Abstract
In an increasingly human-dominated landscape, effective management of disturbance-maintained ecosystems, such as grasslands and savannas, is critical to the conservation of biodiversity. Yet, the response of individual organisms to landscapes created by disturbances and management is rarely studied. In this study, we examined the endangered Karner blue butterfly, Lycaeides melissa samuelis, in a heterogeneous oak savanna. Our objective was to quantify the butterfly’s habitat use and behavior to assess the effects of prescribed burning. The oak savanna management in Ohio, USA divides each Karner blue site (n = 4) into three units. Each one-third unit is then burned, mowed, or unmanaged in an annual rotation within each site, and the result is a fire return interval of ~3 years. Our surveys measured habitat use, while behavior observations quantified reproduction and foraging for the two annual broods. Our habitat use results showed burned treatments were recolonized quickly, but there was not a clear selection for burned treatments. Foraging rates were similar in all treatments; however, females oviposited significantly less in unmanaged treatments (only 5 of 127 ovipositions). This oviposition preference was likely due to habitat degradation and the availability of recently burned, early successional habitat. Since Karner blues avoided reproduction in units unburned for ≥4 years, these units could be burned to create high quality early successional habitat. These results demonstrate how behavioral decisions can be pivotal forces driving spatial population dynamics. Our case study demonstrates how a fine-scale landscape perspective combined with measurements of behavioral processes can assist with management decision-making.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Active management is critical for maintaining ecosystems, communities, and species in an increasingly human-dominated landscape (also see Ratti and Garton 1996; Meffe et al. 2006). Management is particularly important in disturbance-maintained ecosystems, since humans have severely altered natural processes, such as fires and seasonal flooding (Nuzzo 1986; Gergel et al. 2002; Huntzinger 2003). Early successional wetlands, grasslands, and savannas are now some of the most imperiled ecosystems in North America (Noss and Peters 1995; Askins 2000) due to the loss of natural disturbances. Disturbances, and management meant to mimic natural disturbances, generally increase heterogeneity within landscapes (Pickett and White 1985; Turner et al. 2001; Fuhlendorf et al. 2006) and result in a landscape with differential habitat quality. However, habitat quality and the effect of management are difficult to determine for many species.
Habitat quality is most often quantified by estimating the resources available, species abundance, density, or reproductive success (Johnson 2007). Rare or elusive species, such as endangered species and invertebrates, present a formidable challenge to abundance and demographic rate estimation. Either long-term data on abundance (Harpole and Haas 1999) or a large number of sample locations for metapopulation studies (Moilanen and Hanski 1998; Bergman and Kindvall 2004; WallisDeVries 2004) are needed to evaluate the effects of management. In addition, few studies have experimentally shown the effects of management for insect species of conservation concern (e.g., Schultz and Crone 1998). Yet, these species often inhabit early successional habitat in need of active management.
In this study, our objective was to quantify the habitat use and behavior of a rare butterfly to identify the effects of management in a disturbance-maintained landscape. While invertebrate studies have previously addressed dispersal behavior (Schultz 1998; Haddad 2000) and patterns on the landscape (Hanski et al. 1996; Yaacobi et al. 2007), very little is known about fine-scale behavioral responses to heterogeneous environments. Assessing management by the behavior of a species has the following advantages (1) behavior provides an immediate, measurable response to rapidly changing environments, such as degrading habitats; (2) these methods can provide a robust sample size within a single year; (3) there is no dependence on finding eggs, larvae, or juveniles for demographic modeling; (4) behavior can be indicative of the fitness of individuals (e.g., Elias et al. 2000; Mack and Clark 2006; Mainguy et al. 2006).
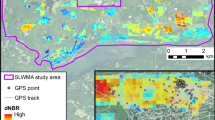
Our investigation focused on the federally endangered Karner blue butterfly (Karner blue), Lycaeides melissa samuelis, in a heterogeneous oak savanna, which has been maintained by annual prescribed burning, mowing, and leaving areas unmanaged. Midwestern oak savanna is highly imperiled since only 0.02% of its historical 11–13 million hectares remain (Nuzzo 1986). Additionally, effective management of the ecosystem is critical to numerous species of concern [e.g., lark sparrows (Chondestes grammacus), red-headed woodpeckers (Melanerpes erythrocephalus), frosted elfins (Callophrys irus), and over 500 savanna plant species]. Prescribed burning is necessary to maintain the structure and composition of oak savanna (Peterson and Reich 2001; Brawn 2006), but has also been found to destroy Karner blue eggs (Swengel 1995). Therefore, managers and biologists require an approach to rapidly assess the effects of savanna degradation and prescribed burning on the Karner blue. The current management strategy in Ohio, USA has differing management treatments within 120 m of each other (Fig. 1), so habitat selection by Karner blues is possible in this system (see Knutson et al. 1999). Our overall objective was to assess the effects of prescribed burning on Karner blues. However, we specifically hypothesized that abundance and oviposition rates would be higher in burned treatments due to the positive response of their host-plant (Grigore and Tramer 1996) and general savanna degradation in unmanaged treatments.
Methods
Species of interest
The Karner blue (Family Lycaenidae) has two broods per year and adults live an average of 3.5 days (Knutson et al. 1999). During May and June, the first brood adults oviposit and the eggs hatch within 5–10 days. The second brood of Karner blues oviposit eggs that overwinter until the following April; management occurs during this overwintering period. The Karner blues’ host-plant, wild blue lupine (Lupinus perennis), is a perennial plant that lives in partially shaded to open areas, nutrient poor soils, and early successional habitats (U.S. Fish and Wildlife Service 2003).
Study area
Karner blues currently occupy four sites in Ohio, USA. These sites are at The Nature Conservancy’s Kitty Todd Preserve located in Lucas County (41° 37′N, 083° 47′W). The region receives a mean precipitation of 840 mm per year, and mean temperatures range from −4.5°C in January to 22°C in July (NOAA 2006). Elevation ranges from 154 to 254 m, and soils are primarily well-drained and sandy. The plant community is globally rare black oak/lupine savanna (NatureServe 2006). Dominant woody vegetation includes Quercus velutina, Q. ellipsoidalis, and Q. alba with a tallgrass prairie herbaceous layer. Each Karner blue site ranged from 0.39 to 2.15 ha in size; tree canopy covered 56–61% of the area at three sites and only 4% of the fourth site.
Management
After the Karner blue was reintroduced into Ohio in 1998, no management activity was performed in occupied Karner blue habitat until 2001. Since 2001, endangered species permits have been issued to burn 1/3 and mow 1/3 of lupine stems (representative of lupine quantity) at each occupied Karner blue site, leaving 1/3 of the lupine stems unmanaged on an annual basis. These management treatments have been rotated annually within each site and result in a fire return interval of ~3 years for each 1/3 unit. For example, unit “A” (1/3 of a site) is burned in year 1, mowed in year 2, and unmanaged in year 3; then the cycle returns and the unit is burned in year 4, etc. In our study, management units corresponded to the following years since burning: 0 years (burned treatments), 1–2 years (mowed treatments), and 4–7 years (unmanaged treatments). Low fuel loads prevented one unit from being burned in 7 years, while the other unmanaged treatments in our study were burned 4 years ago. These unmanaged units were scheduled to be burned the year after our study. In 2005, approximately a third of each occupied site’s lupine stems were burned, mowed, or left unmanaged in accordance with the rotation (Fig. 1). Burning occurred in the winter of 2004–2005, and mowing occurred in March 2005.
Sampling design
Despite management aimed solely at the quantity of host-plants, we wanted to account for shade and area differences that could affect our results. Therefore, we measured unit area, percent of the unit shaded, percent of lupine stems shaded, and total lupine stems within each management unit, and four study sites were included in our study (totaling 12 individual units). We measured habitat use with daily Karner blue surveys within units, and behavior observations were then related to the units, including the management treatment in the year of our study.
Between November 2004 and April 2005, we flagged the border of each burned, mowed, and unmanaged treatment to facilitate their identification. After the lupine bloomed in May, we used a GPS unit (Trimble Pro XRS, Trimble, Sunnyvale, CA) with an accuracy <1 m to map lupine areas according to their management treatment. This defined each management unit, and guided vegetation surveys. Lupine plants covering <1 m2 and >10 m from other lupine were excluded from these maps. GPS points were imported to ArcGIS 8.3 (ESRI, Redlands, CA) and we created polygons of lupine management units.
In May 2005, vegetation surveys were performed on a 10 × 10 m or 10 × 15 m grid, depending on the size of the unit. At each survey location, we recorded the number of lupine stems within a 1 m2 quadrat and took a digital photograph of canopy cover (Nikon Coolpix2000, El Segundo, CA). Lupine density was estimated by counting individual stem sprouts, since individual lupines can be difficult to distinguish (Grigore and Tramer 1996). Canopy cover was estimated by transferring digital photographs to Adobe PhotoShop 7.0 (Adobe Systems, San Jose, CA), and the percent canopy cover was estimated using the procedure of Klingenbock et al. (2000). We then calculated the percentage of management unit area with >15% canopy cover.
We used modified Pollard–Yates transects (Thomas 1983; Pollard and Yates 1993) to count Karner blues in each management unit for the first and second brood in 2005. One to three trained observers performed surveys along transects throughout all areas of lupine within a particular management unit, and the number of Karner blue females and males within 3.5 m of the observer were recorded. These counts were performed daily when weather was conducive to butterfly surveys (Pollard and Yates 1993). The sex was recorded for each observed butterfly in addition to the initial management unit where the butterfly was observed.
When a female Karner blue was observed, we performed a 15 min behavior observation. During the second brood, 16 of 121 observations were performed for only 10 min since a larger population of butterflies was anticipated. During behavior observations, we recorded “foraging” or “not foraging” at 1 min intervals. Foraging was defined as when a butterfly was directly on a flower head and the proboscis was extended for any period of time. If a Karner blue foraged at any time during a minute of observation, the minute was counted as foraging. This accounted for butterflies foraging, and then briefly searching for more flowers, before continuing to forage. Ovipositions occurred when a Karner blue crawled down a plant stem, usually lupine, flexed its abdomen, and deposited an egg. After observations, we recorded total time of observation, number of ovipositions in each management unit, and visually estimated the percent canopy cover of oviposition locations.
Analysis
SAS 9.1 (SAS Institute 2004) was used for all data analysis and we used α = 0.05. We used SAS Generalized Linear Mixed Models (Proc GLIMMIX) with a Poisson distribution to analyze survey data. The lupine and canopy cover measurements, at the scale of management units, were used to account for differences in unit area, stems, and percent of area shaded. A priori, we decided to use site as a random effect, area (m2) as a covariate, and percent of unit shaded and current management treatment as main effects in the models. We used Karner blue abundance as the dependent variable. Type III tests for fixed effects determined if each main effect should be included in the final model via a backwards selection process (Der and Everitt 2002). Contrasts were used to compare burned versus mowed and burned/mowed versus unmanaged treatments when applicable.
Behavior analysis determined if female Karner blues were in a particular management unit for oviposition or foraging behavior. A priori, we discarded observations ≤5 min since not all butterflies could be followed after the initial observation, and no butterflies oviposited during these short observation periods. For oviposition analysis, we assumed each butterfly had an opportunity to oviposit in the initial survey management unit it was observed within. Given that Grundel et al. (1998) reported only 8.4% of females moved >10 m during 10 min observations, this was a reasonable assumption. However, on four of 183 observations (2%), we had butterflies oviposit in two management units. We treated each of these as two separate observations, since the butterflies had an opportunity to oviposit in both units. To analyze oviposition data, we used a GLIMMIX with a negative binomial distribution, which uses an extra parameter to adjust for the variance in the data set (Quinn and Keough 2002). In this model, we used site as a random effect, brood as a covariate, and initial management treatment and percent of unit shaded as a main effects to explain total ovipositions.
Since flower species, flower abundance, and Karner blue foraging time differed by brood (Pickens 2006), we analyzed the foraging rate separately for each brood. We used a GLIMMIX model with a Possion distribution to fit the data. The foraging rate distribution was underdispersed, so we corrected the model with the SAS quasi-likelihood function to adjust the scale parameter based on the Pearson χ2 (Quinn and Keough 2002). We used site as a random effect with management treatment and percent of unit shaded as main effects. Foraging was not recorded per management unit in the field, so we assumed that foraging was done in the initial survey management unit. A priori, we eliminated the four observations when a butterfly oviposited in multiple management units.
Results
We performed 168 management unit surveys for the first brood and 203 surveys for the second brood. There were 146 males and 58 females observed in the first brood; 130 males and 124 females were observed in the second brood. We used area of unit and percent of unit shaded as variables instead of host-plant stems and percent of stems shaded, since unit area and stems were highly correlated (Pearson correlation, n = 12, r = 0.70, P = 0.011), and area explained more variation in Karner blue abundance.
For the first brood of Karner blues, neither males nor females showed any difference in their use of the three management treatments (Table 1). For the second brood females, management treatment was a significant effect. Furthermore, the contrasts revealed that burned treatments were favored over mowed treatments (Table 1). We observed no difference in female abundance between burned/mowed (managed) and unmanaged treatments. Second brood males were not influenced by management treatments. Area of unit was positively associated with Karner blue male and female abundance in both broods. The percent of unit shaded was negatively associated with both broods of females and the second brood of males. This corresponds to more butterflies in more open, sunny units.
Our behavior observations determined that management treatments directly influenced Karner blue oviposition behavior, while Karner blue foraging rates were unaffected by treatments. Forty-six ovipositions were observed from 60 observations for the first brood, and 81 ovipositions were observed from 122 observations during the second brood. Overall, management treatments had an effect on oviposition rates (F 2,175 = 3.75, P = 0.026), but shade had no effect on ovipositions (F 1,174 = 1.06, P = 0.304). Our contrasts revealed that Karner blues had a higher oviposition rate in burned and mowed treatments compared to unmanaged treatments (F 1,175 = 7.43, P = 0.007) (Fig. 2). A total of 5 of the 127 ovipositions were in unmanaged treatments, and all five occurred at one study site. There was no difference in oviposition rate between the burned and mowed management treatments (F 1,175 = 0.00, P = 0.963) (Fig. 2). Oviposition rates did not differ between broods (F 1,175 = 0.47, P = 0.494). At the individual scale, we observed two ovipositions by one butterfly on lupine shaded by canopy cover. All other ovipositions were on host-plants with <16% canopy cover.
In contrast to reproductive behavior, foraging behavior was not influenced by management treatment. Foraging rate (foraging min/total min observed) did not differ by management treatment for the first brood (F 2,45 = 0.41, P = 0.664) or the second brood (F 2,89 = 1.33, P = 0.269) (Fig. 3). Likewise, shade was not associated with foraging rate for the first brood (F 1,47 = 0.13, P = 0.719) or second brood (F 1,91 = 3.80, P = 0.054). Although not statistically significant, second brood females tended to forage more when in open, sunny units.
Discussion
In this endangered insect population, quantifying the fine-scale habitat use and behavior of the Karner blue showed that butterflies avoided reproduction in units left unburned for ≥4 years. Our results indicated butterflies recolonized burned units quickly, presumably as a result of the close proximity to the other management units. In addition, second brood females used burned treatments more than mowed treatments. Most importantly, Karner blues had a very low oviposition rate in unmanaged treatments compared to burned and mowed treatments (Fig. 2). This included no observed ovipositions in three of the four unmanaged units. Meanwhile, oviposition rates were similar for both burned and mowed treatments. The foraging analysis showed Karner blues foraged at similar rates in all management treatments. These combined results can be directly applied to plan for prescribed burning with the objective of maximizing preferred reproductive habitat for Karner blues.
Our survey analysis had mixed results in regards to management. We found female Karner blues of both broods were more abundant in open, sunny units compared to more shaded units. We believe caution should be used in interpreting this result because the site with the most Karner blues had virtually no tree cover. Therefore, no selection of shaded units was possible at the site. The shade variable may have also confounded more clear management effects, but this remains uncertain. Alternatively, Karner blues could simply be moving randomly at the scale of our sites, and then choosing oviposition locations selectively. The positive association of area and Karner blue abundance supports this conclusion (Table 1). We found that Karner blues foraged on nectar plants without regard to management treatment and this may reflect localized conditions such as the location of plantings (i.e., oak savanna restoration) and the preferred habitats of nectar plants (e.g., sunny vs. shaded). For instance, large New Jersey tea (Ceanothus americanus) were located in distinct locations year after year, regardless of annual management treatments (pers. observation). Grundel et al. (1998) also found that Karner blues used canopy openings extensively for foraging. Therefore, our survey results may not show clear management effects because of the Karner blues’ differing requirements for foraging and reproduction.
Karner blue behavioral decisions within units gave us critical insights about the use of management treatments for reproduction and foraging. Karner blues oviposited more frequently in the burned and mowed treatments compared to the unmanaged treatments. We did not examine the causal factor for this preference, but there are a multitude of oviposition studies that indicate butterflies use cues such as host-plant nitrogen (Myers 1985; Ellis 2003; Prudic et al. 2005), size of host-plants or leaves (2003), ants (Pierce and Elgar 1985; Fraser et al. 2002), and host-plant scent (Feeny et al. 1989) to determine oviposition preferences. However, few studies use this lab-based information in a landscape or a conservation context. The burned, mowed, and unmanaged treatments in our study did not have different host-plant quality according to leaf nitrogen and water analysis (Pickens and Root 2008), so differential larval survival was unlikely within the year of our study. However, we expected annual degradation of unburned oak savanna and the loss of host-plants due to encroaching woody vegetation (Peterson and Reich 2001), increases in leaf litter, and changes in herbaceous species composition (Tester 1996). For example, leaf litter depth was (mean ± SD) 0.69 cm ± 0.77 for burned, 2.2 ± 0.95 for mowed, and 3.81 ± 1.22 for unmanaged treatments (Pickens 2006). Woody vegetation such as sassafras (Sassafras albidum) and black oaks (Quercus velutina) also invaded most of our management units that had been unburned for ≥4 years. In fact, Grigore and Tramer (1996) did find lower L. perennis abundance in areas without regular burning, and one possible mechanism for this decrease is low host-plant survival due to oak savanna degradation. Therefore, we hypothesize that host-plant survival is variable based on successional stage, and Karner blues may prefer to oviposit in habitat conditions conducive to high host-plant survival (e.g., low leaf litter, little woody vegetation).
Habitat degradation has been modeled for several species (Doak 1995; Schultz and Crone 1998; Akcakaya et al. 2004), but the direct effect of degradation remains difficult to measure (Doak 1995). Specifically, data are lacking on the behavioral response of species in landscapes that change both temporally and spatially. While habitat selection is fundamental to landscape ecology (Morris and Brown 1992), we found specific behavioral decisions to be critical for understanding the spatial dynamics of the Karner blue in response to a temporally changing, successional landscape. Invertebrates and other disturbance-dependent organisms (e.g., plants, birds) are likely to experience inversions of habitat quality; for example, land managers burn poor quality, late successional habitat to create high quality habitat for early successional species. In our study, we had a human-induced inversion of habitat quality via prescribed burning. Basically, unmanaged units in 2004 were burned the year of our study (2005). Karner blues ovipositing in burned and mowed units in 2004 had their larvae emerge in mowed and unmanaged units in 2005. These butterflies could then recolonize the burned unit in our study year. Our results show Karner blues prefer not to oviposit in units that are unburned for ≥4 years. To manage for this reproductive preference, burning must be performed regularly and a source population must be able to quickly colonize burned treatments. This conclusion is similar to previous studies of plants (MacDougall and Turkington 2006) and birds (Jenkins et al. 2003) where the proximity of revitalized habitat is critical for species persistence. The proximity of our management treatments was within daily movement distances of Karner blues (see Knutson et al. 1999), and allowed for a plastic behavioral response to the environment, which is likely to benefit the species.
Conclusions
Our investigation showed that quantifying insect behavior can assist in determining the effects of management. Our study contributes to the growing number of case studies, which are necessary to understand the effects of disturbance on populations (Brawn 2006). This information is vitally important when prescribed burns are needed to sustain oak savanna, while minimizing short-term negative effects to sensitive populations. Our behavioral approach was successful in identifying critical reproductive habitat in the landscape, and the results can be directly applied to make adaptive management decisions in this disturbance-maintained ecosystem. We recommend burning oak savanna with a 3–4 year fire return interval based on the Karner blue’s oviposition preferences. This interval may differ in habitats with more or less habitat degradation. Oak savanna birds and other insects should also benefit from regular burning, since fire suppression is a major reason for oak savanna loss in Ohio and elsewhere (Nuzzo 1986). In regard to other species in disturbance-maintained landscapes, we suggest that the behavior of the organism is given strong consideration in management decision-making. In particular, the abundance of insects in a habitat does not necessarily indicate reproduction or reproductive preferences. Fine-scale studies of habitat use and behavior will continue to further our understanding of how species react to disturbance and heterogeneity.
References
Akcakaya RH, Radeloff VC, Mladenoff DJ, He HS (2004) Integrating landscape and metapopulation modeling approaches: viability of the sharp-tailed grouse in a dynamic landscape. Conserv Biol 18:526–537. doi:10.1111/j.1523-1739.2004.00520.x
Askins RA (2000) Restoring North America’s birds: lessons from landscape ecology, 2nd edn. Yale University Press, New Haven
Bergman KO, Kindvall O (2004) Population viability analysis of the butterfly Lopinga achine in a changing landscape in Sweden. Ecography 27:49–58. doi:10.1111/j.0906-7590.2004.03629.x
Brawn JD (2006) Effects of restoring oak savannas on bird communities and populations. Conserv Biol 20:460–469. doi:10.1111/j.1523-1739.2006.00310.x
Der G, Everitt B (2002) A handbook of statistical analyses using SAS, 2nd edn. Chapman & Hall, London
Doak DF (1995) Source-sink models and the problem of habitat degradation: general models and applications to the Yellowstone grizzly. Conserv Biol 9:1370–1379. doi:10.1046/j.1523-1739.1995.09061370.x
Elias SP, Fraser JD, Buckley PA (2000) Piping plover brood foraging ecology on New York barrier islands. J Wildl Manag 64:346–354. doi:10.2307/3803232
Ellis S (2003) Habitat quality and management for the northern brown argus butterfly Aricia Artaxerxes (Lepidoptera: Lycaenidae) in North East England. Biol Conserv 113:285–294. doi:10.1016/S0006-3207(02)00376-2
Feeny P, Stadler E, Ahman I, Carter M (1989) Effects of plant odor on oviposition by the black swallowtail butterfly, Papilio polyxenes (Lepidoptera: Papilionidae). J Insect Behav 2:803–827. doi:10.1007/BF01049402
Fraser AM, Tregenza T, Wedell N, Elgar MA, Pierce NE (2002) Oviposition tests of ant preference in a myrmecophilous butterfly. J Evol Biol 15:861–870. doi:10.1046/j.1420-9101.2002.00434.x
Fuhlendorf SD, Harrell WC, Engle DM, Hamilton RG, Davis CA, Leslie J, David M (2006) Should heterogeneity be the basis for conservation? Grassland bird response to fire and grazing. Ecol Appl 16:1706–1716. doi:10.1890/1051-0761(2006)016[1706:SHBTBF]2.0.CO;2
Gergel SE, Dixon MD, Turner MG (2002) Consequences of human-altered floods: levees, floods, and floodplain forests along the Wisconsin River. Ecol Appl 12:1755–1770. doi:10.1890/1051-0761(2002)012[1755:COHAFL]2.0.CO;2
Grigore MT, Tramer EJ (1996) The short-term effect of fire on Lupinus perennis (L.). Nat Areas J 16:41–48
Grundel R, Pavlovic NB, Sulzman CL (1998) Habitat use by the endangered Karner blue butterfly in oak woodlands: the influence of canopy cover. Biol Conserv 85:47–53. doi:10.1016/S0006-3207(97)00165-1
Haddad N (2000) Corridor length and patch colonization by a butterfly, Junonia coenia. Conserv Biol 14:738–745. doi:10.1046/j.1523-1739.2000.99041.x
Hanski I, Moilanen A, Pakkala T, Kuussaari M (1996) The quantitative incidence function model and persistence of an endangered butterfly metapopulation. Conserv Biol 10:578–590. doi:10.1046/j.1523-1739.1996.10020578.x
Harpole DN, Haas CA (1999) Effects of seven silvicultural treatments on terrestrial salamanders. For Ecol Manage 114:349–356
Huntzinger M (2003) Effects of fire management practices on butterfly diversity in the forested western United States. Biol Conserv 113:1–12. doi:10.1016/S0006-3207(02)00356-7
Jenkins CN, Powell RD, Bass OL Jr, Pimm SL (2003) Why sparrow distributions do not match model predictions. Anim Conserv 6:39–46. doi:10.1017/S1367943003003068
Johnson MD (2007) Measuring habitat quality: a review. Condor 109(3):489–504. doi:10.1650/8347.1
Klingenbock A, Osterwalder K, Shine R (2000) Habitat use and thermal biology of the “Land Mullet” Egernia major, a large Scincid lizard from remnant rain forest in Southeastern Australia. Copeia 4:931–939. doi:10.1643/0045-8511(2000)000[0931:HUATBO]2.0.CO;2
Knutson RL, Kwilosz JR, Grundel R (1999) Movement patterns and population characteristics of the Karner blue butterfly (Lycaeides melissa samuelis) at Indiana Dunes National Lakeshore. Nat Areas J 19:109–120
MacDougall AS, Turkington R (2006) Dispersal, competition, and shifting patterns of diversity in a degraded oak savanna. Ecology 87:1831–1843. doi:10.1890/0012-9658(2006)87[1831:DCASPO]2.0.CO;2
Mack GG, Clark RG (2006) Home-range characteristics, age, body size, and breeding performance of female mallards (Anas platyrhynchos). Auk 123:467–474. doi:10.1642/0004-8038(2006)123[467:HCABSA]2.0.CO;2
Mainguy J, Gauthier G, Giroux JF, Bety J (2006) Gosling growth and survival in relation to brood movements in greater snow geese (Chen caerulescens atlantica). Auk 4:1077–1089. doi:10.1642/0004-8038(2006)123[1077:GGASIR]2.0.CO;2
Meffe GK, Groom MJ, Carroll RC (2006) Ecosystem approaches to conservation. In: Groom MJ, Meffe GK, Carroll RC (eds) Principles of conservation biology, 3rd edn. Sinnauer associates inc, Sunderland, pp 467–508
Moilanen A, Hanski I (1998) Metapopulation dynamics: effects of habitat quality and landscape structure. Ecology 79:2503–2515
Morris DW, Brown JS (1992) The role of habitat selection in landscape ecology—introduction. Evol Ecol 6:357–359. doi:10.1007/BF02270697
Myers JH (1985) Effect of physiological condition of the host plant on the ovipositional choice of the cabbage white butterfly, Pieris rapae. J Anim Ecol 54:193–204. doi:10.2307/4630
NatureServe (2006) NatureServe Explorer: An online encyclopedia of life. [web application], http://www.natureserve.org/explorer Version 4.6. NatureServe, Arlington, VA
NOAA (2006) National weather service forecast office: climate data-Toledo OH. National Oceanic and Atmospheric Administration, Asheville
Noss RF, Peters RL (1995) Endangered ecosystems of the United States: a status report and plan for action. Defenders of Wildlife, Washington
Nuzzo VA (1986) Extent and status of midwest oak savanna: presettlement and 1985. Nat Areas J 6:6–36
Peterson DW, Reich P (2001) Prescribed fire in oak savanna: fire frequency effects on stand structure and dynamics. Ecol Appl 11:914–927. doi:10.1890/1051-0761(2001)011[0914:PFIOSF]2.0.CO;2
Pickens BA (2006) The consequences of a management strategy for the endangered Karner blue butterfly. MS thesis, Bowling Green State University
Pickens BA, Root KV (2008) Factors affecting host-plant quality and nectar use for the Karner blue butterfly: implications for oak savanna restoration. Nat Areas J 28:210–217. doi:10.3375/0885-8608(2008)28[210:FAHQAN]2.0.CO;2
Pickett S, White PS (1985) The ecology of natural disturbance and patch dynamics. Academic Press, San Diego
Pierce NE, Elgar MA (1985) The influence of ants on host plant selection by Jalmenus evagoras, a myrmecophilous Lycaenid butterfly. Behav Ecol Sociobiol 16:209–222. doi:10.1007/BF00310983
Pollard E, Yates TJ (1993) Monitoring butterflies for ecology and conservation, 1st edn. Chapman & Hall, London
Prudic K, Oliver J, Bowers M, Schmitz O (2005) Soil nutrient effects on oviposition preference, larval performance, and chemical defense of a specialist insect herbivore. Oecologia 143:578–587. doi:10.1007/s00442-005-0008-5
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, New York
Ratti JT, Garton EO (1996) Research and experimental design. In: Bookhout TA (ed) Research and management techniques for wildlife and habitats, 5th edn. The Wildlife Society, Bethesda, pp 1–23
SAS Institute Inc (2004) Release: SAS 9.1 Edition. SAS Institute, Cary
Schultz CB (1998) Dispersal behavior and its implications for reserve design in a rare Oregon butterfly. Conserv Biol 12:284–292. doi:10.1046/j.1523-1739.1998.96266.x
Schultz CB, Crone EE (1998) Burning prairie to restore butterfly habitat: a modeling approach to management tradeoffs for the Fender’s blue. Restor Ecol 6:244–252. doi:10.1046/j.1526-100X.1998.00637.x
Swengel AB (1995) Observations of spring larvae of Lycaeides melissa samuelis (Lepidoptera: Lycaenidae) in central Wisconsin. Gt Lakes Entomol 28:155–170
Tester JR (1996) Effects of fire frequency on plant species in oak savanna in east-central Minnesota. Bull Torrey Bot Club 123:304–308. doi:10.2307/2996779
Thomas JA (1983) A quick method for estimating butterfly numbers during surveys. Biol Conserv 27:195–211. doi:10.1016/0006-3207(83)90019-8
Turner MG, Gardner RH, O’Neill RV (2001) Landscape ecology in theory and practice. Springer, New York
US Fish and Wildlife Service (2003) Karner blue butterfly recovery plan (Lycaeides melissa samuelis). Department of the Interior, U.S. Fish and Wildlife Service, Great Lakes- Big Rivers Region (Region 3)
WallisDeVries MF (2004) A quantitative conservation approach for the endangered butterfly Maculinea alcon. Conserv Biol 18:489–499
Yaacobi G, Ziv Y, Rosenzweig ML (2007) Effects of interactive scale-dependent variables on beetle diversity patterns in a semi-arid agricultural landscape. Landscape Ecol 22:687–703. doi:10.1007/s10980-006-9061-7
Acknowledgments
The Ohio Department of Natural Resources, Division of Wildlife provided funding support for this project through a State Wildlife Grant; an additional grant was provided by the Ohio Biological Survey. We are thankful for the many people who were supportive of this project: G. Haase (The Nature Conservancy), P. Tolson and C. Ellsworth (Toledo Zoo) were especially helpful. We are grateful for the data collection efforts of E. Knurek, M. Ricci, and R. Kip. J. Bouzat, C. Pickens and H. Michaels of Bowling Green State University (BGSU) provided valuable insights throughout this project. We thank H. Gee, S. Kang, P. Newell (LSU), and 2 anonymous reviewers for their revisions of the manuscript, along with M. Kaller (LSU) for statistical advice. Endangered species permits from the U.S. Fish and Wildlife Service were obtained through the Ohio Division of Wildlife. We are appreciative of the BGSU Geology Department and the BGSU Statistical Consulting Center for providing equipment and expertise.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pickens, B.A., Root, K.V. Behavior as a tool for assessing a managed landscape: a case study of the Karner blue butterfly. Landscape Ecol 24, 243–251 (2009). https://doi.org/10.1007/s10980-008-9302-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-008-9302-z