Abstract
Denervation causes muscle atrophy and incapacity in humans. Although electrical stimulation (ES) and stretching (St) are commonly used in rehabilitation, it is still unclear whether they stimulate or impair muscle recovery and reinnervation. The purpose of this study was to evaluate the effects of ES and St, alone and combined (ES + St), on the expression of genes that regulate muscle mass (MyoD, Runx1, atrogin-1, MuRF1 and myostatin), on muscle fibre cross-sectional area and excitability, and on the expression of the neural cell adhesion molecule (N-CAM) in denervated rat muscle. ES, St and ES + St reduced the accumulation of MyoD, atrogin-1 and MuRF1 and maintained Runx1 and myostatin expressions at normal levels in denervated muscles. None of the physical interventions prevented muscle fibre atrophy or N-CAM expression in denervated muscles. In conclusion, although ES, St and ES + St changed gene expression, they were insufficient to avoid muscle fibre atrophy due to denervation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rehabilitation of denervated muscles still remains a clinical challenge (Sheffler and Chae 2007; Ashley et al. 2008) because muscle recovery after denervation is a very slow and often incomplete process. Muscle denervation is caused mainly by degenerative modifications in the skeletal muscle and leads to functional limitations during daily activities, including muscle fibre atrophy (Bodine et al. 2001; Dow et al. 2004; Russo et al. 2007) and the loss of muscle force generation capacity (Dow et al. 2004).
Adult muscle maintenance is strongly linked to an orchestrated process of molecular regulation between protein synthesis and degradation (Glass 2005; Lagirand-Cantaloube et al. 2008). The process of denervation decreases or ceases muscle activity, which leads to severe consequences, such as higher rates of protein breakdown compared to protein synthesis and muscle atrophy (Glass 2003; Jones et al. 2004; Liu et al. 2007). Several genes, such as myogenic regulatory factors (MRFs), are directly involved in this process, and myogenic differentiation 1 (MyoD) plays an important role in it. MyoD has been shown to be highly sensitive to both increased and reduced mechanical stress and to the absence or presence of electrical activity (Legerlotz and Smith 2008). An increase in MyoD expression could also be considered an attempt by the muscle to regain sensitivity to neural activity, given the increased expression of acetylcholine receptors after denervation (Buonanno et al. 1992).
The atrophy observed in denervated muscles involves a common proteolytic program via activation of the ubiquitin-proteasome pathway (Bodine et al. 2001; Jackman and Kandarian 2004). In this program, there are two important enzymes responsible for identifying the proteins which will be degraded by the proteasome: atrogin-1 (Gomes et al. 2001) also known as muscle atrophy F-Box (MAFbx; Bodine et al. 2001) and muscle-specific RING finger protein-1 (MuRF1; Bodine et al. 2001). Atrogin-1 contains an F-box domain, a characteristic motif found in a family of E3 ubiquitin ligases called SCF (skp1-Cul1-F-box; Glass 2003). MuRF1 has a RING domain at its amino-terminal end, as well as two coiled-coil domains in its central region (Cao et al. 2005).
In multiple models of skeletal muscle atrophy, including denervation (Bodine et al. 2001), atrogin-1 and MuRF1 are up-regulated, and they are essential to accelerated muscle protein loss, regulating muscle size via protein breakdown (Bodine et al. 2001; Gomes et al. 2001). Another important process responsible for the maintenance of cellular homeostasis is autophagy; this is a tightly regulated pathway for the degradation and recycling of proteins transported to lysosomes (Malicdan et al. 2009). Runt-related transcription factor 1 (Runx-1) is involved in this pathway. Runx-1 expression in innervated muscle is minimal, and it occurs in the absence of electrical activity as in the case of denervation (Zhu et al. 1994). Furthermore, it has been demonstrated that the expression of Runx-1 is required for the maintenance of denervated myofibre structure, preventing autophagy and wasting of skeletal muscle (Wang et al. 2005). Therefore, it is important to investigate Runx-1 expression in denervated muscle under therapeutic modalities of intervention.
Myostatin is also relevant in muscle mass control. It is a member of the transforming growth factor-beta (TGF-β) family, and it has been shown to be a strong negative regulator of muscle growth (Sharma et al. 2001). Its role in denervated muscles seems to inhibit hypertrophy of skeletal muscle rather than to induce atrophy (Wehling et al. 2000; Zhang et al. 2006).
Regarding the functional recovery of denervated muscles, two important strategies are used in human rehabilitation: electrical stimulation (ES) and stretching (St). Previous studies reported that ES is able to prevent muscle fibre atrophy (Dow et al. 2004, 2005) and control molecular modifications (Kostrominova et al. 2005; Russo et al. 2007) inherent to denervation. It appears that the total number of contractions per day is the most important aspect of avoiding degenerative modifications in denervated muscles (Dow et al. 2004; Kostrominova et al. 2005). Previous data have proposed that a few contractions (20 contractions every 48 h) were able to reduce the accumulation of MyoD and atrogin-1 mRNA in denervated muscles of rats, but failed to prevent muscle fibre atrophy (Russo et al. 2007). In contrast, Dow et al. showed that 200 muscle contractions applied daily are sufficient to prevent muscle fibre atrophy and degeneration in denervated extensor digitorum longus muscles of rats indicating a protective effect of ES (Dow et al. 2004).
Nevertheless, ES remains a controversial tool for the treatment of denervated muscles (Eberstein and Eberstein 1996). Some studies have shown that ES can have an ambiguous effect on nerves, either stimulating or impairing recovery according to the electrical parameters used (Lu et al. 2008). Therefore, it would be interesting to analyse the molecular markers involved in this process, e.g. N-CAM, to determine whether ES hinders reinnervation. In innervated muscles, N-CAM expression is low and basically restricted to the neuromuscular junction (Covault and Sanes 1986) or to marking N-CAM+ satellite cells (Ishido et al. 2006), while in denervated muscles, the N-CAM expression is increased around and inside atrophic fibres (Gosztonyi et al. 2001).
Stretching is well described as a potent stimulus capable of inducing protein synthesis (Goldspink 1999; Goldspink et al. 2002; Salvini et al. 2006) and maintaining range of motion and flexibility in skeletal muscle (Feland et al. 2001; Batista et al. 2008). It is largely recommended in physical activities and rehabilitation, however few studies have evaluated its effects on denervated muscle adaptation. Loughna and Morgan showed that stretching could modulate myosin heavy chain (MyHC) gene expression through the tension generated by the mechanical signal, independently of innervation, but in a muscle-specific manner (Loughna and Morgan 1999). Sakakima and Yoshida observed that stretching applied 40 min a day, six times a week, reduced atrophy in slow-twitch fibres only during the first 2 weeks in the denervated soleus muscle of rats (Sakakima and Yoshida 2003). However, these protocols do not reflect the usual recommendations for the rehabilitation of denervated muscles in humans, e.g. long periods of immobilization in the stretched position. Others (Peviani et al. 2007) have proposed, instead, short bouts of stretching maintained only for a few minutes.
Another therapeutic strategy to improve muscle mass is the combination of ES and St (Williams et al. 1986). Together they promote a potent hypertrophic effect, inducing a dramatic increase in protein synthesis in innervated muscles. This increase in protein content occurs due to a load increment on the muscles and because ES induces muscle shortening while maintaining a lengthened position due to St (Williams et al. 1986).
Thus, the purpose of the present study was to investigate the effects of ES, St and their combination on gene expression regulation, muscle fibre cross-sectional area (CSA), and N-CAM expression in denervated rat muscles. This study brings new information on how physical interventions that are widely used in peripheral nerve rehabilitation act in denervated skeletal muscles.
Materials and methods
Animal care and experimental groups
Thirty male 3-month-old Wistar rats (265.8 ± 17.6 g) were used. The animals were housed in plastic cages in a room with controlled environmental conditions and had free access to water and standard food. The University’s Ethics Committee approved the experimental procedures, and the study was conducted in accordance with the National Guide for Care and Use of Laboratory Animals.
The animals were randomly divided into five groups: (1) normal (N; n = 6), in which the animals received no intervention and remained free in the cage for 7 days; (2) denervated tibialis anterior (TA) muscle (D; n = 6), in which the animals were submitted to two electrical excitability evaluations (pre-denervation and on the sixth day); (3) denervated TA muscle submitted to both electrical excitability evaluation and ES daily (D + ES, n = 6); (4) denervated TA muscle submitted to daily St (D + St, n = 6) and two electrical excitability evaluations (pre-denervation and on the sixth day); (5) denervated TA muscle submitted to electrical excitability evaluation and to ES plus St daily (D + ES + St, n = 6). The contralateral muscles were also investigated and considered as an internal control of each group. The TA muscle was chosen because it is a superficial muscle that allows the identification of minimal visible contractions and the application of ES directly to the overlying skin (selective electrical treatment) using surface electrodes.
The rats were anaesthetised with an intraperitoneal injection of xylazine (12 mg/kg) and ketamine (95 mg/kg) for the surgical and denervation processes, electrical parameter evaluations, ES, St, ES + St interventions, and muscle removal. The rats were euthanised with an overdose of anaesthetic on the seventh day of the experimental procedures. This period of denervation was chosen because the atrophy of rat skeletal muscles is high during the first weeks after denervation (Bodine et al. 2001).
Denervation process
The denervation process consisted of a sciatic nerve axonotmesis conducted as previously described (Jungnickel et al. 2006; Seo et al. 2006). Shortly after the rat was anaesthetised, a small incision was made through the skin and fascia near the right trochanter between the gluteus maximus and biceps femoris muscles to expose the sciatic nerve. Next, a flat-tipped watchmaker’s tweezer number five (D.L. Micof) was used to crush the sciatic nerve twice for 10 s with a 5 s interval between crushes. After that, the fascia and skin were sutured, distal to proximal, with silk thread. This procedure promoted distal hind-limb muscle denervation, including the TA muscle. The same researcher performed the crush to ensure that the damage was inflicted on the same area and with comparable duration and pressure in all animals of the denervated groups. This model of nerve injury is characterised by axonal degeneration and no sign of muscle reinnervation during the first 10 days after the nerve injury (Carmignoto et al. 1983; Carter et al. 1998).
Electrical evaluation and electrical stimulation procedures
The ES was performed as previously described by Russo et al. (2004, 2007, 2008). In brief, the rat skin was shaved, cleaned, and covered with a layer of conductive gel, where two electrodes were positioned: an indifferent electrode (5 cm round self-adhesive electrode) on the animal’s back, and an active electrode (3 mm round metallic electrode) small enough to stimulate only the TA muscle. During the ES procedure, this electrode was in contact with the skin overlying the TA muscle and perpendicular to the muscle fibres.
The electrical parameters (rheobase, chronaxie and accommodation indexes) were evaluated before each ES treatment and also as presented in experimental group descriptions (Russo et al. 2007). Briefly, rheobase is the minimal electrical stimulus intensity necessary to produce a muscle contraction (rectangular phasic current; pulse interval, 1 s; interpulse interval, 2 s). Chronaxie is the minimal pulse duration necessary to induce a muscle contraction (rectangular phasic current; interpulse interval, 2 s; amplitude, twice the rheobase value). Accommodation is the muscle’s ability not to respond to slowly incrementing electrical pulses (exponential phasic current; pulse interval, 1 s; interpulse interval, 2 s). The values identified during the electrical evaluation were used to determine the ES parameters for each session, as previously described (Russo et al. 2004, 2007). For chronaxie > 1 ms, an exponential phasic current was used (frequency, 50 Hz; pulse duration, twice the chronaxie value; time ON = 1 s and OFF = 3 s). Briefly, the D and D + St groups were submitted to muscular excitability evaluations pre-denervation and on the day before euthanasia (sixth day). In contrast, the D + ES and D + ES + St groups were submitted to a total of seven daily muscular excitability evaluations from pre-denervation until the sixth day.
After the muscle excitability evaluation, the amplitude necessary to induce a maximal contraction of the TA muscle (maximal full flexion of the right ankle) was identified. The ES sessions were applied daily for 6 days, beginning 24 h after denervation, and produced 200 maximal contractions of the TA muscle. These maximal contractions were divided into four sets of 50 contractions, with a duration of 3.2 min per set and 10 min rest (without ES) between sets to minimise muscle fatigue (Cummings 1990). The ES protocol can be seen in the diagram (Fig. 1). It has been previously demonstrated that 200 muscle contractions are effective to maintain muscle mass and strength (Dow et al. 2004) and to reduce the gene expression generally increased during denervation (Kostrominova et al. 2005).
Schematic representation of the electrical stimulation of the tibialis anterior (TA) muscle including electrical evaluation (EE). a EE was performed at the initial moment followed by 4 sets of ES lasting 3.2 min each with a 10 min rest interval between sets. b Fifty muscle contractions were electrically induced throughout a 3.2 min set. The TA remained in contraction for 1 s and in relaxation for 3 s
Stretching procedures
After the rat was anaesthetised, the right ankle was held with tape in full plantar flexion to stretch the right TA modified from Gomes et al. (2006). Each St session was performed once a day and consisted of four sets of 3.2 min each, with a 10 min rest between sets. The set duration (3.2 min) was chosen to mimic the period of time used in the ES protocol and to allow subsequent comparisons between ES, St and their combination (ES + St).
Combined ES and St
To verify the possible effects of combined ES and St in one of the groups, the right TA muscle was submitted concomitantly to both stimuli (D + ES + St). Thus, the TA muscle was held in the full-stretched position with a piece of tape as described above and submitted to ES as previously mentioned (4 sets of 3.2 min with a 10 min rest; Fig. 1). The piece of tape was removed during the rest interval.
Muscle and nerve biopsies
The animals were weighed, and the right TA muscle and the sciatic nerve were carefully dissected and removed.
Sciatic nerve histology
To confirm the injury, nerve histology was performed. The stump of sciatic nerve was prepared for light microscopy. Samples were fixed in 10% formalin for 3 h and then washed in phosphate buffered saline (PBS) until embedded. The specimens were dehydrated and embedded in paraffin and cut at 7 μm, perpendicular to the main nerve axis. Sections were stained with Masson’s Trichrome following manufacturer instructions, and observed under a light microscope (Axiolab, Carl Zeiss, Jena, Germany) equipped with a Carl Zeiss AxioCam HRc camera.
Muscle evaluation
The muscles were divided in half at the middle of the belly. The distal fragment was used for the histological and morphometric measurements. The proximal fragment was immediately frozen in liquid nitrogen and stored at −80°C (Forma Scientific, Marietta, OH) for the mRNA analysis.
Morphometric analysis
The histological cross-sections (10 μm) from the middle belly of each TA muscle (n = 6 per group) were obtained in a cryostat (Micron HE 505, Jena, Germany), stained with Toluidine Blue/1% Borax (TB) and viewed under light microscopy (Axiolab, Carl Zeiss, Jena, Germany) to evaluate general muscle morphology and the total number of muscle fibres. Images from five different regions were obtained using a light microscope equipped with a digital camera (Sony DSC S75, Tokyo, Japan) to view the muscle fibre CSA. The CSA of 70 randomly chosen fibres from each image was measured using the Axiovision 3.0.6 SP4 software (Carl Zeiss, Jena, Germany) with a total of 350 muscle fibres per animal. Furthermore, one image of all muscle cross-sections was acquired at low magnification, and all muscle fibres were counted using the UTHSCSA Image Tool 3.0 software (developed by the University of Texas Health Science Center at San Antonio and available on the website http://ddsdx.uthscsa.edu/dig/itdesc.html).
Immunostaining
The primary antibody used for immunostaining was rabbit anti-neural cell adhesion molecule (N-CAM) affinity-purified PAb (1:100 dilution, catalogue no. AB5032; Chemicon International, Temecula, CA), and the secondary antibody was rhodamine red goat anti-rabbit IgG (1:150 dilution, catalog no. Rb394; Molecular Probes, Eugene, OR). The muscle cross-sections used for immunostaining against the N-CAM were fixed with 4% paraformaldehyde in 0.2 M phosphate buffer (PB) for 10 min at room temperature, blocked with 0.1 M glycine in PBS for 5 min, and permeabilized in 0.2% Triton X-100-PBS for 10 min. The slides were incubated with a solution containing the primary antibody, 3% normal goat serum, and 0.3% Triton X-100—0.1 M PB overnight in a moisture chamber (4°C). After washing the slides with 0.1 M PB (3 times for 10 min each), we added a solution containing the secondary antibody and 0.3% Triton X-100-0.1 M PB for 2 h in a dark room. The slides were washed in 0.1 M PB (3 times for 10 min each). The slides were mounted with Vectashield mounting medium for fluorescence with 4,6-diamidino-2-phenylindole (catalogue no. H-1200; Vector Laboratories) applied to the coverslips. Analyses were performed by obtaining photomicrographs of the stained sections using a fluorescence microscope (Axiolab, Carl Zeiss, Jena, Germany) equipped with a rhodamine filter. Both 20× and 40× magnification was used.
RNA isolation and analysis
One frozen fragment from each muscle was homogenised, and the total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The extracted RNA was dissolved in tris–HCl and ethylene-diaminetetracetic acid (TE) pH 7.6 and quantified spectrophotometrically. The purity was assessed by determining the ratio of the absorbance at 260 nm to the absorbance at 280 nm. All samples had ratios above 2.2. The integrity of the RNA was then confirmed by analysis of the 18S and 28S ribosomal RNA, stained with ethidium bromide (Invitrogen, Carlsbad, CA), under violet ultra light.
Reverse transcription
We reverse transcribed 1 μg of RNA to synthesise cDNA. A reverse transcription (RT) reaction mixture containing 1 μg of cellular RNA, 5× reverse transcription buffer, a dNTP (Promega, Madison, WI) mixture containing 0.2 mmol L−1 each of dATP, dCTP, dGTP and 0.1 mol L−1 of dTTP, 1 μl of oligo (dT) primer (Invitrogen, Carlsbad, CA) and 200 U of M-MLV RT enzyme (Promega, Madison, WI) was incubated at 70°C for 10 min, 42°C for 60 min and finally heated at 95°C for 10 min before quick chilling on ice.
Oligonucleotide primers
Oligonucleotide primers were designed for GAPDH (GenBank, AF106860) using the Primer Express Software 2.0 (Applied Biosystems, Foster City, CA). Primers have been previously described for MyoD, atrogin-1 and myostatin (Peviani et al. 2007) and for GAPDH (Russo et al. 2008). Both the MuRF1 and Runx1 sequences are described by Durigan et al. (2009) and Smith et al. (2005), respectively. All of the sequences were synthesised using Imprint (Campinas, São Paulo, Brazil).
Analyses by real-time polymerase chain reactions
The RNA transcript levels for the different experimental and control muscles were analysed simultaneously, and the reactions were carried out in duplicate in the Lightcycler (Rotor Gene 3000, Cobertt Research, San Francisco, USA) using fluorescent dye SYBR green detection (Applied Biosystems, Foster City, CA) and 180 nM of each primer in a final volume of 50 μl. Thermal cycling conditions to target genes have been previously described (Peviani et al. 2007; Russo et al. 2008). For each gene, all samples were amplified simultaneously in duplicate in one assay run. Data were analysed using the comparative cycle threshold (Ct) method. The target gene expression was normalised to GAPDH because this gene is not affected by denervation (Russo et al. 2007, 2008; Lima et al. 2009). In addition, the negative controls contained RNA but no M-MLV RT, ensuring that the PCR product was not a result of amplified genomic DNA. A blank was also performed with no template sample, only water, primers and SYBR green (Durigan et al. 2008).
Statistics
Shapiro–Wilk’s W and Levene’s test were applied to evaluate the normality and homogeneity of the results, respectively. One-way ANOVA was used to identify possible differences between groups. When differences were observed, the Tukey test was performed. For all tests, the significance level was set at 5% (P ≤ 0.05).
Results
Nerve morphology
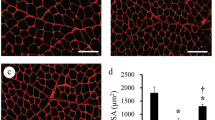
Figure 2 shows light imaging of the sciatic nerve of the N group (Fig. 2a). Bundles of normal fibres with well-defined axons and myelin can be observed (Fig. 2a). All denervated groups showed a similar pattern of nerve degeneration. Figure 2b was obtained from the D + St group and represents all of the injured nerve groups. Many myelin digestion chambers are noted in the endoneurium, indicating axonal degeneration (Fig. 2b). Furthermore, the myelinated fibres are undergoing myelin breakdown, confirming the degeneration (Fig. 2b). None of the physical agents seem to accelerate or retard axonal degeneration.
Sciatic nerve sections from N (a) and D + St (b) stained with Masson’s Trichrome. White arrows indicate normal nerve fibres. Asterisks show empty spaces in the endoneurium indicating axonal degeneration. No relevant difference was noted in those groups submitted to crush nerve injury with or without the physical interventions
Electrical parameters
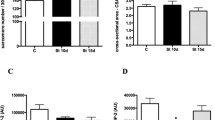
Pre-denervation measurements of the D, D + ES, D + St and D + ES + St groups were obtained and considered as the normal values of rheobase, chronaxie and accommodation (Fig. 3). Denervation decreased rheobase values in D + ES and D + ES + St starting on the third and fifth days, respectively, compared to pre-denervation (P < 0.05, Fig. 3a). After 6 days of denervation, all groups showed a significant decline in rheobase (D: −50%; D + ES: −43%; D + St: −39%; D + ES + St: −46%) compared to pre-denervation (P < 0.05), and there was no difference between denervated groups (P > 0.05; Fig. 3a).
Electrical variables of the tibialis anterior (TA) muscles obtained from electrical evaluations (EE) throughout the 7 days of denervation. Pre-denervation (PD) data obtained from all denervated groups were considered as normal values of rheobase, chronaxie and accommodation. The data are presented as mean ± standard deviation. D—denervated TA muscle submitted to EE at PD and on the 6th day; D + ES—denervated TA muscle submitted to EE and to electrical stimulation (ES) daily; D + St—denervated TA muscle submitted to electrical evaluation at PD and on the 6th day and stretched daily; D + ES + St—denervated TA muscle submitted to EE and to ES while stretched daily. a Rheobase values: *P < 0.05 when D + ES is compared to PD values; † P < 0.05 when D + ES + St is compared to PD values; ♦ P < 0.05 when all groups are compared to themselves. b Chronaxie values: *P < 0.05 when D + ES is compared to PD values; † P < 0.05 when D + ES + St is compared to PD values; § P < 0.05 when D + ES is compared to D + ES + St; ♦ P < 0.05 when all groups are compared to themselves. c Accommodation values: ‡ P < 0.05 when both D + ES and D + ES + St are compared to PD values; ♦ P < 0.05 when all groups are compared to themselves. Note that all groups reduced both rheobase and accommodation values on the 6th day compared to PD. Chronaxie increased in all denervated groups, and it was higher than 1 ms on the 6th day confirming that the TA muscles were denervated
Chronaxie values increased exponentially on the fourth day in D + ES and on the fifth day in D + ES + St compared to pre-denervation (P < 0.05; Fig. 3b). The highest chronaxie values were reached by all groups on the sixth day compared to pre-denervation values (D: 5.6 times; D + ES: 7.6 times; D + St: 5.4 times; D + ES + St: 6.9 times). Also, no difference was found among the groups on the sixth day (P > 0.05, Fig. 3b).
Accommodation decreased its values on the first day after denervation in D + ES and D + ES + St compared to pre-denervation (P < 0.05; Fig. 3c), and it remained steady until the sixth day. At the end, all groups showed a significant reduction in accommodation values compared to pre-denervation (D: −73%; D + ES: −64%; D + St: −64%; D + ES + St: −51%), and no difference was found among the groups (P > 0.05, Fig. 3c).
Body and TA muscle weights
Only the N and D groups gained body weight during the experimental period (Table 1), nevertheless no difference was noted in the final body weight between the groups (P > 0.05). Denervation decreased TA muscle weight (D: −25.6 ± 5%; D + ES: −26.6 ± 4%; D + St: −27.9 ± 2%; D + ES + St: −24 ± 2%) compared to the N group (P < 0.05, Table 1). No difference was found in TA muscle weight among the denervated groups (P > 0.05).
Muscle morphology
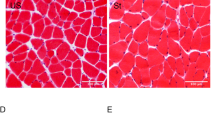
Figure 4 shows evident muscle fibre atrophy in all denervated groups (Fig. 4b–e) compared to the N group (Fig. 4a), which was confirmed by CSA measurements (D: −32.3%; D + ES: −40.9%; D + St: −36.6%; D + ES + St: −37.4%, P < 0.05; Table 1). There was no difference in muscle fibre CSA among the denervated groups (P > 0.05, Table 1). The total number of muscle fibres was investigated, and no difference was found among the experimental groups (P > 0.05, Table 1). Electrical stimulation similarly increased the number of central nuclei in D + ES and D + ES + St compared to the N group (D + ES: P = 0.0007; D + ES + St: P = 0.001; Table 1; Fig. 4c, e, f), and also when compared to D and D + St (P < 0.05). D and D + St and N did not change the number of central nuclei when these three groups were compared (P > 0.05, Table 1).
Muscle-fibre cross-sections of the tibialis anterior (TA) muscle stained with toluidine blue (TB). a N, normal TA muscle; b D, denervated TA muscle; c D + ES, denervated TA muscle submitted to electrical stimulation (ES) daily; d D + St, denervated TA muscle stretched daily; e D + ES + St, denervated TA muscle submitted to ES and stretching (St) at the same time; f Central nuclei observed in the D + ES + St group at high magnification (100×). White arrows show central nuclei in muscle fibres observed in both electrically stimulated groups (c and e). All denervated groups (b–e) showed significant muscle fibre atrophy compared to normal muscles (a). Denervated muscles submitted to ES showed a few muscle fibres with central nucleus (c, e–f). Bar: 100 μm
N-CAM immunostaining
The pattern of N-CAM expression in normal muscles was slight and restricted to nerve terminations, vessels and to the periphery of a few muscle fibres (Fig. 5a). In contrast, all denervated groups showed an increase in N-CAM. Groups D and D + ES + St are represented in Fig. 5b and c, respectively. N-CAM could be observed around several atrophic muscle fibres, and it was expressed in all denervated groups without any marked difference among them.
Immunofluorescence analysis of neural cell adhesion molecule (N-CAM) performed in the tibialis anterior (TA) muscle. N-CAM expression and localization in normal muscles (a), in denervated muscles (b), and submitted to electrical stimulation and stretching (c). N-CAM expression was restricted to the neuromuscular junction (a, white arrows). Denervation increased the N-CAM expression around most muscle fibres (b and c). No significant difference was found in N-CAM expression and localization among denervated groups (data not shown). Bar: 100 μm
MyoD, Runx1, atrogin-1, MuRF1 and myostatin gene expressions by real-time RT-PCR
MyoD gene expression: MyoD expression was up-regulated in all denervated groups (D, D + ES, D + St, and D + ES + St) on the seventh day compared to N (Fig. 6a, P < 0.05). This increase was substantial in group D, where MyoD reached the highest levels compared to D + ES, D + St, and D + ES + St (Fig. 6a, P < 0.05). However, D + ES had the lowest MyoD levels compared to D + St and D + ES + St (Fig. 6a, P < 0.05). No difference was found between D + St and D + ES + St (Fig. 6a, P > 0.05).
MyoD (a), Runx1 (b), atrogin-1 (c), MuRF1 (d) and myostatin (e) mRNA levels in the tibialis anterior (TA) rat muscle. The data are presented as mean ± standard deviation. Groups are described in the legend of Fig. 4. *P < 0.05 compared to N; § P < 0.05 compared to D; † P < 0.05 compared to D + St and D + ES + St. ‡ P < 0.05 compared to all groups. Note that group D had the highest values of the target genes compared to the other groups. ES, St and their combination reduced the accumulation of MyoD, atrogin-1 and MuRF1 in denervated groups (a, c, d) and maintained Runx1 and myostatin at normal levels (b and e). Electrical stimulation per se had the lowest values of MyoD, atrogin-1 and MuRF1 gene expression among the denervated groups (a, c, d)
Runx1 gene expression: Runx1 increased its expression in group D compared to the other groups (Fig. 6b; P < 0.05). This increment was 70 times greater compared to normal values. ES, St and their combination maintained Runx1 expression at normal levels (Fig. 6b; P > 0.05).
Atrogin-1 gene expression: The atrogin-1 mRNA levels increased in all denervated groups compared to N (Fig. 6c, P < 0.05). Nevertheless, group D had the highest values of atrogin-1 compared to the other denervated groups (P < 0.05). The D + ES group displayed the lowest levels of atrogin-1 when compared to the other denervated groups (almost three times lower than D; Fig. 6c, P < 0.05). The D + St and D + ES + St groups reduced the atrogin-1 accumulation in almost half compared to group D (Fig. 6c, P < 0.05) with no difference between the stretched groups (D + St vs. D + ES + St, P > 0.05).
MuRF1 gene expression: Following the same pattern observed in atrogin-1 gene expression, MuRF1 increased exponentially due to denervation (Fig. 6d). ES, St and their combination decreased the MuRF1 expression in denervated muscles compared to D (Fig. 6d, P < 0.05). Interestingly, D + ES showed normal levels of MuRF1 expression (D + ES vs. N: P = 0.42).
Myostatin gene expression: Only group D increased myostatin mRNA levels compared to N (Fig. 6e, P < 0.05) and to the other denervated groups (Fig. 6e, P < 0.05). Myostatin gene expression remained unchanged in the D + ES, D + St, and D + ES + St groups which showed similar levels to those found in the N group (Fig. 6e, P > 0.05).
Contralateral muscles
To evaluate the possible effect of repetitive anaesthesia in experimental groups, the contralateral TA muscles were compared among the groups and also to the ipsilateral of the N group. No difference in any of the comparisons was found (P > 0.05, data not shown).
Discussion
The present study provided new information about the effects of ES, St and their combination on denervated rat muscles. The study showed that ES and St reduced the accumulation of MyoD, atrogin-1 and MuRF1 in denervated muscles and maintained Runx1 and myostatin gene expressions at normal levels, affecting important pathways of muscle regulation. Together, these results suggest that both ES-induced muscle contraction and muscle stretching are able to regulate MyoD, Runx1, atrogin-1, MuRF1 and myostatin expressions in denervated muscles.
The increase in MyoD gene expression due to denervation has already been reported (Eftimie et al. 1991; Hyatt et al. 2003; Ishido et al. 2004; Russo et al. 2007). This increment has been interpreted as an attempt to prevent muscle atrophy, because the role of MyoD is closely related to satellite cell activation and proliferation (for review see Legerlotz and Smith 2008).
Atrogin-1, MuRF1 (Bodine et al. 2001) and myostatin (Liu et al. 2007) gene expressions are also up-regulated in rat denervated muscles. In several models of disuse, atrogin-1 and MuRF1 mRNA levels increased rapidly before muscle weight loss was detectable and maintained a high expression during the period when overall proteolysis was accelerated, indicating that atrogin-1 and MuRF1 play an important role in the initiation and maintenance of accelerated proteolysis (Bodine et al. 2001; Zhang et al. 2007).
Myostatin showed a direct relationship between atrophy and its mRNA and protein levels in rat denervated muscles 7 days after the nerve crush injury (Liu et al. 2007). The authors concluded that myostatin inhibited hypertrophy rather than induced atrophy itself. This inhibitory mechanism is probably related to the decrease in satellite cell proliferation rates (Joulia-Ekaza and Cabello 2006).
The results of the present study indicate that both ES and St regulate gene expression in denervated rat muscles. There are a few mechanisms that could explain this regulation, e.g. it has been proposed that MyoD and other MRFs are tightly regulated by electrical activity and serve as intermediaries between electrical activity and the expression of other muscle-specific genes, such as alpha acetylcholine receptors (α-AChR; Hyatt et al. 2003; Legerlotz and Smith 2008). The present study showed clearly that MyoD expression is down-regulated by ES in denervated muscles. In addition, this study demonstrated for the first time that MyoD expression is affected by intermittent St in denervated muscles. Previous studies have already showed that passive St can up-regulate MyoD gene expression in innervated muscles (Gomes et al. 2006; Peviani et al. 2007). Based on this information, it is reasonable to assume that ES-induced contractions and St-induced tension affect the hypertrophic pathways.
Interestingly, this study provides new information about Runx1 expression in vivo. A 50–100-fold up-regulation in Runx1 expression was reported after 5 days of denervation (Zhu et al. 1994). Similar results were observed in our experiments with a 73-fold up-regulation after 7 days of denervation. It has been shown that this up-regulation is an attempt to prevent the autophagy and to minimise muscle wasting (Zhu et al. 1994; Wang et al. 2005). These increments in Runx1 expression occur in the absence of electrical muscle activity (Wang et al. 2005). Furthermore, Runx1 was recently presented as a transcription factor target of MyoD, which corroborates our results given that MyoD expression was elevated after denervation (Ishibashi et al. 2005). It is worth noting that all therapy strategies reduced Runx1 expression to normal values, which could be attributed to muscle tension caused by St and ES. Therefore, our results suggest that all physical stimuli affected the autophagic pathway because it jeopardised Runx1 expression. However, future studies are necessary to understand the effect of these interventions in a long-term protocol in the denervated muscle.
Considering that ES and St are both able to induce protein synthesis through the IGF1 pathway (Williams et al. 1986), it is possible to assume that muscle activity could down-regulate atrogin-1 expression in a compensatory way. It is well described that the IGF1/PI3K/Akt pathway can regulate atrogin-1 and MuRF1 expression via FoxO phosphorylation. Akt or protein kinase B inhibits protein degradation in skeletal muscle by promoting the phosphorylation and inactivation of FoxO, changing its subcellular localization and preventing the expression of FoxO-dependent, atrophy-related genes such as atrogin-1 and MuRF1 (Stitt et al. 2004). Furthermore, MuRF1 has been found in association with the myofibrillar component titin, a giant protein present in the sarcomere from the M- to the Z-line. At the M-line, it is a target component of the myofibrillar apparatus for degradation in atrophying muscle (Cao et al. 2005). Thus, it is reasonable to assume that tension forces caused either by ES or St regulated the MuRF1 family expression.
Regarding the results of myostatin, it is possible that mechanical stress could regulate myostatin expression. Muscle changes in length stress are transmitted throughout titin to a specific portion called titin-cap or telethonin which can interfere with cellular signalling mechanisms (Tskhovrebova and Trinick 2003). One of these mechanisms involves the inactivation of myostatin by preventing the latent complex formation and its secretion (Joulia-Ekaza and Cabello 2006). Considering the results of the present study, it is possible to suggest that both ES and St increased mechanical stress in the muscle fibres, which could down-regulate myostatin via titin-cap. Nevertheless, further studies are necessary to assess these hypotheses.
Another interesting point is that the combination of St and ES did not have a cumulative effect on the expression of the examined genes. It seems that tension stimulus provoked by stretching was enough to induce muscle adaptation. However, studies that isolate the muscle fibres are needed to investigate the sarcomere tension induced by these stimuli.
The present study applied ES and St protocols as prescribed in the rehabilitation of peripheral nerve injuries in humans. Electrical stimulation was applied in single sessions using surface electrodes and considering the muscle excitability changes that guided the stimulation protocol. The total of 200 contractions per day was used in the present study because this amount of stimulation was shown to cease muscle fibre atrophy (Dow et al. 2004) and molecular changes (Kostrominova et al. 2005) in 2-month denervated EDL muscles of rats. Nevertheless, it seems that the rest interval between sessions is the main factor to protect muscle mass in denervated muscles (Dow et al. 2005). Twenty-hour intervals between each session of 100 contractions were not able to stop muscle fibre atrophy (Dow et al. 2005). The present data corroborate this premise because 200 contractions were also insufficient to avoid muscle atrophy. Good results were found only when a rest interval of 8 h between sets was applied (Dow et al. 2005), showing that long rest periods between contractions may be sufficient to induce degenerative changes in denervated muscles, and the short interval between contractions is the key regulator of denervation atrophy.
In addition, St as applied in rehabilitation or as recommended in sports activities can be performed intermittently and not chronically as used in the literature (Goldspink et al. 1991). It seems that the total amount of time the muscles are maintained in the stretched position is also a determining factor in avoiding muscular degenerative modifications due to denervation. Forty minutes of St per day delayed muscle atrophy in the first 2 weeks post-denervation in rats (Sakakima and Yoshida 2003); in contrast, protocols using short intervals of St stimulation have not been previously investigated.
Future studies should investigate different sessions of stimulation using surface electrodes throughout the day to confirm the results found by Dow et al. (2005). Moreover, alternative ES and St throughout the day could be a good strategy to treat denervated muscles by distributing contractile stimuli. Despite the protective effects on gene expression regulation, all physical interventions failed to stop muscle atrophy in the denervated muscles, which is due to the decrease in muscle fibre CSA rather than the number of muscle fibres.
In contrast, the accumulation of ubiquitin-ligases atrogin-1/MuRF1 and myostatin in group D did not cause more muscle fibre atrophy. The same applies to the down-regulation of these genes by ES, St and ES + St. These data suggest that low levels of these genes can induce muscle fibre atrophy. In addition, it is possible to interpret the down-regulation of MyoD, Runx1, atrogin-1, MuRF1 and myostatin in denervated groups submitted to physical interventions as a strategy to spare the gene machinery during denervation, e.g., contractile stimuli alone could affect the muscle homeostasis. However, other studies focusing on long-term evaluations should be conducted to verify these hypotheses.
An interesting finding was the presence of central nuclei in muscle fibres from the groups submitted to ES with or without St, showing that the regenerative machinery was activated by ES. Moreover, the N-CAM localization around the atrophic muscle fibres in all denervated groups showed that, in spite of gene expression regulation, ES, St and their combination, none of the physical interventions impaired the expression of this neural adhesion factor, which is essential to the muscle fibre reinnervation process (Gosztonyi et al. 2001). Nerve histology also corroborated these findings.
Another relevant clinical aspect reported in the present study was the short-term change in muscle excitability. Denervated muscles become hypoexcited after the sixth day of denervation, and neither ES nor St changed muscle excitability. Additionally, the increase in chronaxie values, combined with the nerve histology, confirmed that all muscles were denervated on the sixth day post-denervation. This statement shows us that denervated muscles (chronaxie higher than 1 ms) do not respond to short-duration electrical pulses. Excitability curves should be performed in denervated muscles to find the best parameters to produce contraction in this type of muscle (Russo et al. 2004, 2007, 2008; Ashley et al. 2005).
In summary, the present study showed that clinical protocols of ES and St were able to down-regulate MyoD, Runx1, atrogin-1, MuRF1 and myostatin in rat denervated muscles, however they failed to stop muscle fibre atrophy. Electrical stimulation seems to stimulate the regenerative process in denervated muscles. This study brought new information about how ES, St and their combination (applied as prescribed in the rehabilitation of denervated muscles in humans) can regulate gene expression and muscle mass, contributing to the design of new therapy strategies.
Abbreviations
- CSA:
-
Cross-sectional area
- ES:
-
Electrical stimulation
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- MRFs:
-
Myogenic regulatory factors
- mRNA:
-
Messenger ribonucleic acid
- MuRF1:
-
Muscle-specific RING Finger protein-1
- MyoD:
-
Myogenic differentiation-1
- N-CAM:
-
Neural cell adhesion molecule
- PCR:
-
Polymerase chain reaction
- Runx1:
-
Runt-related transcription factor 1
- St:
-
Stretching
- TB:
-
Toluidine blue/1% borax
References
Ashley Z, Sutherland H, Lanmuller H et al (2005) Determination of the chronaxie and rheobase of denervated limb muscles in conscious rabbits. Artif Organs 29:212–215
Ashley Z, Sutherland H, Russold M et al (2008) Therapeutic stimulation of denervated muscles: the influence of pattern. Muscle Nerve 38:875–886
Batista LH, Camargo PR, Oishi J, Salvini TF (2008) Effects of an active eccentric stretching program for the knee flexor muscles on range of motion and torque. Rev Bras Fisiot—Braz J Phys Ther 12:176–182
Bodine SC, Latres E, Baumhueter S et al (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294:1704–1708
Buonanno A, Apone L, Morasso MI et al (1992) The MyoD family of myogenic factors is regulated by electrical activity: isolation and characterization of a mouse Myf-5 cDNA. Nucleic Acids Res 20:539–544
Cao PR, Kim HJ, Lecker SH (2005) Ubiquitin-protein ligases in muscle wasting. Int J Biochem Cell Biol 37:2088–2097
Carmignoto G, Finesso M, Siliprand R, Gorio A (1983) Muscle reinnervation—I. Restoration of transmitter release mechanisms. Neuroscience 8:393–401
Carter AJ, Kristmundsdottir F, Gilmour J, Glasby MA (1998) Changes in muscle cytoarchitecture after peripheral nerve injury and repair. A quantitative and qualitative study. J Hand Surg 23:365–369
Covault J, Sanes JR (1986) Distribution of N-CAM in synaptic and extrasynaptic portions of developing and adult skeletal muscle. J Cell Biol 102:716–730
Cummings J (1990) Electrical stimulation in denervated muscle. In: Gersh M (ed) Electrotherapy in rehabilitation, 3rd edn. Churchil Livingstone, New York, pp 269–290
Dow DE, Cederna PS, Hassett CA et al (2004) Number of contractions to maintain mass and force of a denervated rat muscle. Muscle Nerve 30:77–86
Dow DE, Faulkner JA, Dennis RG (2005) Distribution of rest periods between electrically generated contractions in denervated muscles of rats. Artif Organs 29:432–435
Durigan JLQ, Peviani SM, Russo TL, Delfino GB, Ribeiro JU, Cominetti MR, Selistre-de-Araujo HS, Salvini TF (2008) Effects of alternagin-C from Bothrops alternatus on gene expression and activity of metalloproteinases in regenerating skeletal muscle. Toxicon 52:687–694
Durigan JLQ, Peviani SM, Russo TL et al (2009) Effects of exercise training on atrophy gene expression in skeletal muscle of mice with chronic allergic lung inflammation. Braz J Med Biol Res 42:339–345
Eberstein A, Eberstein S (1996) Electrical stimulation of denervated muscle: is it worthwhile? Med Sci Sports Exerc 28:1463–1469
Eftimie R, Brenner HR, Buonanno A (1991) Myogenin and MyoD join a family of skeletal muscle genes regulated by electrical activity. Proc Natl Acad Sci USA 88:1349–1353
Feland JB, Myrer JW, Schulthies SS, Fellingham GW, Measom GW (2001) The effect of duration of stretching of the hamstring muscle group for increasing range of motion in people aged 65 years or older. Phys Ther 81:1110–1117
Glass DJ (2003) Molecular mechanisms modulating muscle mass. Trends Mol Med 9:344–350
Glass DJ (2005) Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol 37:1974–1984
Goldspink G (1999) Changes in muscle mass and phenotype and the expression of autocrine and systemic growth factors by muscle in response to stretch and overload. J Anat 194:323–334
Goldspink DF, Easton J, Winterburn SK, Williams PE, Goldspink GE (1991) The role of passive stretch and repetitive electrical stimulation in preventing skeletal muscle atrophy while reprogramming gene expression to improve fatigue resistance. J Card Surg 6:218–224
Goldspink G, Williams P, Simpson H (2002) Gene expression in response to muscle stretch. Clin Orthop Relat Res 1:S146–S152
Gomes MD, Lecker SH, Jagoe RT et al (2001) Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA 98:14440–14445
Gomes AR, Soares AG, Peviani S et al (2006) The effect of 30 minutes of passive stretch of the rat soleus muscle on the myogenic differentiation, myostatin, and atrogin-1 gene expressions. Arch Phys Med Rehabil 87:241–246
Gosztonyi G, Naschold U, Grozdanovic Z, Stoltenburg-Didinger G, Gossrau R (2001) Expression of Leu-19 (CD56, N-CAM) and nitric oxide synthase (NOS) I in denervated and reinnervated human skeletal muscle. Microsc Res Tech 55:187–197
Hyatt JP, Roy RR, Baldwin KM, Edgerton VR (2003) Nerve activity-independent regulation of skeletal muscle atrophy: role of MyoD and myogenin in satellite cells and myonuclei. Am J Physiol Cell Physiol 285:C1161–C1173
Ishibashi J, Perry RL, Asakura A, Rudnicki MA (2005) MyoD induces myogenic differentiation through cooperation of its NH2- and COOH-terminal regions. J Cell Biol 171:471–482
Ishido M, Kami K, Masuhara M (2004) In vivo expression patterns of MyoD, p21, and Rb proteins in myonuclei and satellite cells of denervated rat skeletal muscle. Am J Physiol Cell Physiol 287:C484–C493
Ishido M, Uda M, Masuhara M, Kami K (2006) Alterations of M-cadherin, neural cell adhesion molecule and beta-catenin expression in satellite cells during overload-induced skeletal muscle hypertrophy. Acta Physiol (Oxf) 187:407–418
Jackman RW, Kandarian SC (2004) The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol 287:C834–C843
Jones SW, Hill RJ, Krasney PA, O’Conner B, Peirce N, Greenhaff PL (2004) Disuse atrophy and exercise rehabilitation in humans profoundly affects the expression of genes associated with the regulation of skeletal muscle mass. FASEB J 18:1025-1-7
Joulia-Ekaza D, Cabello G (2006) Myostatin regulation of muscle development: molecular basis, natural mutations, physiopathological aspects. Exp Cell Res 312:2401–2414
Jungnickel J, Haase K, Konitzer J, Timmer M, Grothe C (2006) Faster nerve regeneration after sciatic nerve injury in mice over-expressing basic fibroblast growth factor. J Neurobiol 66:940–948
Kostrominova TY, Dow DE, Dennis RG et al (2005) Comparison of gene expression of 2-mo denervated, 2-mo stimulated-denervated, and control rat skeletal muscles. Physiol Genomics 22:227–243
Lagirand-Cantaloube J, Offner N, Csibi A et al (2008) The initiation factor eIF3-f is a major target for atrogin1/MAFbx function in skeletal muscle atrophy. EMBO J 27:1266–1276
Legerlotz K, Smith HK (2008) Role of MyoD in denervated, disused, and exercised muscle. Muscle Nerve 38:1087–1100
Lima SC, Caierão QM, Peviani SM, Russo TL, Somazz MC, Salvini TF, Teodori RM, Minamoto VB (2009) Muscle and nerve responses after different intervals of electrical stimulation sessions on denervated rat muscle. Am J Phy Med Rehabil 88:126–135
Liu M, Zhang D, Shao C, Liu J, Fei Ding, Gu X (2007) Expression pattern of myostatin in gastrocnemius muscle of rats after sciatic nerve crush injury. Muscle Nerve 35:649–656
Loughna PT, Morgan MJ (1999) Passive stretch modulates denervation induced alterations in skeletal muscle myosin heavy chain mRNA levels. Pflugers Arch 439:52–55
Lu MC, Ho CY, Hsu SF et al (2008) Effects of electrical stimulation at different frequencies on regeneration of transacted peripheral nerve. Neurorehabil Neural Repair 22:367–373
Malicdan MC, Noguchi S, Nishino I (2009) Monitoring autophagy in muscle diseases. Methods Enzymol 453:379–396
Peviani SM, Gomes AR, Moreira RF, Moriscot AS, Salvini TF (2007) Short bouts of stretching increase myo-D, myostatin and atrogin-1 in rat soleus muscle. Muscle Nerve 35:363–370
Russo TL, França C, Castro C, Salvini TF (2004) Alterations of chronaxie, rheobase and accommodation in denervated skeletal muscle submitted to electrical stimulation. Rev Bras Fisiot—Braz J Phys Ther 8:169–175
Russo TL, Peviani SM, Freria CM, Gigo-Benato D, Geuna S, Salvini TF (2007) Electrical stimulation based on chronaxie reduces atrogin-1 and MyoD gene expressions in denervated rat muscle. Muscle Nerve 35:87–97
Russo TL, Peviani SM, Durigan JLQ, Salvini TF (2008) Electrical stimulation increases matrix metalloproteinase-2 gene expression but does not change its activity in denervated rat muscle. Muscle Nerve 37:593–600
Sakakima H, Yoshida Y (2003) Effects of short duration static stretching on the denervated and reinnervated soleus muscle morphology in rat. Arch Phys Med Rehabil 84:1339–1342
Salvini TF, Coutinho EL, Russo TL, DeLuca C (2006) One-minute bouts of passive stretching after immobilization increase sarcomerogenesis in rat soleus muscle. Braz J Morphol Sci 23:271–277
Seo TB, Han IS, Yoon JH, Hong KE, Yoon SJ, Namgung UK (2006) Involvement of Cdc2 in axonal regeneration enhanced by exercise training in rats. Med Sci Sports Exerc 38:1267–1276
Sharma M, Langley B, Bass J, Kambadur R (2001) Myostatin in muscle growth and repair. Exerc Sport Sci Rev 29:155–158
Sheffler LR, Chae J (2007) Neuromuscular electrical stimulation in neurorehabilitation. Muscle Nerve 35:562–590
Smith N, Dong Y, Lian J et al (2005) Overlapping expression of Runx1 (Cbfa2) and Runx2 (Cbfa1) transcription factors supports cooperative induction of skeletal development. J Cell Physiol 203:133–143
Stitt TN, Drujan D, Clarke BA et al (2004) The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14:395–403
Tskhovrebova L, Trinick J (2003) Titin: properties and family relationships. Nat Rev Mol Cell Biol 4:679–689
Wang X, Blagden C, Fan J, Nowak SJ, Taniuchi I, Littman DR, Burden SJ (2005) Runx1 prevents wasting, myofibrillar disorganization, and autophagy of skeletal muscle. Genes Dev 19:1715–1722
Wehling M, Cai B, Tidball JG (2000) Modulation of myostatin expression during modified muscle use. FASEB J 14:103–110
Williams P, Watt P, Bicik V, Goldspink G (1986) Effect of stretch combined with electrical stimulation on the type of sarcomeres produced at the ends of muscle fibers. Exp Neurol 93:500–509
Zhang D, Liu M, Ding F, Gu X (2006) Expression of myostatin RNA transcript and protein in gastrocnemius muscle of rats after sciatic nerve resection. J Muscle Res Cell Motil 27:37–44
Zhang P, Chen X, Fan M (2007) Signaling mechanisms involved in disuse muscle atrophy. Med Hypotheses 69:310–321
Zhu X, Yeadon JE, Burden SJ (1994) AML1 is expressed in skeletal muscle and is regulated by innervation. Mol Cell Biol 14:8051–8057
Acknowledgments
This project was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, 07/03160-8) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). D Gigo-Benato and G B Delfino are PhD grant holders supported by FAPESP (Process numbers: 06/52931-4 and 08/03499-8, respectively). T L Russo and J L Q Durigan are post-doctoral grant holders supported by FAPESP (08/05237-0; 08/09408-4, respectively), and S M Peviani is a post-doctoral grant holder supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The authors are grateful to Teresa F. F. Piassi for her technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Russo, T.L., Peviani, S.M., Durigan, J.L.Q. et al. Stretching and electrical stimulation reduce the accumulation of MyoD, myostatin and atrogin-1 in denervated rat skeletal muscle. J Muscle Res Cell Motil 31, 45–57 (2010). https://doi.org/10.1007/s10974-010-9203-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-010-9203-z