample sites were located following a stratified random design considering each of the four secondary forest cover classes (276 sampling sites in total). Of the total number of sites 51 fell in “vegetation cover 1” class, 77 in “vegetation cover 2”, 86 in “vegetation cover 3”, and 62 in “vegetation cover 4”. Each sampling site consisted of two concentric circular plots: all woody plants >5 cm in DBH [diameter at breast (1.3 m) height], hereafter referred to as adults, were sampled in a 200 m2 plot; whereas 1–5 cm DBH woody plants, hereafter referred to as juveniles, were sampled in a nested 50 m2 subplot. We measured the diameter of all juvenile and adult stems and the height of all individuals. In every sampling unit we computed the number of adults, juveniles and all woody plant species per sample site (i.e. species density sensu Gotelli and Colwell 2001), as a measure of local or α species diversity. To calculate above ground biomass from tree diameter and height, two allometric equations developed for tropical forests of Mexico were employed: one for trees ≥10 cm in DBH (Cairns et al. 2003) and the other for trees <10 cm in DBH (Hughes et al. 1999). Stand age of secondary forest sites was determined by interviewing local ≥ 40 year-old residents, who owned or used the land for traditional slash-and-burn agriculture, which is the predominant land use in the study area and has been practiced since the Pre-Classic Mayan period (600 a.d. http://www.kiuic.org/english_f/research.htm).
Calculation of landscape pattern metrics
Several indices are calculated at the patch type level using the software FRAGSTATS (McGarigal et al. 2002) and all of them were initially considered. Since many of these indices are redundant or represent an alternative formulation of the same information, Pearson correlation coefficients between each pair of metrics, as well as the correlation of these metrics with plant species richness and biomass values were computed to select the metrics to be included in this study (Hargis et al. 1998). The selection of the metrics was made considering variables that quantify different aspects of landscape configuration, the potential explanatory power of the metric for plant species composition and biomass, and how common such measurements are in landscape ecology literature (Mazerolle and Villard 1999). Based on these criteria, six indices were selected in this study: patch density—PD-, edge density—ED-, mean area weighted shape index—SHAPE_AM-, mean area weighted proximity index—PROX_AM-, mean area weighted Euclidean nearest neighbor distance—ENN_AM- and total edge contrast index—TECI-. A description of each metric can be found in McGarigal et al. (2002).
The 23 landscapes units of this study were considered for the computation of the indices at the patch type level. The patch type approach of this study requires calculation of individual metrics for each given patch, regardless of how many individual patches make up that particular patch type. In other words we calculated different metrics for each vegetation cover in every one of the 23 landscapes units. To calculate the proximity index, a search radius of 30 pixels (300 m) was selected, which coincides with empirically derived evidence about the average size of a patch. The weighted edge contrast between vegetation cover classes, required to compute the total edge contrast index, was calculated as the inverse of the Morisita-Horn similarity index between each pair of vegetation cover classes (Magurran 1988).
Spatial data
A set of explanatory spatial variables was generated from the geographical coordinates of the sampling sites, using a principal coordinate of neighbor matrices (PCNM) analysis (Borcard et al. 2004). This set of variables (called PCNM vectors) represents a spectral decomposition of the spatial relationships among the sampling sites that correspond to all spatial scales that can be perceived for the data (Borcard et al. 2004). PCNM vectors are also uncorrelated variables that can be used as predictors in regression analysis to describe spatial relationships in community data, because they are not subject to multicollinearity troubles (Borcard and Legendre 2002).
The general procedure to obtain the set of explanatory spatial variables involves the following steps: (a) Calculation of a Euclidean distance matrix comprised of geographical distances between site locations, (b) Modification of the Euclidean distance matrix by replacing distances greater than the larger distances between adjacent sites with an arbitrary large number as suggested by Borcard et al. (2004), (c) Performing a principal coordinate analysis on the modified distance matrix and (d) the principal coordinate axes that correspond to positive eigenvalues are retained as the set of explanatory PCNM variables. The PCNM function from the R software ‘spacemakeR’ library was used to perform this analysis (Dray et al. 2006). The PCNM analysis produced 136 eigenvectors (PCNM vectors), among which 60 eigenvectors had positive values and a significant autocorrelation (P > 0.001) tested by Moran’s I (Borcard et al. 2004).
Species density and biomass among vegetation classes
We evaluated differences in plant community structure (species density and biomass) among vegetation classes obtained from a supervised classification using one-way ANOVA, and Tukeys’s test for post-hoc comparisons. Dependent variables were transformed (ln X, or ln X + 1) as needed to meet the assumptions of normality and homogeneity of variances (Zar 1999).
Variation partitioning
Following Borcard et al. (2004), we used regression analysis to partition the variation in species density and biomass into independent variance components, but we expanded the method to three groups of variables (stand age, landscape structure, and spatial dependence) instead of two. Hence, the total explained variance (the variance explained using the three sets of explanatory variables) was divided into seven non-overlapping fractions, namely the unique stand age (Uage), unique landscape structure (Uland), unique spatial (Uspatial), mixed stand age-landscape structure (Mage+land), mixed stand age-spatial (Mage+spatial), mixed landscape structure-spatial (Mland+spatial), and mixed stand age-landscape structure-spatial (Mage+land+spatial) fractions. The four mixed fractions (Mage+land, Mage+spatial, Mland+spatial, Mage+land+spatial) refer to variance shared by the three groups of explanatory variables (Fig. 2).
To quantify the effects of landscape structure, stand age and spatial variables on species density and biomass, we performed the following procedures: (a) A model using landscape structure variables was fit to the response variables using multiple regression. (b) A model using stand age as explanatory variable was fit to the response variables using simple regression analysis. Dependent variables (species density and stand biomass) were formally tested for normality and homogeneity of variances in the residuals (Zar 1999). The explanatory variables were stand age and a group of patch-type metrics, which needed to be transformed with 1/x, log10 (x), log10 (x + 1) and sqrt (x) as necessary to meet the assumptions of linearity (Zar 1999). (c) A multiple regression model using spatial variables (significant PCNM vectors) derived from a PCNM analysis of plot locations was fit to the response variables (species density and biomass). Linear trends were checked by conducting a regression model of response variables with x and y spatial locations of each site. In case of significant linear trends, detrended residuals were used as response variables for the three previous models. (d) Three multiple regression models were performed using all possible combinations of two groups of explanatory variables (stand age, landscape structure and space) and an additional regression model that combined the three sets of explanatory variables. In all four regression models we exclusively selected significant variables of previous regression models (a–c). All multiple regression analyses were carried out using forward selection. Finally, variation partitioning was performed using the following equations:
Spatial structure of stand age and landscape patterns at different spatial scales
The PCNM and multiple regression analyses were used for identifying relationships between response variables (species density and biomass) and explanatory variables (landscape structure and stand age) at multiple spatial scales by first identifying significant spatial structures in response variables across the gradient of scales defined by the data set, and then relating the significant spatial structures to the explanatory variables (Borcard et al. 2004). The following procedure was used to characterize relationships among response and explanatory variables at multiple scales: (a) we performed multiple regression analyses between the two response variables and all significant PCNM vectors indicated in the “Spatial data” section, in order to detect scale-dependant spatial variability in species density and biomass. The R 2 value of these regression models indicates the percentage of spatial variance accounted for by the complete gradient of scales. (b) We partitioned the global spatial model for each response variable into several additive submodels. To identify these submodels a variogram analysis of the significant PCNM vectors was performed using the GS + software (Robertson 2000). The global spatial model was partitioned into three arbitrarily defined submodels based on the range of the variograms of PCNM vectors (Fig. 3) corresponding to the following spatial scales: a very broad scale submodel ranging from 8,000 to 10,500 m corresponded to PCNM vectors 1 to 5; a broad scale submodel ranging from 2,000 to 8,000 m corresponded to 11 PCNM vectors (6–16); and a local scale submodel <2000 m, corresponded to 44 PCNM vectors (17–60). (c) A set of three multiple regression analysis were performed for each response variable using as explanatory variables the PCNM vectors corresponding to each spatial submodel defined before. We also calculated the predicted values of each response variable (species density and biomass) corresponding to each spatial scale. These predicted values may be considered as surrogates of the variability of the response variable at a given scale. (d) Finally, a multiple regression model using stand age and landscape structure as explanatory variables was fit to the two predicted response variables for each spatial submodel.
Results
Land cover map
The land cover thematic map of the study area is shown in Fig. 1. This landscape covers a total area of 37,242 ha; 94.3% is covered by forest in any of the four vegetation classes, and only 5.7% is covered by agriculture, urban areas and roads. Vegetation class 4 (>15 year-old secondary forest on hills) was the most abundant land cover type covering 11,545 ha (31% of the total area), followed by vegetation class 3 (>15 year-old secondary forest on flat areas), which occupied 27.3% of the area (10,158 ha), and vegetation classes 1 (3–8 year-old forest) and 2 (9–15 year-old forest), with 18.5 and 17.5% of the area, and 6,900 and 6,525 ha, respectively. The overall accuracy calculated for the map was 75.6%, and the Kappa index was 0.7.
Species density and biomass among vegetation classes
Species density and biomass differed significantly at least in one of the four vegetation classes for all woody plants (F[3,275] = 46.9, P < 0.001 and F[3,275] = 105.8, P < 0.001 respectively), as well as for adults (F[3,275] = 90.9.9, P < 0.001 and F[3,275] = 105.8, P < 0.001) and juveniles (F[3,275] = 14.1, P < 0.001 and F[3,275] = 18.5, P < 0.001) separately. Post hoc Tukey’s HSD tests revealed that species density and biomass differed significantly among all four vegetation classes for all woody and adult plants, but not for juveniles (Fig. 4). The mean values of species density and stand biomass of adults and all plants showed a consistent pattern: the youngest vegetation class (3–8 years old) had fewer species and less biomass than the older successional stages (>15 years old). Species density was higher in vegetation class 4 than vegetation class 3, whereas biomass showed the opposite pattern, indicating that topography influences these variables (Fig. 4).
Variation partitioning
The variation explained by stand age, landscape structure and spatial dependence varied between response variables, and between adults and juveniles (Fig. 5). Total variation explained by the models was consistently higher for biomass (34–71%) compared to species density (28–52%), and highest for adults, lowest for juveniles, and intermediate for all plants (Fig. 5). Variation partitioning for adults and all individuals revealed that stand age was the single most important factor accounting for 47% of the total variation in biomass. In contrast, for species density, landscape structure and spatial dependence combined contributed equally or even more than stand age (Fig. 5). On the other hand, variation partitioning for juveniles showed a stronger influence of spatial dependence compared to stand age and landscape structure, accounting for 18 and 19% of the total variation in stand biomass and species density, respectively.
Relating species density and biomass with stand age and landscape structure
Multiple regression results indicated statistically significant relationships between species density and stand age, PD, ED, SHAPE_AM and TECI (Table 1). Stand age was positively related to all response variables, except for juvenile biomass, which showed a negative relationship, and the relationships were consistently stronger for adults compared to juveniles. Relationships involving PD and SHAPE_AM were consistently negative, indicating that species density decreases as the number of patches increases and as the shape of a patch type becomes more irregular. Stand biomass was best explained by stand age, PROX_AM, ENN_AM and TECI (Table 1). Biomass in all groups consistently responded negatively to ENN_AM and TECI and positively to PROX_AM, indicating that biomass decreases with distance between patch types of the same vegetation or as the perimeter of the focal patch type increases its contrast with other patch types (Table 1).
Spatial structure of stand age and landscape patterns at different spatial scales
The two response variables of vegetation community structure (species density and stand biomass) displayed spatial variability across 32 of the 60 significant PCNMs (Table 2). For all individuals, adults and juveniles, these variables accounted for 20, 20 and 26% of among-site variability in biomass, and for 12, 18 and 19% of among-site variability in species density, respectively.
The relationships between explanatory variables (stand age and landscape structure) and species density and stand biomass at different spatial scales are given in Table 3. At the very broad scale (8,000–10,500 m), explanatory variables accounted for 4–14% of variation. For all individuals, stand age was the variable that contributed most to above ground biomass (b = 0.192 for adults and all individuals, b = −0.187 for juveniles), whereas landscape structure metrics contributed more than stand age to species density (for all woody plants b = 0.413 and 0.266 for edge density and stand age, respectively; Table 3). At the broad scale (2,000–8,000 m), stand age was the explanatory variable that contributed most to both species density and biomass (for all woody plants b = 0.271 and 0.231 for species density and biomass, respectively, while for adults b = 0.271 and 0.224 for species density and biomass, respectively). Finally, at the local scale (<2,000 m), only one landscape metric (ENN_AM for adults and all plants, PROX_AM for juveniles) contributed significantly to among-site variation in biomass, whereas no significant relationship was found between explanatory variables and species density.
Discussion
Exploring how patterns of species density and biomass change across spatial scales is important for conservation and management of TDF as it may help reveal factors that influence biological diversity and carbon storage. Environmental and spatial factors differed in their relative contribution to variation in plant biomass and species density when considering all individuals and adults. Stand age was the most important variable affecting plant biomass. The association between both variables was a positive, supporting the intuitive notion of greater biomass in older vegetation patches. Previous research in southern Yucatan has demonstrated that above ground biomass increases rapidly with forest age (Read and Lawrence 2003; Eaton and Lawrence 2009). In contrast, landscape structure and spatial variables had a comparable or even stronger influence on species density than stand age. These results are consistent with recent findings in tropical forests showing that long-term monitoring data follow chronosequence patterns for basal area (Kalacska et al. 2004; Chazdon et al. 2007), but not for species density, indicating that stand age largely determines basal area– and biomass, whereas species density seems to be strongly affected by other factors operating at the local and landscape level, such as soil fertility and proximity to seed sources (Chazdon et al. 2007).
On the other hand, juvenile biomass and species density showed consistent patterns of environmental and spatial influence. Pure spatial dependence appears to be the most important predictor of juvenile species density and biomass. Although, this result could imply strong dispersal/recruitment limitation (Cottenie 2005), we believe that variation in space likely had a considerable environmental component that was not detected because some relevant environmental variables, such as topographic position or soil properties (Jones et al. 2008), were not considered in our analysis. Guariguata and Ostertag (2001) point out that microhabitat differences and soil nutrients can strongly affect composition and growth of colonizing species during tropical secondary forest succession. Failing to include such important environmental variables undoubtedly limits our ability to accurately gauge the relative contribution of stand age, landscape structure and spatial dependence to variation in plant biomass and species density.
Stand age showed contrasting relationships with juvenile biomass compared to juvenile species density. Species density increased with stand age (R 2 = 0.04; P < 0.01), whereas biomass decreased as stand age increased (R 2 = 0.13, P > 0.01; data not shown). The negative effect of stand age on biomass is partly due to a decline in juvenile stem density during succession (data not shown). Several researchers have found similar patterns (Denslow and Guzman 2000; Capers et al. 2005). This decline could result from asymmetric competition with adults during tropical forest succession, as many small individuals that dominate in early stages are progressively replaced by few large ones as a result of thinning (Finegan 1996; Chazdon 2008). Opposite patterns of basal area and stem density as a function of stand age were found for adults versus juveniles (unpublished data), which is consistent with this interpretation. In contrast, the increase in juvenile species density with stand age (despite decreasing stem density) can be partly attributed to recruitment of new canopy tree species that reach adult reproductive size in older compared to younger stands (Capers et al. 2005).
Although stand age was the main predictor of above ground biomass for adults and all individuals, landscape variables were also important predictors of biomass, especially for juveniles. Plant biomass consistently decreased as the degree of contrast between a patch type and its neighbor classes increased or as Euclidian distance among patch types also increased. A greater degree of contrast between a patch type and its neighbor patches reflects a landscape that is more fragmented. Similarly, a greater distance between a patch type and its neighbors suggest a patch type that is more isolated. In contrast, as the value of proximity indices increases, it is expected that patch types form clusters of similar fragments. This indicates that the focal patch type is less isolated and the landscape is less fragmented. Therefore, the results of this study suggest that above ground biomass decreases when a landscape is more fragmented and a patch type is more isolated. These patterns concur with recent findings indicating that habitat fragmentation substantially reduces forest biomass (Laurance et al. 1998; Nascimento and Laurence 2004), possibly due to enhanced tree mortality and proliferation of disturbance-adapted species such as lianas (D’Angelo et al. 2004).
On the other hand, the strong positive association between species density and ED (edge density) suggest that patch types with more edges have greater woody species diversity. Compared to forest interior habitats, edges have different microclimatic conditions that facilitate the establishment and growth of pioneer and light-demanding species as well as disturbance-adapted species such as lianas (Laurance et al. 1998). Therefore, small disturbances distributed in the forest could promote spatial variation of patch types that create a variety of physical environments for plants, allowing an increase in habitat diversity, and hence in species diversity (Honnay et al. 2003). However, we found a strong negative association between species density and both PD and SHAPE_AM, indicating that patch types with a greater number of patches and more irregular forms have lower species density. This suggests that large and frequent disturbances can increase the degree of fragmentation with negative impacts on plant diversity. These two seemingly contradictory results suggest that moderate levels of disturbance and fragmentation may enhance plant diversity in a forest-dominated landscape, whereas high levels of disturbance and fragmentation can have a strong negative impact on diversity (Hill and Curran 2003). Thus, our results suggest that forest biomass would likely decrease with any form of land use resulting in forest fragmentation and decreased representation of old-growth forests in the landscape. However, low levels of land use, such as traditional slash-and-burn agriculture practiced in small patches surrounded by old-growth forest (which currently predominate in our study area) could actually enhance plant α diversity, by promoting habitat heterogeneity. Yet, higher levels of land use, such as more extensive agriculture or pastures for livestock, that result in higher fragmentation, isolation and reduction of old-growth forests are also likely to reduce plant α diversity.
PCNM submodels allowed us to link the spatial distribution of species density and biomass to stand age and landscape structure at different spatial scales. The results suggest that biomass response to environmental variables is scale dependent. At the very broad and broad scales (2,000–10,500 m), stand age contributed most to, and was positively associated with biomass for all woody plants and adults-largely reflecting growth of the largest individuals, but negatively associated for juveniles-largely reflecting a decline in juvenile stem density during succession (Capers et al. 2005; Rico-Gray and García-Franco 1992). However, at the local scale, biomass was unaffected by stand age and influenced by landscape structural variables for all individuals, as well as for adults and juveniles. Although the local scale ranged from 1 to 2000 m, the range of most PCNM variables was <500 m (Fig. 5), which coincides with the empirically observed mean patch size in this study. Thus, the lack of association between stand age and plant biomass at the local scale could simply reflect the small variation in stand age, at very local (<500 m) scales.
Our results suggest that species density responses to environmental variables are also scale dependent. Landscape structure variables and stand age were more related to species density at the very broad and broad scales, respectively, whereas no significant association was found at the local scale. The latter result may also be related to mean patch size, and suggests that, within a patch, local factors, such as competition among individuals and species ability to acquire limited resources could be more important for species diversity than stand age (Bell et al. 2000).
Conclusion
The patterns of distribution of biomass and species density in the TDF studied can be explained by different factors. Stand age was the most important variable contributing to explain variation in biomass, whereas landscape structure and spatial variables had a comparable or even stronger influence on species density than stand age. Although stand age was the main predictor of above ground biomass, the latter also decreases as the landscape becomes more fragmented and isolated. Plant species density was affected by two main factors: (1) the quality of the surrounding habitats, which reflects the degree of fragmentation between the focal patch type and its neighbors, (2) habitat diversity, which may enhance species density of a particular type of fragment. We also found that environmental and landscape structure variables associated with both species density and biomass are scale dependent. Finally, we recommend that future research incorporates other environmental variables, such soil site characteristics, that have substantial variation within as well as among patches in a landscape (Tuomisto et al. 2003), and could be used to more fully understand how different environmental variables explain plant diversity and biomass at different spatial scales.
References
Bautista F, Batllori-Sampedro E, Ortiz-Pérez MA, Palacio-Aponte G, Castillo-González M (2003) Geoformas, agua y suelo en la Península de Yucatán. In: Colunga P, Larque A (eds) Naturaleza y sociedad en el área maya. Academia Mexicana de Ciencias y Centro de Investigación Científica de Yucatán, Yucatán, México
Bell G, Lechowicz MJ, Waterway MJ (2000) Environmental heterogeneity and species diversity of forest sedges. J Ecol 88:67–87
Bellier E, Monestiez P, Durbec JP, Candau JN (2007) Identifying spatial relationships at multiple scales: principal coordinate of neighbour matrices (PCNM) and geostatistical approches. Ecography 30:385–399
Borcard D, Legendre P (2002) All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecological Modelling 153:51–68
Borcard D, Legendre P, Avois-Jacquet C, Tuomisto H (2004) Dissecting the spatial structure of ecological data at multiple scales. Ecology 85:1826–1832
Boscolo D, Metzger JP (2009) Is bird indidence in Atlantic forest fragments influenced by landscape patterns at multiple scales? Landscape Ecol 24:907–918
Brown JH, Lomolino MV (1998) Biogeography, 2nd edn. Sinauer Associates, Sunderland, MA, USA
Brown S, Lugo AE (1990) Tropical secondary forests. J Trop Ecol 6:1–32
Buschbacher R (1986) Tropical deforestation and pasture development. Bioscience 36:22–28
Cairns MA, Olmsted I, Granados J, Argaez J (2003) Composition and aboveground tree biomass of a dry semi-evergreen forest on Mexico’s Yucatan Peninsula. For Ecol Manag 186:125–132
Campbell JB (1987) Introduction to remote sensing. The Guilford Press, New York
Capers RS, Chazdon RL, Redondo-Brenes A, Vilchez-Alvarado B (2005) Succesional dynamics of woody seedling communities in wet tropical secondary forests. J Ecol 93:1071–1084
Carnevali G, Ramírez MI, González-Iturbe JA (2003) Flora y Vegetación de la Península de Yucatán. In: Colunga P, Larque A (eds) Academia Mexicana de Ciencias y Centro de Investigación Científica de Yucatán, Yucatán, México
Chavez PS (1996) Image-based atmospheric corrections revisited and improved. Photogram Eng Remote Sens 62:1025–1036
Chazdon RL (2008) Chance and determinism in tropical forest succession. In: Carson W, Schnitzer S (eds) Tropical Forest Community Ecology. Blackwell Publishing, Oxford, UK, pp 384–408
Chazdon RL, Letcher SG, Breugel MV, Martínez-Ramos M, Bongers F, Finegan B (2007) Rates of change in tree communities of secondary Neotropical forests following major disturbances. Phil. Trans. R. Soc. B 362:273–289
Clark DB, Palmer MW, Clark DA (1999) Edaphic factors and the landscape-scale distributions of tropical rain forest trees. Ecology 80:2662–2675
Cottenie K (2005) Integrating environmental and spatial processes in ecological community dynamics. Ecol Lett 8:1175–1182
Cushman SA, McGarigal K (2004) Patterns in the species environment relationship depend on both scale and choice of response variables. Oikos 105:117–124
D’Angelo SA, Andrade ACS, Laurance SG, Laurance WF, Mesquita RCG (2004) Inferred causes of tree mortality in fragmented and intact Amazonian forest. J Trop Ecol 20:243–246
Dale MRT, Fortin MJ (2002) Spatial autocorrelation and statistical tests in ecology. Ecoscience 9:162–167
Denslow JS, Guzman GS (2000) Variation in stand structure, light and seedling abundance across a tropical moist forest chronosequence, Panama. J Veg Sci 11:201–212
Dray S, Legendre P, Peres-Neto PR (2006) Spatial modelling: a comprehensive framework for principal coordinate analysis of neighbour matrices (PCNM). Ecol Model 196:483–493
Eaton JM, Lawrence D (2009) Loss of carbon sequestration potential after decades of shifting cultivation in the Southern Yucatán. For Ecol Manag 258:949–958
FAO (2007) State of the World’s forests 2007. FAO, Roma, Italia
Finegan B (1996) Pattern and process in neotropical secondary rain forests: the first 100 years of succession. Trends Ecol Evol 11:119–124
Flores J, Espejel I (1994) Tipos de vegetación de la Península de Yucatán. Etnoflora Yucatanense, Fascículo 3. México 135
Gaston KJ (2000) Global patterns in biodiversity. Nature 405:220–227
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391
Groeneveld J, Alves LF, Bernacci LC, Catharino ELM, Metzget JP, Püzt S, Huth A (2009) The impact of fragmentation and density regulation on forest succession in the Atlantic rain forest. Ecol Model 220:2450–2459
Guariguata MR, Ostertag DR (2001) Neotropical secondary forest succession: changes in structural and functional characteristics. For Ecol Manag 148:185–206
Gustafson EJ (1998) Quantifying landscape spatial patterns: what is the state of the art? Ecosystems 1:143–156
Hargis CD, Bossonette JA, David JL (1998) The behavior of landscape metrics commonly used in the study of habitat fragmentation. Landscape Ecol 13:167–186
Hernández-Stefanoni JL, Dupuy JM (2008) Effects of landscape patterns on species density and abundance of trees in a tropical subdeciduos forest of the Yucatan Peninsula. For Ecol Manag 255(11):3797–3805
Hill JL, Curran PJ (2003) Area, shape and isolation of tropical forest fragments: effects on tree species diversity and implications for conservation. J Biogeogr 30:1391–1403
Honnay O, Piessens K, Van Landuyt W, Hermy M, Guilinck H (2003) Satellite based land use and landscape complexity indices as predictors for regional plant species diversity. Landsc Urban Plan 63(4):241–250
Hughes RF, Kauffman JB, Jaramillo-Luque VJ (1999) Biomass, carbon, and nutrient dynamics of secondary forests in a humid tropical region of México. Ecology 80:1892–1907
Jensen JR (2000) Remote sensing of environment: an earth resource perspective. Prentice Hall, New Jersey, p 544
Jones MM, Tuomisto H, Borcard D, Legendre P, Clark DB, Olivas PC (2008) Explaining variation in tropical plant community composition: influence of environmental and spatial data quality. Oecologia 155:593–604
Kalacska M, Sánchez-Azofeifa GA, Calvo-Alvarado JC, Quezada, Rivard B, Janzen DH (2004) Species composition, similarity and diversity in three successional stages of a seasonally dry tropical forest. For Ecol Manag 200:227–247
Lamb D, Erskine PD, Parrotta JA (2005) Restoration of degraded tropical forest landscapes. Science 310:1628–1632
Laurance WF, Laurance SG, Delamonica P (1998) Tropical forest fragmentation and greenhouse gas emissions. For Ecol Manag 110:173–180
Laurance WF, Vasconcelos HL, Lovejoy TE (2000) Forest loss and fragmentation in the Amazon: implications for wildlife conservation. Oryx 34:39–45
Lebrija-Trejos E, Bongers F, Pérez-García, Meave J (2008) Successional change and resilience of a very dry tropical deciduous forest following shifting agriculture. Biotropica 40:422–431
Magurran AE (1988) Ecological diversity and its measurement. Princeton University Press, Princeton, N. J
Mazerolle MJ, Villard MA (1999) Patch characteristics and landscape context as predictor of species presence and abundance: a review. Ecoscience 6(1):117–124
McGarigal K, Cushman SA, Neel MC, Ene E (2002) FRAGSTATS: spatial pattern analysis for categorical maps. University of Massachusetts, USA
Miles L, Newton AC, Defries RS, Ravilious C, May I, Blyth S, Kapos V, Gordon JE (2006) A global overview of the conservation status of tropical dry forests. J Biogeogr 33:491–505
Nascimento HEM, Laurence WF (2004) Biomass dynamics in Amazonian forest fragments. Ecol Appl 14(4):s127–s138
Portillo-Quintero CA, Sánchez-Azofeifa GA (2010) Extent and conservation of tropical dry forests in the Americas. Biol Conserv 143:144–155
Quesada M, Sánchez-Azofeifa GA, Alvarez-Añorve M, Stoner KE, Avila-Cabadilla L, Calvo-Alvarado J, Castillo A, Espirito-Santo MM, Fagundes M, Fernandes GW, Gamon J, Lopezaraiza-Mikel M, Lawrence D, Morellato LPC, Powers JS, Neves FD, Rosas-Guerrero V, Sayago R, Sánchez-Montoya G (2009) Succession and management of tropical dry forests in the Americas: review and new perspectives. For Ecol Manag 258:1014–1024
Read L, Lawrence D (2003) Recovery of biomass following shifting cultivation in dry tropical forests of the Yucatán. Ecol Appl 13:85–97
Rico-Gray V, García-Franco JG (1992) Vegetation and soil seed bank of successional stages in tropical lowland deciduous forest. J Veg Sci 3:617–624
Robertson GP (2000) GS + : geostatistics for environmental science. Gamma Design Software. Plainwell, Michigan
Ruiz J, Fandiño MC, Chazdon RL (2005) Vegetation structure, composition, and species richness across 56-year chronosequence of dry tropical foresto n Providencia Island, Colombia. Biotropica 37:520–530
Sánchez-Azofeifa GA, Quesada M, Rodriguez JP, Nassar JM, Stoner KE, Castillo A, Garvin T, Zent EL, Calvo-Alvarado JC, Kalacska MER, Fajardo L, Gamon JA, Cuevas-Reyes P (2005) Research priorities for neotropical dry forests. Biotropica 37:477–485
Thomlinson JR, Serrano MI, del M. López T, Aide TM (1996) Land-use dynamics in a post-agriculture Puerto Rican landscape (1936–1988). Biotropica 28:525–536
Tilman D (1994) Competition and biodiversity in spatially structured habitats. Ecology 75:2–16
Toledo VM, Batis AI, Becerra R, Martínez E, Ramos CH (1995) La selva útil: etnobotánica cuantitativa de los grupos indígenas del trópico húmedo de México. Interciencia 20:177–187
Trejo I, Dirzo R (2000) Deforestation of seasonally dry tropical forest: a national and local analysis in Mexico. Biol Conserv 94:133–142
Tuomisto H, Poulsen AD, Ruokolainen K, Moran RC, Quintana C, Celi J, Canas G (2003) Linking floristic patterns with soils heterogeneity and satellite imagery in Ecuadorian Amazonia. Ecol Appl 13(2):352–371
Whittaker RH (1960) Vegetation of the Siskiyou Mountains, Oregon and California. Ecol Monogr 30:279–338
Whittaker RJ, Willis KJ, amd Field R (2001) Scale and species richness: towards a general, hierarchical theory of species diversity. J Biogeogr 28:453–470
Wu J (1999) Hierarchy and scaling: extrapolating information along scaling ladder. Can J Remote Sens 25:367–380
Zar JH (1999) Biostatistical Analysis. Prenctice Hall, New York
Aknowledgements
We thank the ejidos of Xkobehnaltún, Xuul and Yaxhachén for allowing us to work in their lands and for their assistance with field work and determination of stand age of sample sites. James Callaghan and Kaxil Kiuic A. C. provided logistic support. T. Spies and two anonymous reviewers provide helpful comments on the manuscript. The study was financially supported by CICY and FOMIX-Yucatán (project YUC-2008-C06-108863).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hernández-Stefanoni, J.L., Dupuy, J.M., Tun-Dzul, F. et al. Influence of landscape structure and stand age on species density and biomass of a tropical dry forest across spatial scales. Landscape Ecol 26, 355–370 (2011). https://doi.org/10.1007/s10980-010-9561-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-010-9561-3