Abstract
Many amphibian species rely on both aquatic and terrestrial habitats to complete their life cycles. Therefore, processes operating both within the aquatic breeding habitat, and in the surrounding uplands may influence species distributions and community composition. Moreover, changes in land use adjacent to breeding site may degrade aquatic habitats. To assess land use effects on pond-breeding amphibian assemblages, we investigated relationships between land use, breeding habitat conditions, and breeding amphibian use of constructed wetlands in urban environments of the Baltimore metropolitan area, USA. Forest and impervious surface associations with species richness and occurrence occurred at spatial scales ranging from 50 to 1,000 m, with strongest relationships at 500 m. Forest and impervious surface cover within 1,000 m of ponds were also related to water and sediment quality, which in turn were capable of explaining a proportion of the observed variation in species richness and occurrence. Taken together, our results suggest that forest and other land covers within relatively proximal distances to ponds (i.e., within 50–1,000 m) may be influencing species richness directly via the provisioning of upland habitat, and indirectly via influences on within pond habitat quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wetlands are often small or located between terrestrial and open water systems. Therefore, surrounding land use affects wetland function in many ways. For organisms such as pond-breeding amphibians with complex life cycles, processes operating over multiple spatial scales influence population dynamics (Wilbur 1980; Semlitsch and Bodie 2003). Adult pond-breeding amphibians aggregate at wetlands, where eggs are laid, and larvae remain in the aquatic environment until they metamorphose into terrestrial or semi-aquatic juveniles. During the non-breeding season, adults and juveniles may utilize terrestrial habitats in close proximity to breeding sites for foraging and refugia (e.g., Bulger et al. 2003; Regosin et al. 2005; Richter et al. 2001; Semlitsch and Bodie 2003); in this context, the terrestrial habitat can be viewed as either complementary or supplementary to aquatic breeding sites (Dunning et al. 1992). Over larger spatial scales, terrestrial landscape structure may influence dispersal and determine metapopulation dynamics (Marsh and Trenham 2001; Rothermel and Semlitsch 2002). Moreover, adjacent landscape structure and human land use govern hydrogeochemical processes that ultimately impact physiochemical and hydrological properties of aquatic habitats (Crosbie and Chow-Fraser 1999; Paul and Meyer 2001; Houlahan and Findlay 2004), which in turn relate to amphibian use (Johnson et al. 2002; Richter and Azous 1995; Snodgrass et al. 2000).
Our objective here was to investigate relationships between adjacent land use, pollutant conditions and amphibian use of wetlands in urban landscapes. We chose as our study sites human-created stormwater management ponds because stormwater ponds are becoming increasingly common features of urban landscapes and may benefit or harm pond breeding amphibians depending on habitat conditions within and surrounding ponds (Stahre and Urbonas 1990; Williams 1990; Campbell 1994; Helfield and Diamond 1997). Stormwater ponds mitigate effects associated with increased runoff and pollutant accumulation on impervious surfaces by intercepting high-velocity runoff and allowing sediment-associated pollutants to accumulate in pond substrata rather than entering natural streams or wetlands (e.g., Bishop et al. 2000; Karouna-Renier and Sparling 2001). Urban environments are characterized by extensive structural changes to a previously natural landscape, and as a result, processes and their scales of influence on amphibian distributions in urban landscapes are likely to differ from those in a natural or less-developed system (e.g., agricultural; Pickett and Cadenasso 1995). Amphibian movements in the terrestrial landscape, whether for dispersal or habitat complementation and supplementation, are hampered by direct loss of habitat, habitat fragmentation, and physical barriers (e.g., roadways; Carr and Fahrig 2001; Cushman 2006), all of which are common features of urban areas. Therefore, we expected that land use adjacent to ponds would have a strong influence on pond habitat quality and thus amphibian use.
Materials and methods
Study sites
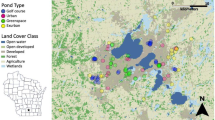
We investigated land cover effects on breeding amphibian use of stormwater management ponds using eighteen stormwater ponds located in the Baltimore–Washington metropolitan area. To assure our study sites included the range of land use conditions found in urban areas, we selected sites to represent three general land use categories: highway (n = 5), residential (n = 6) and commercial (n = 7). To define conditions expected at constructed wetlands that received little or no runoff, we included two human-constructed wetlands that receive little runoff from developed areas (referred to hereafter as open space ponds). The mean distance between ponds was 13.33 km, and to the best of our knowledge, ponds did not dry during our study period (Spring 2002–2005). All ponds were constructed between 1984 and 1991, and are part of a longer-term investigation of wildlife use of stormwater ponds in the Washington D.C. area (Casey et al. 2007; Karouna-Renier and Sparling 1997, 2001; Sparling et al. 2004). Because all ponds have non-overlapping drainage basins we considered individual ponds to be independent.
Species richness and community structure
Throughout the 2002, 2003 and 2004 amphibian breeding seasons ponds were surveyed using a combination of survey methods (unconstrained searches, calling surveys and larval surveys). Unconstrained searches were conducted four times per pond (April, May, June and August 2002) by the USGS Patuxent Wildlife Research Center for all ponds except one, which was added to the study in 2003. Unconstrained searches involved actively searching and dip-netting for adult and larval amphibians in and along pond edges and were terminated after all areas of ponds were searched. During 2003 and 2004, early (mid-February to late-March), mid- (early-May to mid-June) and late season (late-June to early-August) calling surveys were performed at all sites. Five-minute calling surveys began at least 30 min after sunset but prior to midnight. To capture additional species potentially missed by calling surveys, we conducted larval surveys at each site during each of four time periods: mid-April to early May 2003, mid- to late June 2003, mid-April to mid-May 2004, and mid- to late July 2004. During larval surveys, we ensured sampling of all pond microhabitat types using dip nets and seines (both with 0.31-cm Ace-style mesh) to sample vegetated and open space areas of ponds, respectively. Dipnet sweeps were 1 m in length, while seine hauls were 4–10 m in length depending on the complexity of vegetation structure. Each larval sampling event was a minimum of 30 min and was terminated 30 min after no new species were encountered. Specimens were identified in the field when possible using adult (Gibbons and Semlitsch 1991) and larval keys (Altig 1970). To verify field identifications, larval vouchers and unidentified larval specimens were euthanized, preserved and returned to the laboratory for identification with the aid of a dissecting microscope.
Land cover
To assess land cover influences on species richness and occurrence in stormwater ponds and to assess the distance at which land cover impacts were greatest, we utilized an ArcView GIS (ESRI, Redlands, CA) software environment to extract land cover, imperviousness and road data at overlapping intervals of 50, 100, 500, 1,000, 1,500, 2,000, 2,500 and 3,000 m from pond edges. Digital aerial photographs (30.48 cm × 30.48 cm pixel size; taken in 2000 or 2002; acquired from local governments) were used to determine pond surface areas and create interval boundaries. Land cover and imperviousness data layers (30 m × 30 m pixel and sub-pixel resolution; National Land-Cover Database 2001; Homer et al. 2004) and a 2002 road layer (Bureau of Transportation Statistics, US Department of Transportation, Washington, DC) were obtained from the Multi-Resolution Land Characteristics Consortium (USGS, EROS Data Center, MRLC Project, Sioux Falls, SD; http://gisdata.usgs.net/website/MRLC/). Land cover and impervious data layers are based on imagery acquired between July 1999 and April 2001. We defined five classes of land cover based on descriptions given in Homer et al. (2004): Developed, Open-Developed, Agricultural, Forest, and Wetlands. Developed includes low-, medium- and high-intensity developed areas, while Open-Developed describes lesser developed areas dominated primarily by vegetation and lawns. Agricultural includes areas dominated by crops, pastures and hay, and Forest and Wetlands combines all forest and wetland types, respectively. We used the Patch Analyst extension in ArcView (Elkie et al. 1999) to determine the proportion of each interval occupied by imperviousness and each land cover class. Road density was calculated for each interval as total road length per interval area.
Within-pond habitat
We characterized local pond habitat in terms of water chemistry and sediment metal concentrations. Using calibrated, hand-held meters, in situ measurements of pH and conductivity were made in the littoral zone at the inflow, middle and outflow areas of ponds. We measured pH during larval surveys and six additional visits to each pond, while conductivity was measured four times from 2002 to 2005. In May 2002 and September 2003, we collected surface water samples from littoral zones at the middle of ponds and shipped them to the USGS National Water Quality Laboratory (NWQL) for analyses of total nitrogen (N), total phosphorous (P), and acid-neutralizing capacity (ANC). Samples for N and P analyses were acidified using H2SO4, and all samples were placed on ice for shipment. We measured As, Cr, Cu, Ni, Pb and Zn concentrations in sediments collected from ponds. Details of sediment sampling and metal analyses can be found in Casey et al. (2007). Briefly, in September 2003 and July 2004, the top 2.5 cm of sediment were collected at the inflow, middle and outflow areas of each pond, composited, and homogenized prior to analysis. To leach potentially bioavailable fractions of trace metals from sediments, we digested sediments with 6 N HNO3 and measured dissolved analyte concentrations using inductively coupled plasma mass spectrometry.
Data analysis
We used an information-theoretic approach (Burnham and Anderson 2002) to investigate relationships between species richness, the occurrence of individual species, land cover, and local environmental characteristics (i.e., wetland size, conductivity, pH, nutrients, and metals). We considered species to be present at a pond if any life stage was detected. At the local scale we constructed a set of models that included size (represented by surface area), nutrient status (represented by nitrogen concentrations), potential for road salt contamination (represented by specific conductance), and degree of metal contamination (represented by Cr, Ni and Zn sediment concentrations) because these factors have been previously linked to amphibian use of wetlands (Findlay and Houlahan 1997; Houlahan and Findlay 2003; Knutson et al. 1999; Richter and Azous 1995). Because of a high degree of correlation between nitrogen and phosphorous levels (r = 0.83), we included only nitrogen in our models to represent nutrient status. We limited our consideration of metals to Cr, Ni, and Zn because only these metals exceeded consensus-based toxicity threshold effects concentrations (MacDonald et al. 2000) at five or more ponds. To relate both species richness and the occurrence of individual species to local and landscape variables we used generalized linear models as executed in the GENMOD procedure of SAS. For species richness we used a normal distribution for the error term as species richness was normally distributed (Shapiro–Wilk’s w = 0.952; P = 0.391). We log-transformed surface area and metal concentrations to more closely approximate the linearity assumption of the model. To avoid over-parameterizing models, we only considered models with one and two local-scale predictor variables. At the landscape scale we considered models including proportion forest cover, proportion impervious surface cover, and road density within buffers from 50 to 3,000 m surrounding ponds. Because of a high degree of correlation among land cover types at any given distance from a pond, we limited models to a single parameter at the landscape scale. For the same reason, we did not investigate models that included local and landscape-scale variables simultaneously. We followed the model comparison criteria outlined in Burnham and Anderson (2002) and used a corrected Akaike’s Information Criterion (AICc) and relative model weights (w i ) to rank models. We used logistic regression with a binomially distributed error term and a logit link function to model individual species occurrence as a function of both land cover and local-scale variables. For all models we calculated the percent of deviance explained as the reduction in deviance for the full model divided by the deviance of the null model (i.e., intercept term only). Only species occurring at 25–75% of the ponds were modeled; Pseudacris crucifer, Rana clamitans and R. catesbeiana were not modeled because of their near ubiquitous distribution (Table 1). Due to similarities in larval characteristics and mating calls between species belonging to the gray tree frog complex (Hyla versicolor and H. chrysoscelis), we grouped these two species together for analyses purposes. We also grouped toad species together for analysis because of their complementary distribution (i.e., Bufo americanus was found mainly in the Piedmont region of the study area and B. woodhousei mainly on the coastal plain; Table 1).
Results
We detected ten anuran species and one caudate species among our sites, with richness values ranging from 0 to 11 (Table 1). The three most commonly occurring species occurred at greater than 80% of the sites, while five species occurred at only 25% of sites. Open space ponds and ponds surrounded by residential land use generally supported the greatest number of species, while ponds surrounded by highways supported the fewest species (Table 1).
Land use surrounding ponds was related to water chemistry and sediment metals levels (Table 2). Forest and impervious surface cover at distances ≥500 m were negatively and positively correlated with pH, respectively. Conductance exhibited a pattern similar to pH, but relationships with forest and impervious surface cover were not as strong. Metal levels in sediments were most strongly correlated with impervious surface cover in close proximity to ponds. Nutrients were the environmental measures that correlated with road densities and strongest relationships occurred at distances from 50 to 1,000 m (Table 2).
At the local scale, metal levels in sediments were the best predictors of amphibian species richness (Table 3). The most supported model for species richness (w i = 0.66) included Zn sediment concentrations and pond surface area, and sediment concentration of Zn was included in all models with weights >0.10. Zn and surface area explained ~43% of the deviance in species richness and Zn levels were negatively correlated with richness while surface area was positively correlated with species richness (Table 4). Although Cr and specific conductivity were included in some models with moderate support (w i > 0.10), the relative support for these models was low in comparison to the model containing Ni and Zn (Table 3).
The most supported model of species richness was indicative of the relationships between local-scale environmental variables and the occurrence of individual species at ponds. Models of occurrence accounted for between 8 and 67% of the deviance in occurrence of individual species, with the occurrence of R. palustris and Bufo americanus/woodhousei, two relatively common species, being poorly predicted (Table 3). With the exception of models for Bufo spp. and Rana utricularia, a negative relationship between Zn sediment concentrations and occurrence was included in one or more of the most supported models and was the most important variable for predicting the occurrences of R. palustris, Acris crepitans, and Notophthalmus viridescens (Table 4). A positive effect of pond surface area was included in the most supported models for both Acris crepitans and Rana sylvatica, two species found in relative few ponds, and appeared to be the most important local-scale environmental variable for occurrence of R. sylvatica. Specific conductivity was the most important local-scale environmental variable for the H. versicolor/chrysoscelis species and Bufo americanus/woodhousei complexes, and negatively influenced the occurrence of both.
In general, land cover within 1,000 m of ponds was a better predictor of species richness and the occurrence of individual species than local-scale environmental variables (Table 3). Species richness and the occurrence of individual species were positively related to forest cover and negatively related to impervious surface cover within 1,000 m of ponds (Table 4), with the majority of the strongest relationships occurring at 500 m (Fig. 1). Road density was only weakly related to species richness or the occurrence of individual species (Table 3). Forest cover within 500 m of ponds was the best predictor of species richness and accounted for ~76% of the deviance in species richness, though impervious surface cover within 500 m of ponds accounted for 69% of the deviance in species richness despite relatively low support for this model (Table 3). Among individual species, the occurrence of R. utricularia, A. crepitans, Bufo americanus/woodhousei, R. sylvatica, and N. viridescens was more closely related to forest cover, while the occurrence of R. palustris and individuals of the H. versicolor/chrysoscelis complex were more closely related to impervious surface cover. For the former group the probability of occurrence declined rapidly as forest cover was reduced from 40 to 30%; in the case of the latter group the probability of occurrence declined rapidly as impervious surface cover increased from 20 to 30% (Fig. 2).
Probability of occurrence of amphibian species at stormwater management ponds as a function of either forest or impervious surface cover surrounding ponds. See Table 3 for specifics of model parameters and fit
Discussion
We found stormwater ponds capable of supporting a relatively diverse community of pond-breeding amphibians under specific landscape configurations. We included two constructed wetlands in relatively undeveloped areas to define expected conditions for constructed wetlands that were not designed to treat stormwater runoff. We found ponds receiving runoff from both commercial and residential land use supported the majority of pond-breeding amphibian species in our region and had similar species richness to our open space ponds when forest cover was >~40% and impervious surface cover <~20% within 1,000 m of ponds. Mole salamanders (Ambystoma maculatum and A. opacum) were the only common species in our region that were not present in any of our ponds, possibly as a result of their limited dispersal ability or the permanent nature of the ponds that we included in our study. Nonetheless, our results do suggest that stormwater ponds can play a role in amphibian conservation and that landscape characteristics within 500 to 1,000 m from a pond can be used to determine which ponds are most likely to host a diverse community of breeding amphibians, or to determine locations for the construction of new stormwater ponds that might maximize their wildlife habitat value.
Forest and impervious surface cover effects on amphibians of stormwater management ponds occurred at spatial scales ranging from 50 to 1,000 m, with peak effects occurring at 500 m from ponds. Previous studies have identified peak land cover effects at a range of spatial scales (i.e., 1,000–3,000 m from wetlands; Findlay and Houlahan 1997; Hecnar and M’Closkey 1998; Houlahan and Findlay 2003; Knutson et al. 1999), but in some cases, the observed scale of peak influence (i.e., 1,000 m) corresponds to the minimum spatial scale utilized (e.g., Knutson et al. 1999). Parris (2006) reported a 12–19% increase in species richness of amphibians with a decrease from 22 to 0% road cover within a 500 m radius of wetlands in the Melbourne, Australia metropolitan area. Although some of the more supported models for individual species included forest or impervious surface cover within 50 and 100 m of ponds, the 30 m × 30 m pixel resolution of the land use/land cover data we used may not have the resolution to discern patterns at these scales, indicating a need for studies investigating relationships at finer scales.
Variation in scales of influence for individual species are likely to be a function of species-specific differences in sensitivity to anthropogenic stressors and in terrestrial habitat use (Houlahan and Findlay 2003; Mazerolle et al. 2005; Price et al. 2005). A direct loss of terrestrial habitat within 500 m of ponds represents a loss of complimentary or supplementary habitat to many amphibian species (e.g., Regosin et al. 2005; Richter et al. 2001; Semlitsch and Bodie 2003). Roadways and high traffic densities impede overland movements of amphibians, such that the amount of suitable terrestrial habitat is further reduced due to inaccessibility and road mortality (Carr and Fahrig 2001; Hels and Buchwald 2001). Therefore, species such as R. sylvatica that require upland habitats or have extensive upland movements may be influenced more by adjacent land cover than species such as R. clamitans that are more tightly tied to the aquatic habitat. Furthermore, species that tolerate desiccating conditions, such as B. americanus and B. woodhousei may tolerate degradation and fragmentation of upland habitat better than more sensitive species such as H. versicolor and H. chrysoscelis.
Within ponds, species richness and the occurrence of individual species was related to sediment metal levels, and to a lesser extent, specific conductance and pond surface area. Water and sediment quality characteristics of stormwater management ponds have been related to urban land use (Karouna-Renier and Sparling 2001). Stormwater ponds are directly linked to impervious surfaces via storm drains, and therefore, non-point source pollutants accumulated on impervious surfaces are easily transported by surface runoff to stormwater ponds (Makepeace et al. 1995). Roadways and high traffic densities are major contributors to a variety of trace metal pollutants and road salts that influence specific conductivity, but other non-point sources of pollutants in urban environments include chromated copper arsenate treated lumber, pesticides, and fertilizers, all of which are commonly used on manicured lawns (Makepeace et al. 1995; Rice et al. 2002). Pollutant exposure risks to amphibians in urban habitats are generally understudied (Snodgrass et al. 2008; Snodgrass et al. in press), but adverse effects of trace metals and organic pollutants on larval amphibians have been previously documented (e.g., Bishop et al. 2000; Rowe et al. 1998; Snodgrass et al. 2005).
In conclusion, land cover (or other associated land use changes) within 1,000 m of ponds was also related to local-scale environmental variables, which in turn were capable of explaining a proportion of the observed variation in species richness and occurrence. Taken together, our results suggest that land cover within relatively proximal distances to ponds (i.e., within 50–1,000 m) may be acting directly on species richness via the provisioning of upland habitat and indirectly via influences on local pond habitat quality. However, because land cover was related to pollutant accumulation and water quality within ponds, assessing the individual contributions of upland habitat loss and pollution of breeding sites to reductions in the habitat value of stormwater ponds for amphibians in urban landscapes will require experimental approaches. Moreover, relationships between land use and pond habitat quality will make it difficult to distinguish the role of land use changes in the disruption of metapopulation dynamics because measures such as road density cannot be used solely as a measure of pond isolation (Parris 2006). Therefore, experiments that investigate the toxicity of wetland sediments and waters to amphibians are needed (Snodgrass et al. 2008), and when combined with field studies should yield insight into the significance of pond pollution in urban wetland systems.
References
Altig R (1970) A key to the tadpoles of the continental United States and Canada. Herpetologica 26:180–207
Bishop CA, Struger J, Shirose LJ, Dunn L, Campbell GD (2000) Contamination, wildlife communities in stormwater detention ponds in Guelph, the Greater Toronto Area, Ontario, 1997 and 1998. Part II - contamination and biological effects of contamination. Water Qual Res J Canada 35:437–474
Bulger JB, Scott NJ, Seymour RB (2003) Terrestrial activity and conservation of adult california red-legged frogs Rana aurora draytonii in coastal forests and grasslands. Biol Conserv 110:85–95. doi:10.1016/S0006-3207(02)00179-9
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Campbell KR (1994) Concentrations of heavy metals associated with urban runoff in fish living in stormwater treatment ponds. Arch Environ Contam Toxicol 27:352–356. doi:10.1007/BF00213171
Carr LW, Fahrig L (2001) Effect of road traffic on two amphibian species of differing vagility. Conserv Biol 15:1071–1078. doi:10.1046/j.1523-1739.2001.0150041071.x
Casey RE, Simon JA, Atueyi S, Snodgrass JW, Karouna-Renier NK, Sparling DW (2007) Temporal trends of trace metals in sediment and invertebrates from stormwater retention ponds. Water Air Soil Pollut 178:69–77. doi:10.1007/s11270-006-9132-z
Crosbie B, Chow-Fraser P (1999) Percentage land use in the watershed determines the water and sediment quality of 22 marshes in the Great Lakes basin. Can J Fish Aquat Sci 56:1781–1791. doi:10.1139/cjfas-56-10-1781
Cushman SA (2006) Effects of habitat loss and fragmentation on amphibians: a review and prospectus. Biol Conserv 128:231–240. doi:10.1016/j.biocon.2005.09.031
Dunning JB, Danielson BJ, Pulliam RH (1992) Ecological processes that affect populations in complex landscapes. Oikos 65:169–175. doi:10.2307/3544901
Elkie PC, Rempel RS, Carr AP (1999) Patch analyst user’s manual: a tool for quantifying landscape structure. Northwest sciences and technology technical manual TM-002. Ontario Ministry of Natural Resources, Thunder Bay, Ontario
Findlay CS, Houlahan J (1997) Anthropogenic correlates of species richness in southeastern Ontario wetlands. Conserv Biol 11:1000–1009. doi:10.1046/j.1523-1739.1997.96144.x
Gibbons JW, Semlitsch RD (1991) Guide to the reptiles and amphibians of the Savannah River site. University of Georgia Press, Athens, Georgia
Hecnar SJ, M’Closkey RT (1998) Species richness patterns of amphibians in southwestern Ontario ponds. J Biogeogr 25:763–772. doi:10.1046/j.1365-2699.1998.2540763.x
Helfield JM, Diamond ML (1997) Use of constructed wetlands for urban stream restoration: a critical analysis. Environ Manage 21:329–341. doi:10.1007/s002679900033
Hels T, Buchwald E (2001) The effect of road kills on amphibian populations. Biol Conserv 99:331–340. doi:10.1016/S0006-3207(00)00215-9
Homer C, Huang C, Yang L, Wylie B, Coan M (2004) Development of a 2001 national land-cover database for the United States. Photogramm Eng Remote Sensing 70:829–840
Houlahan JE, Findlay CS (2003) The effects of adjacent land use on wetland amphibian species richness and community composition. Can J Fish Aquat Sci 60:1078–1094. doi:10.1139/f03-095
Houlahan JE, Findlay CS (2004) Estimating the ‘critical’ distance at which adjacent land-use degrades wetland water and sediment quality. Landscape Ecol 19:677–690. doi:10.1023/B:LAND.0000042912.87067.35
Johnson CM, Johnson LB, Richards C, Beasley V (2002) Predicting the occurrence of amphibians: An assessment of multiple-scale models. In: Scott JM, Heglund PJ, Morrison ML, Haufler JB, Raphael MG, Wall WA, Samson FB (eds) Predicting species occurrences: issues of accuracy and scale. Island Press, Covelo, California, pp 157–170
Karouna-Renier NK, Sparling DW (1997) Toxicity of stormwater treatment pond sediments to Hyalella azteca (Amphipoda). Bull Environ Contam Toxicol 58:550–557. doi:10.1007/s001289900370
Karouna-Renier NK, Sparling DW (2001) Relationships between ambient geochemistry, watershed land-use and trace metal concentrations in aquatic invertebrates living in stormwater treatment ponds. Environ Pollut 112:183–192. doi:10.1016/S0269-7491(00)00119-6
Knutson MG, Sauer JR, Olsen DA, Mossman MJ, Hemesath LM, Lannoo MJ (1999) Effects of landscape composition and wetland fragmentation on frog and toad abundance and species richness in Iowa and Wisconsin, USA. Conserv Biol 13:1437–1446. doi:10.1046/j.1523-1739.1999.98445.x
MacDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39:20–31. doi:10.1007/s002440010075
Makepeace DK, Smith DW, Stanley SJ (1995) Urban stormwater quality—summary of contaminant data. Crit Rev Environ Sci Technol 25:93–139
Marsh DM, Trenham PC (2001) Metapopulation dynamics and amphibian conservation. Conserv Biol 15:40–49. doi:10.1046/j.1523-1739.2001.00129.x
Mazerolle MJ, Desrochers A, Rochefort L (2005) Landscape characteristics influence pond occupancy by frogs after accounting for detectability. Ecol Appl 15:824–834. doi:10.1890/04-0502
Parris KM (2006) Urban amphibian assemblages as metacommunities. J Anim Ecol 75:757–764. doi:10.1111/j.1365-2656.2006.01096.x
Paul MJ, Meyer JL (2001) Streams in the urban landscape. Annu Rev Ecol Syst 32:333–365. doi:10.1146/annurev.ecolsys.32.081501.114040
Pickett STA, Cadenasso ML (1995) Landscape ecology: spatial heterogeneity in ecological systems. Science 269:331–334. doi:10.1126/science.269.5222.331
Price SJ, Marks DR, Howe RW, Hanowski JM, Niemi GJ (2005) The importance of spatial scale for conservation and assessment of anuran populations in coastal wetlands of the western Great Lakes, USA. Landscape Ecol 20:441–454. doi:10.1007/s10980-004-3167-6
Regosin JV, Windmiller BS, Homan RN, Reed JM (2005) Variation in terrestrial habitat use by four poolbreeding amphibian species. J Wildl Manage 69:1481–1493. doi:10.2193/0022-541X(2005)69[1481:VITHUB]2.0.CO;2
Rice KC, Conko KM, Hornberger GM (2002) Anthropogenic sources of arsenic and copper to sediments in a suburban lake, Northern Virginia. Environ Sci Technol 36:4962–4967. doi:10.1021/es025727x
Richter KO, Azous AL (1995) Amphibian occurrence and wetland characteristics in the Puget-Sound Basin. Wetlands 15:305–312
Richter SC, Young JE, Seigel RA, Johnson GN (2001) Postbreeding movements of the dark gopher frog, Rana sevosa goin and netting: implications for conservation and management. J Herpetol 35:316–321. doi:10.2307/1566123
Rothermel BB, Semlitsch RD (2002) An experimental investigation of landscape resistance of forest versus old-field habitats to emigrating juvenile amphibians. Conserv Biol 16:1324–1332. doi:10.1046/j.1523-1739.2002.01085.x
Rowe CL, Kinney OM, Nagle RD, Congdon JD (1998) Elevated maintenance costs in an anuran (Rana catesbeiana) exposed to a mixture of trace elements during the embryonic and early larval periods. Physiol Zool 71:27–35
Semlitsch RD, Bodie JR (2003) Biological criteria for buffer zones around wetlands and riparian habitats for amphibians and reptiles. Conserv Biol 17:1219–1228. doi:10.1046/j.1523-1739.2003.02177.x
Snodgrass JW, Casey RE, Joseph D, Simon JA (2008) Microcosm investigations of stormwater pond sediment toxicity to embryonic and larval amphibians: variation in sensitivity among species. Environ Pollut 154:291–297. doi:10.1016/j.envpol.2007.10.003
Snodgrass JW, Casey RE, Simon JA, Gangapura K (in press) Ecotoxicology of amphibians and reptiles in urban environments: an overview of potential exposure routes and bioaccumulation. In: Mitchell JC, Jung-Brown RE, Mitchell JC, Jung-Brown RE, Walls SC (eds) Urban herpetology. Herpetological conservation vol 3. Society for the Study of Amphibians and Reptiles, Salt Lake City, Utah
Snodgrass JW, Hopkins WA, Jackson BP, Baionno JA, Broughton J (2005) Influence of larval period on responses of overwintering green frog (Rana clamitans) larvae exposed to contaminated sediments. Environ Toxicol Chem 24:1508–1514. doi:10.1897/04-339R1.1
Snodgrass JW, Komoroski MJ, Bryan AL, Burger J (2000) Relationships among isolated wetland size, hydroperiod, and amphibian species richness: implications for wetland regulations. Conserv Biol 14:414–419. doi:10.1046/j.1523-1739.2000.99161.x
Sparling DW, Eisemann JD, Kuenzel W (2004) Contaminant exposure and effects in red-winged blackbirds inhabiting stormwater retention ponds. Environ Manage 33:719–729. doi:10.1007/s00267-003-0058-6
Stahre P, Urbonas B (1990) Stormwater detention: for drainage, water quality and CSO management. Prentice Hall, Englewood Cliffs, NJ
Wilbur HM (1980) Complex life cycles. Annu Rev Ecol Syst 11:67–93. doi:10.1146/annurev.es.11.110180.000435
Williams M (1990) Wetlands: a threatened landscape. Basil Blackwell, Cambridge, MA
Acknowledgements
We thank Donald C. Forester and Richard A. Siegel for advice and comments on earlier drafts of the manuscript. We thank Robin Jung, Evan Grant, and Priya Nanjappa for project guidance, logistical support, and field assistance. Steve Lev assisted with ICPMS. Isaac Chellman, Sarah Faust, Sheera Schneider, Lindsay Funk, Stephanie Atueyi, Tammy Fuehrer, Debra Joseph, Clint Otto, Robin Rauch and Amanda Richardson assisted with field collections. Acquisition of aerial photography was made possible by Vargis LLC, local county governments (Anne Arundel County, Howard County, and Prince George’s County, MD), and the Maryland State Highway Administration. Project funding was provided by the Northeast region of the USGS Amphibian Research and Monitoring Initiative (ARMI).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Simon, J.A., Snodgrass, J.W., Casey, R.E. et al. Spatial correlates of amphibian use of constructed wetlands in an urban landscape. Landscape Ecol 24, 361–373 (2009). https://doi.org/10.1007/s10980-008-9311-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-008-9311-y