Abstract
The manufacture of flexible devices is of immense interest due to their eco-friendliness, economic prospects and wide range of applications in biocompatible devices. Therefore, this paper reports the characterization and properties of chlorinated natural rubber (Cl-NR)/polyindole (PIN) blend with different contents of nickel oxide (NiO) nanoparticles. The formation of blend nanocomposites was analysed by FT-IR, UV spectroscopy, XRD, FE-SEM, DSC and TGA. The FT-IR and UV spectra proved the effective interfacial interaction between nanoparticles and the blend matrix, with a shift in UV peak intensity with the attachment of NiO at 433 cm−1. The XRD pattern revealed the regular arrangement of the blend matrix due to the presence of nanofiller, resulting in the semicrystalline structure of the composite system. The SEM micrographs revealed a homogeneous distribution of NiO in the blend matrix. The increased degradation temperature in the TGA demonstrated the enhanced thermal stability of the NiO-filled Cl-NR/PIN blend. DSC results showed that the glass transition temperature of the composite increased with NiO content in the blend matrix. The dielectric properties and AC electrical conductivity increased significantly with increasing temperatures, as well as with the addition of nanoparticles up to 5 mass % loading. The inclusion of NiO improved the tensile strength and hardness while reducing the elongation-at-break of nanocomposites. The blend nanocomposite with enhanced thermal stability, glass transition temperature, tensile strength, AC conductivity and dielectric properties enables them to be used in highly flexible electronic applications where traditional elastomeric materials fall short.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The optical and electrical properties of conducting polymers like polyindole, polypyrrole, polyaniline, etc. make them an outstanding candidate for applications in electrochromic displays, field effect transistors, solar cells, sensors and batteries [1, 2]. For better application of conducting polymers in these respects, their processability and mechanical properties must be improved. In recent research scenarios, the blending of different polymers and the formation of polymer composites have been adopted to enhance the inherent material properties [3, 4]. The common process of blending causes the merging of the highly conducting nature of conducting polymers with the highly flexible nature of elastomers [4]. Various studies are accessible in the literature on the blending of elastomers, low-density polyethylene, polypropylene and polystyrene with several conducting polymers [5, 6]. Natural rubber is a versatile elastomer with excellent elasticity, resilience and biocompatibility [7]. The reactive double bonds present in the backbone of NR provide an excellent opportunity for different chemical modifications such as epoxidation, hydrogenation and chlorination [8,9,10]. Among the various chemical modification techniques, chlorination results in more attractive mechanical properties with excellent flame and oil resistance [11, 12]. Moreover, the chlorinated segments in NR can be blended more strongly with conducting polymers because there is strong polar–polar interaction between the components of the blend and polar segments of conducting polymers, which cannot be afforded by unmodified non-polar natural rubber.
Various scientific groups have been conducting extensive research in recent years to build supercapacitors, electrical sensors and energy storage devices using polyindole [13, 14]. The lack of flexibility of these conducting materials makes their processability more time-consuming and uneconomical. Thus, the blending of polyindole with flexible and high-strength chlorinated natural rubber finds its explicit position in future flexible electronic devices. The rapid redox process possible in pi-conjugated conducting polymer systems offers pseudo-capacitance to the materials [15]. The extended pi-electron conjugation possible in the backbone of polyindole offers high conductivity to the material. In addition to this, polyindole has quick charge–discharge behaviour, very high specific capacitance value and easy synthesis [16]. Thus, this polyindole performs as an excellent raw material in the field of energy storage device fabrication [17]. PIN is one of the conducting polymers that is not explored as much as that of polypyrrole and polyaniline. The major negative aspects of PIN include structural instability, low mechanical stability, mechanical strain over repeated cycles, material degradation and long-term usage leading to poor capacity and collapse of capacitors [18, 19]. The weak association of polymer chains in PIN leads to mechanical instability and structural irregularity [20]. To overcome the above-mentioned loopholes and make PIN technically feasible by blending it with high-strength chlorinated natural rubber.
The mechanical and electrical properties of rubber blends can be improved further by adding metal oxide nanofiller for the development of innovative conducting materials. Among metal oxides, nickel oxide (NiO) is the least expensive and has a high surface-to-volume ratio, resulting in better interaction with the blend matrix than typical macro and micro fillers [21]. NiO is a p-type semiconductor with a wide range of applications in optical, electrical and magnetic devices due to the existence of Ni+ and Ni++ in the metal oxide [15]. The inclusion of NiO nanoparticles into the conducting polymer significantly increases the dielectric constant as compared to the parent polymer [22]. Furthermore, adding a trace of NiO to the chlorinated NR/polyindole blend can increase the thermal stability and conductivity of flexible elastomers. Therefore, the current study concentrated on the in situ synthesis of chlorinated natural rubber with indole monomer in the presence of various contents of nickel oxide nanoparticles. The effect of nickel oxide nanoparticles on the structural properties, glass transition temperature, thermal stability, temperature AC conductivity and dielectric properties of Cl-NR/PIN blend nanocomposites are analysed.
Experimental
Materials and methods
Natural rubber (ISNR 5) was acquired from rubber research institute (Kottayam, India). The indole, toluene, chloroform, sodium hydroxide, methanol and CTAB (cetyltrimethylammonium bromide) were purchased from Merck India. Sigma-Aldrich supplied the NiO nanoparticles with a particle size of less than 50 nm and a conductivity of 5.1 × 10−2 Scm−1. All the chemicals and solvents used were of analytical reagent grade. Chlorinated NR with 15% chlorine content was prepared via an in situ reaction of chloroform with aqueous alkali in the presence of CTAB as a phase transfer catalyst [23].
Preparation of Cl-NR/PIN blend/NiO nanocomposites
The Cl-NR/PIN (90/10) blend nanocomposites were prepared by the in situ chemical oxidative polymerization of indole with Cl-NR solutions containing different mass fractions of NiO (1, 3, 5, 7 and 10 mass %) using ammonium persulfate (APS) as an oxidising agent. For that, Cl-NR was dissolved in toluene under constant stirring to get a uniform solution. The indole monomer was added to the solution of Cl–NR in an optimised ratio of 9:1, and the mixture was stirred for 30 min to get a homogeneous solution. Then, the homogeneous solution was kept under ultrasonication for 40 min to evenly distribute the nanofiller and the setup was kept under stirring for 12 h under room temperature conditions. The obtained product was washed with deionised water and methanol to eliminate the unreacted monomers and oxidising agents. Finally, the film was dried overnight in a vacuum oven at 70 °C.
Characterisations of samples
The UV–VIS absorption spectra of Cl-NR/PIN blend with different contents of NiO nanocomposites in toluene were recorded on Shimadzu-2600 spectrophotometers. A Fourier transform infrared spectrometer was used to investigate the structural properties of blend nanocomposites (FT-IR series model JASCO 4100). The Brucker D8 Advanced Diffractometer was used to examine X-ray diffraction using Cu Kα radiation, and the data were evaluated in the 2θ range between 5° to 90° at a speed rate of 2° min−1. The FESEM images of blend/ NiO nanocomposite were obtained using the Carl Zeiss VP-500 model. The glass transition temperature of rubber blend nanocomposites was recorded in Shimadzu DSC-50, and the sample was heated at 10 °C min−1. A Hitachi STA7200 thermogravimetric analyzer was used to analyse the thermal stability of the blend nanocomposites, and the sample was heated from 25 to 600 °C at a heating rate of 10 °C min−1. The AC conductivity and dielectric constant of composites in the frequency region of 100–106 Hz were obtained using a HIOKI 3570 model impedance analyser (two electrode system). For dielectric measurements, the films are cut in circular shapes with a thickness of 0.5 mm and a diameter of 1.2 cm. The effect of temperature on AC conductivity was investigated by varying the temperature from 30 to 90 °C while maintaining a constant heating rate of 1 °C min−1. The tensile strength of nanocomposites was evaluated using a universal testing machine (Zwick UTM) at a crosshead speed of 30 mm min−1. A Zwick H04- 3150 hardness tester (Zwick GmbH) was used to measure the hardness of the samples.
Results and discussions
Ultraviolet–visible (UV–vis) spectroscopy
The optical characteristics of Cl- NR/PIN blend system with various loadings of nickel oxide nanoparticles is shown in Fig. 1. The major absorption band of Cl-NR/PIN system is observed at 286 nm, which corresponds to the π–π* transition. The absorption band shifted to 286.5, 287, 288, 288.2, 289 nm, respectively, for 1, 3, 5, 7 and 10 mass % nickel oxide loaded blend composites. The intensity of the UV peak is found to increase with filler loading up to a threshold filling of 5 mass %. Beyond this equilibrium filling level (7 and 10 mass % NiO loading), there is a slight decrease in the intensity of absorption for composites. The bathochromic shift observed for composites indicates the extended conjugation of the blended system. The formation of clusters in the composites at higher loadings causes a slight reduction in UV absorption.
FT-IR analysis
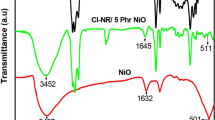
Figure 2 depicts the characteristic bands of nano NiO, the Cl-NR/PIN blend, and its nanocomposites with varying NiO loadings. The FTIR spectra of Cl-NR/PIN blend show the broadband in the range of 3164 to 3618 cm−1 is attributed to the N–H stretching vibrations and the band near 1654 cm−1 is ascribed to the N–H deformations of PIN [24]. The CH, CH2 and CH3 stretching vibrations are present at 2847 cm−1, 2918 cm−1 and 2967 cm−1, respectively [25]. The unique C–Cl stretching band corresponds to the halogenated segment of NR and is observed at 845 cm−1 along with a considerable band at 1053 cm−1, indicating the presence of a cyclopropyl ring in the dichlorocarbene modified NR [26]. The FT-IR spectra of NiO show major bands located at 3434, 1632 cm−1 assigned to the hydroxyl group of water molecules absorbed on the surface of NiO along with a characteristic Ni–O stretching band at 498 cm−1 [27]. The composites with 5 and 10 mass % filler loading exhibit all the characteristic bands corresponding to the Cl-NR/ PIN blend with the appearance of a new band at 492 cm−1, indicating the effective incorporation of nano Ni–O in the blend matrix. The O–H stretching bands in the nanomaterial are merged with the N–H stretching and N–H deformation bands in the composite system.
X-ray diffraction (XRD)
The XRD pattern of the Cl-NR/PIN blend exhibits a broad peak at 2θ = 19.3° confirming the presence of semicrystalline polyindole in the chlorinated NR system (Fig. 3). The nano nickel oxide exhibits five major peaks in the XRD, which are in good agreement with the standard spectrum of JCPDS, 04–0835. The face-centred lattice arrangement is evident from the crystalline peaks at 2θ = 37.32° (111), 43.45° (200), 62.83° (220), 75.20° (311) and 79.37° (222) [28]. The composite with 5 mass % NiO loading shows the crystalline peaks of nanofiller at 2θ = 18.7°, 21.57° and 29.83°. These additional peaks, along with the semicrystalline peak of Cl-NR/PIN blend illustrate the uniform arrangement of nanofiller in the blend matrix. It can be seen that the intensity of the crystalline peaks of blend nanocomposite increases with the loading of nanoparticles. Thus, the presence of more crystalline peaks in the composites with increased filler loading is evidence of the filler–polymer polar interaction possible at their interface [29]. The Scherrer equation is used to calculate the particle size of NiO in the blend nanocomposites and it is found in the range of 25 to 35 nm [30]. Thus, from the above discussions, it can be concluded that the nickel oxide nanoparticles can impart a semicrystalline nature to the blend system. The percentage crystallinity of NiO nanoparticles, Cl-NR/PIN blend and its nanocomposites are evaluated by the relation
Pure NiO and Cl-NR/PIN show a crystallinity of 89.42% and 25.30% respectively. However, the crystallinity of Cl-NR/PIN was increased to 33.82% and 38.19%, respectively, for 5 and 10 mass% NiO nanoparticles incorporated composites.
Scanning electron microscopy (SEM)
The dispersion of nano NiO in the polymer blend is evaluated directly from the scanning electron microscopic images obtained for the composites and parent blend systems. Figure 4 shows the irregular surface morphology of the Cl-NR/PIN blend system. It is observed that the surface of Cl-NR/PIN blend shows a porous structure with some irregularities. This porous and uneven surface pooves the formation of the successful blend between the Cl-NR and PIN. The composites with 3, 5 and 10 mass % NiO loading at 200 nm magnification shown in the figure indicate that the porous irregular surface of the blend is changed to a more regular one with the uniform filling of spherically shaped nano NiO. The nano metal oxide possesses a higher surface area which can firmly adhere to a polar polymer blend and thereby lead to the encapsulation of NiO in the blend matrix. Here, a localised distribution of spherical particles in the blend matrix at 3 mass % loading and for composite with 5 mass % filler content, a more uniform distribution is observed. Due to the stronger filler—filler adhesion than that of polymer—filler, few clusters of nanofillers are seen at higher filler loadings (10 mass%). Thus, it can be concluded that the gradual shift in SEM micrographs of composites with increasing filler loading indicates that the 5 mass% composite is the filling threshold level where uniform filling is observed.
Differential scanning calorimetry (DSC)
DSC curves of Cl-NR/PIN and its composites with 3, 7 and 10 mass% NiO are shown in Fig. 5. It is observed that the pure Cl-NR/PIN blend shows the glass transition temperature (Tg) at –16.65 °C. The main factors influencing the Tg values are the mobility of macromolecular polymer chains as well as the interaction between the blend components and the nanoparticles [31]. Therefore, the Tg of the blend system is affected by the involvement of metal oxide nanofillers. It is evident from the plot that the Tg values are significantly increased with the addition of nanoparticles to the blend system. The composite with a 5 mass % filler loading shows a glass transition temperature at –14.03 °C and composites with 7 and 10 mass % NiO loadings showed the Tg at –12.01 and –9.08 °C, respectively. This glass transition temperature trend demonstrates that the filler particles are regularly arranged between the blend segments, resulting in an increase in crystallinity or regularity of the material. Thus, the DSC data also confirms the strong filler–matrix interactions.
Thermogravimetric analysis (TGA)
A TGA analysis has been performed to explore the effect of nanofillers on the thermal stability of the Cl-NR/PIN blend. The thermal degradation pattern of Cl-NR/PIN blend and its composites with two different loading levels is exhibited in Fig. 6. All the composites show two stages of mass loss in the curve. The initial mass loss observed between 180–250 °C is due to the removal of oligomers, dopant ions and unreacted monomers in the Cl-NR/PIN [32]. The second degradation region observed at 290–500 °C is the elimination of the rubber blend. The initial decomposition of the blend occurs at 205 °C, while the composite with 3, 7 and 10 mass % loading shows degradation at 217 °C, 225 °C and 231 °C, respectively. This can be attributed to the strong interfacial adhesion between the nanofillers and the blend matrix, which increases the strong intermolecular bonding, thereby preventing the degradation of the polymer chain. The uniform dispersion of nanoparticles inside the blend system imparts higher thermal stability for the nanocomposites [33]. Moreover, the residual amount of all the Cl-NR/PIN nanocomposites at higher temperature is apparently higher than the unfilled blend, which indicates that the presence of nanofillers improved the thermal stability of the blend nanocomposites. The protective layer formed by the uniform dispersion of nanofillers on the surface of the blend system suppresses the decomposition of Cl-NR/PIN blend nanocomposites [34].
Dielectric constant (\({\varvec{\epsilon}}\) r)
The variation of the real part of dielectric permittivity of Cl-NR/PIN blend nanocomposite is exhibited in Fig. 7. The dielectric constant for all the samples is high at lower frequencies. This is because space charge polarisation exists at the grain boundaries, generating a potential barrier. The charge then accumulated at the grain boundary, resulting in greater values of the real part of permittivity [35]. A similar trend in the variation of dielectric constant is reported by Bagat et al. in polyindole/poly vinyl acetate blend [36]. The decrease in dielectric constant with increasing frequency is caused by ionic, electronic and orientation polarisations [37]. The ionic polarisation caused by the externally applied electric field induces positive ion displacement rather than negative ions [37]. The material structure and arrangement of atoms usually cause the orientation polarisation and it is normally observed at frequencies of 1 kHz and 1 MHz. Electronic polarisations are caused by the displacement of the electron cloud with respect to the nucleus of an atom. Among these, orientation polarisation is important in the case of the present system as it requires a longer time than other possible polarisations [38]. Therefore, the dielectric constant value decreases with increasing frequency and reaches a frequency-independent constant value because, at these frequency domains, the dipoles fail to orient with the rapidly alternating applied field, resulting in lower orientation polarization. The increase in dielectric constant with increased filler loading is attributed to the higher dielectric permittivity values of the NiO nanoparticles and to the heterogeneity of the systems. The higher dielectric constant of composite systems encourages the semiconducting behaviour of the blend system. The maximum dielectric constant value is observed for a 5 mass % loading, thus it is considered the level of threshold filling for this blend system.
AC conductivity
The variation of AC electrical conductivity with angular frequency for Cl-NR/PIN blend composites is depicted in Fig. 8. The conductivity of the blend system increases with increasing filler loading until the 5 mass % threshold is reached. This trend in the variation of AC conductivity can be confidently connected to the hopping mechanism in the applied external field. All the plots show an imperfect linear trend in the variation of AC conductivity with a frequency indicating the non-ohmic type of conduction in these systems. The conductivity in the low-frequency region is caused by the interfacial polarisations and only a limited number of charge carriers can migrate effectively over larger distances. However, at higher frequencies, charge transport is greater, leading to increased conductivity [39]. A similar result is obtained in chlorinated natural rubber/metal oxide nanocomposites [23]. The composites show a significant increase in AC conductivity and this specifies the decisive role of conducting NiO filler in improving the bulk conductivity of blend composites. The incorporated nanoparticles cause an increase in charge carriers inside the blend and thereby form a wide conducting network across the relatively low-conducting blend system. This large network of conducting electrons facilitates the easy movement of charge carriers across the Cl-NR/PIN blend system and the system behaves like a common semiconductor. The defects formed inside the blend by the nano NiO also improve the conductivity. The slight depreciation observed at higher filler loadings (7 and 10 mass %) beyond threshold filling is caused by the breakage of a conductive network by the formation of agglomerates of filler particles.
Temperature-dependent AC conductivity and activation energy
The variation of AC conductivity of Cl-NR/PIN and its composites at different temperature conditions are plotted in Fig. 9. The nature of all the plots is the same at all temperature conditions. The nonperfect linear trend of all the plots indicates their semiconducting nature and the non-ohmic type of conduction possible in these systems [40]. The lower frequency plateau region observed in AC conductivity plots represents the DC conductivity of the samples. This DC conductivity in such materials arises from the motion of charge carriers that are not firmly bound in the system. At higher frequencies, the conductivity is aroused by the mobility of bounded charge carriers and in that region, the charged species obeys Jonscher’s universal power law (σAC(ω) = Aωs) [41]. Here the exponential factor ‘s’ indicates the degree of interaction between the charge carriers and the type of conduction in those systems and its values usually lie between 0 and 1. The hopping of charge carriers at low temperatures is very low, and as temperature increases, the thermally activated hopping of charge carriers occurs and resulting in high conductivity in materials [41]. As the thermal energy increases, the mobility of charge carriers increases and the number of transition sites increases which causes elevated conduction in all the systems and proves the semiconducting behaviour of all the systems. The emergence of new transit states facilitates the easy conduction of electricity by overcoming the energy barriers for hopping. Table 1 indicates the s values obtained based on the power law equation and it is observed that the ‘s’ value decreases with temperature and with the increase in filler loading. This trend illustrates the small polaron hopping of charge carriers [42].
Activation energy can be defined as the energy required to overcome the potential barrier to facilitate the easy movement of charge carriers across the material. The Arrhenius equation is given as.
Here \({\sigma }_{0}\) and K are constants known as the preexponential factor and the Boltzmann constant. Ea is the activation energy which is a constant for a sample at a constant temperature and T is the absolute kelvin temperature [42]. The plot of log \({\sigma }_{\text{AC}}\) with 1000/T is displayed in Fig. 10. Charge carrier mobility is low in low-frequency and low-temperature zones and the 5 mass% composite shows the lowest activation energy in all the temperatures.
Mechanical properties
Table 2 shows the tensile strength, elongation at break and hardness of the NiO filled Cl-NR/PIN blend nanocomposites. The strong interaction between the nanoparticles and the blend segments is maximum for 5mass % sample which leads to higher tensile strength. Previous research has described this type of interaction between NiO and the polymer matrix [43]. The aggregation of nanoparticles in the polymer blend matrix causes a slight loss in tensile strength beyond 5 mass % NiO loading. The inclusion of NiO clearly reduces the elongation at break of composites, demonstrating greater resistance to stretching as a result of the effective filler–polymer interaction. The concentration of nanoparticles in the polymer is a key factor in determining the attribute of hardness. The hardness of the blend composite increases with the loading of nanoparticles. Soft composites with hardness values less than 50 can be beneficial in a range of applications, including soft and flexible electronics and hard composites for automotive applications. Based on the findings, we can conclude that the composites developed in this study had a substantially lower hardness than 65, enabling the Cl-NR/PIN/NiO composites to be used in flexible applications [44].
Conclusions
The effect of nano NiO loading on the structural, morphological, thermal, electrical and dielectric properties of the Cl-NR/ PIN blend system was investigated in the present study. The UV–Vis absorbance intensity increased with filler loading up to a threshold loading level of 5 mass % and beyond that, a slight depreciation was observed due to the clustering of filler particles. A peak at 433 cm−1 indicated the effective incorporation of NiO nanofillers in the Cl-NR/PIN blend system. The amorphous nature of the Cl-NR/PIN blend was reduced by the incorporation of nanofiller, as evident from the XRD patterns of the composites. The thermal stability of nanocomposites was much enhanced compared to that of the bare blend system as illustrated by the increased degradation temperature of composites in TGA curves. The dispersion of nanoparticles in the Cl-NR/PIN blend was clearly visible in the FE-SEM micrographs. The AC electrical conductivity of blend system was increased with nanofiller addition and maximum electrical conductivity was observed for a 5 mass % loaded composite. Non-perfect linear plots obtained for the blend nanocomposites explain the non-ohmic type of conduction possible in these systems. All composite systems follow the same trend of AC conductivity variation, which proves the hopping conduction in these semiconducting systems. Optimum AC conductivity was observed for composite with a 5 mass % filler loading and at higher filler loadings, the continuous network of conducting electrons was disturbed by the filler lumps and thus, a reduction was observed in AC conductivity values. The temperature has a positive effect on conductivity; as the temperature rises, the activation energy decreases and therefore the conductivity increases. The decreasing trend of the exponential factor obtained from the Power law equation proved the small polaron hopping conduction possible in these composite systems. The interfacial polarisations possible at the interface of filler and blend system were approved by the frequency independent region in the plot of dielectric constant with frequency. The inclusion of metal oxide nanoparticles increases the tensile strength and hardness of nanocomposites while decreasing the elongation at break. The high values of dielectric constant for composites indicate that these materials possess a semiconducting nature and these materials can be used in various industries where energy storage devices are fabricated.
References
Amorim DRB, Bellucci FS, Job AE, Guimaraes IS, Cunha HN. Electrical, structural and thermal properties of new conductive blends (PANICG) based on polyaniline and cashew gum for organic electronic. J Therm Anal Calorim. 2019;136:1615–29. https://doi.org/10.1007/s10973-018-7778-6.
Yamani K, Berenguer R, Benyoucef A, Morallon E. Preparation of polypyrrole (PPy)-derived polymer/ZrO2 nanocomposites. J Therm Anal Calorim. 2019;135:2089–100. https://doi.org/10.1007/s10973-018-7347-z.
Sofiah AG, Samykano M, Shahabuddin S, Kadirgama K, Pandey AK. An experimental study on characterization and properties of eco-friendly nanolubricant containing polyaniline (PANI) nanotubes blended in RBD palm olein oil. J Therm Anal Calorim. 2021;145:2967–81. https://doi.org/10.1007/s10973-020-09891-6.
Suvarna S, Furhan, Ramesan MT. (2023) Structural, conductivity, mechanical and wettability properties of copper alumina reinforced chlorinated polyethylene/polyvinyl chloride blend nanocomposites. Res Chem Intermed. 49(5):1891-908. https://doi.org/10.1007/s11164-022-04881-9
Camillo EC, Constantino CJ, Teruya MY, Alves N, Mattoso LH, Job AE. Dependence of the electrical conductivity and elastomeric properties on sample preparation of blends of polyaniline and natural rubber. J Appl Polym Sci. 2005;97:1498–503. https://doi.org/10.1002/app.21899.
De Paoli MA, Gazotti WA. Conductive polymer blends: preparation, properties and applications. Macromol Symposia. 2002;189:83–104. https://doi.org/10.1002/masy.200290008.
Silva MJ, Soares VO, Dias GC, Santos RJ, Job AE, Sanches AO, Malmonge JA. Study of thermal and mechanical properties of a biocomposite based on natural rubber and 45S5 Bioglass® particles. J Therm Anal Calorim. 2018;131:735–42. https://doi.org/10.1007/s10973-016-5933-5.
Masek A, Diakowska K, Zaborski M. Physico-mechanical and thermal properties of epoxidized natural rubber/polylactide (ENR/PLA) composites reinforced with lignocellulose. J Therm Anal Calorim. 2016;125:1467–76. https://doi.org/10.1007/s10973-016-5682-5.
Nghia PT, Onoe H, Yamamoto Y, Kawahara S. Hydrogenation of natural rubber having epoxy group. Colloid Polym Sci. 2008;286:993–8. https://doi.org/10.1007/s00396-008-1859-1.
Gnecco S, Pooley A, Lefimil C, Pino C, Valenzuela L. Chlorination of low-molecular-weight Euphorbia lactiflua natural rubber. Polym Bull. 1997;39:605–12. https://doi.org/10.1007/s002890050192.
Suvarna S, Furhan, Parvathi K, Ramesan MT. (2023) Role of copper alumina nanoparticles on the performance of polyvinylchloride nanocomposites. J Vinyl Addt Technol. 29: 17–28. https://doi.org/10.1002/vnl.21939
Ramesan MT, Suvarna S. Effect of Cu-Al2O3 nanoparticles on the performance of chlorinated polyethylene nanocomposites. Prog Rubber Plast Recycl Technol. 2023;31:81–95. https://doi.org/10.1177/14777606221136152.
Wang W, Ren G, Wang M, Liu Y, Wu S, Shen J. A novel composite for energy storage devices: core–shell MnO2/polyindole nanotubes supported on reduced graphene oxides. J Mater Sci Mater Electron. 2018;29:5548–60. https://doi.org/10.1007/s10854-018-8523-4.
Mudila H, Prasher P, Kumar M, Kumar A, Zaidi MG, Kumar A. Critical analysis of polyindole and its composites in supercapacitor application. Mater Renew Sustain Energy. 2019;8:9. https://doi.org/10.1007/s40243-019-0149-9.
Zhu D, Zhou Q, Liang A, Zhou W, Chang Y, Li D, Wu J, Ye G, Xu J, Ren Y. Two-step preparation of carbon nanotubes/RuO2/polyindole ternary nanocomposites and their application as high-performance supercapacitors. Front Mater Sci. 2020;14:109–19. https://doi.org/10.1007/s11706-020-0497-5.
Jiajia W, Zhanwen D, Ping X, Zhijiang C. Fabrication of flexible polyindole/carbon nanotube/bacterial cellulose nanofiber nonwoven electrode doped by D-tartaric acid with high electrochemical performance. Cellulose. 2020;27:6353–66. https://doi.org/10.1007/s10570-020-03199-2.
Khati K, Joshi I, Bisht A, Zaidi MG. Haemoglobin/polyindole composites: the novel material for electrochemical supercapacitors. Bull Mater Sci. 2019;42:20. https://doi.org/10.1007/s12034-018-1700-5.
Zhou Q, Zhu D, Ma X, Xu J, Zhou W, Zhao F. High-performance capacitive behavior of layered reduced graphene oxide and polyindole nanocomposite materials. RSC Adv. 2016;6:29840–7. https://doi.org/10.1039/c5ra27375g.
Hashemi SA, Naderi HR, Mousavi SM, Bahrani S, Arjmand M, Dimiev AM, Ramakrishna S. Synergic effect of laser-assisted graphene with silver nanowire reinforced polyindole/polypyrrole toward superior energy density. Carbon. 2022;188:276–8. https://doi.org/10.1016/j.carbon.2021.12.028.
Anjitha T, Anilkumar T, Mathew G, Ramesan MT. Zinc ferrite @ polyindole nanocomposites: synthesis, characterization and gas sensing applications. Polym Compos. 2019;40:2802–11. https://doi.org/10.1002/pc.25088.
Lee H, Park SC, Roh JS, Moon GH, Shin JE, Kang YS, Park HB. Metal–organic frameworks grown on a porous planar template with an exceptionally high surface area: promising nanofiller platforms for CO 2 separation. J Mater Chem A. 2017;5:22500–5.
Zhang H, Cao D, Jiang Z, Yu H, Shi A, Bai X. Trunk-Leaf Vein” structure inspired synthesis of mesoporous carbon @ nickel oxide/nickel ternary composite for sustainable supercapacitor electrode. Appl Surf Sci. 2022;571:151324. https://doi.org/10.1016/j.apsusc.2021.151324.
Parvathi K, Ramesan MT. High performance chlorinated natural rubber/ zinc ferrite nanocomposite prepared through industrial compounding technique. Polym Bull. 2023;80:3165–82. https://doi.org/10.1007/s00289-022-04201-6.
Talbi H, Ghanbaja J, Billaud D, Humbert B. Vibrational properties and structural studies of doped and dedoped polyindole by FTIR. Raman EEL Spect, Polym. 1997;38:2099–106. https://doi.org/10.1016/S0032-3861(96)00759-8.
Rolere S, Liengprayoon S, Vaysse L, Sainte-Beuve J, Bonfils F. Investigating natural rubber composition with fourier transform infrared (FT-IR) spectroscopy: a rapid and non-destructive method to determine both protein and lipid contents simultaneously. Polym Test. 2015;43:83–93. https://doi.org/10.1016/j.polymertesting.2015.02.011.
Parvathi K, Ramesan MT. Insights into structural, thermal, electrical and mechanical properties of copper alumina reinforced chlorinated natural rubber nanocomposites. J Thermoplast Compos Mater. 2022. https://doi.org/10.1177/08927057221083495.
Parvathi K, Ramesan MT. Structure, properties and antibacterial behaviour of nickel oxide reinforced natural rubber nanocomposites for flexible electronic applications. J Appl Polym Sci. 2022;139:e53120. https://doi.org/10.1002/app.53120.
Saikia JP, Paul S, Konwar BK, Samdarshi SK. Nickel oxide nanoparticles: a novel antioxidant. Colloids Surf, B. 2010;78:146–8. https://doi.org/10.1016/j.colsurfb.2010.02.016.
Kumar YR, Deshmukh K, Naseer Ali MM, Abhijay G, Al-Onazi WA, Al-Mohaimeed AM, Khadheer PSK. Structure, morphology and modelling studies of poly vinyl alcohol nanocomposites reinforced with nickel oxide nanoparticles and graphene quantum dots. Environ Res. 2022;203:111842. https://doi.org/10.1016/j.envres.2021.111842.
Holzwarth U, Gibson N. The Scherrer equation versus the ‘Debye-Scherrer equation.’ Nature Nanotech. 2011;6:534. https://doi.org/10.1038/nnano.2011.145.
Khalil AM, Rabie ST. Mechanical, thermal and antibacterial performances of acrylonitrile butadiene rubber/polyvinyl chloride loaded with Moringa oleifera leaves powder. J Therm Anal Calorim. 2021;143:2973–81. https://doi.org/10.1007/s10973-019-09194-5.
Palaniappan S, John A. Facile synthesis of bis (indolyl) methanes using polyindole salt as reusable catalyst. J Mol Catal A Chem. 2005;242:168–72. https://doi.org/10.1016/j.molcata.2005.07.041.
Sankar S, Ramesan MT. Thermal, optical and temperature-dependent electrical properties of poly(aniline-co-pyrrole)/copper alumina nanocomposites for optoelectronic devices. J Therm Anal Calorim. 2022;147:13375–87. https://doi.org/10.1007/s10973-022-11670-4.
Mi HY, Li Z, Turng LS, Sun Y, Gong S. Silver nanowire/thermoplastic polyurethane elastomer nanocomposites: thermal, mechanical, and dielectric properties. Mater Design. 2014;56:398–404. https://doi.org/10.1016/j.matdes.2013.11.029.
Pan C, Markvicka EJ, Malakooti MH, Yan J, Hu L, Matyjaszewski K, Majidi C. A liquid-metal–elastomer nanocomposite for stretchable dielectric materials. Adv Mater. 2019;31:1900663. https://doi.org/10.1002/adma.201900663.
Bhagat DJ, Dhokane GR. Novel synthesis and DC electrical studies of polyindole/poly(vinyl acetate) composite films. Chem Phys Lett. 2015;619:27–31. https://doi.org/10.1016/j.cplett.2014.11.052.
Zhu L. Exploring strategies for high dielectric constant and low loss polymer dielectrics. J Phys Chem Lett. 2014;5:3677–87. https://doi.org/10.1021/jz501831q.
Liu R, Wang J, Li Q, Li S, Zhang S, Ding X. Copper phthalocyanine oligomer grafted acrylic elastomer nanocomposites with high dielectric constants. J Appl Polym Sci. 2014. https://doi.org/10.1002/app.39975.
Saji J, Khare A, Choudhary R, Mahapatra S. Impedance analysis, dielectric relaxation, and electrical conductivity of multi-walled carbon nanotube-reinforced silicon elastomer nanocomposites. J Elastomers Plast. 2015;47:394–415. https://doi.org/10.1177/0095244313514991.
Dutta K, De SK. Electrical conductivity and dielectric properties of SiO2 nanoparticles dispersed in conducting polymer matrix. J Nanopart Res. 2007;9:631–8. https://doi.org/10.1007/s11051-006-9184-4.
Jegadheeswaran S, Sundaramahalingam A, Pohekar SD. High-conductivity nanomaterials for enhancing thermal performance of latent heat thermal energy storage systems. J Therm Anal Calorim. 2019;138:1137–66. https://doi.org/10.1007/s10973-019-08297-3.
Ramesan MT, Jayakrishnan P, Sampreeth T, Pradyumnan PP. Temperature-dependent AC electrical conductivity, thermal stability and different DC conductivity modelling of novel poly(vinyl cinnamate)/zinc oxide nanocomposites. J Therm Anal Calorim. 2017;129:135–45. https://doi.org/10.1007/s10973-017-6140-8.
Meera K, Ramesan MT. Performance of boehmite nanoparticles reinforced carboxymethyl chitosan/polyvinyl alcohol blend nanocomposites tailored through green synthesis. J Polym Environ. 2023;31:447–60. https://doi.org/10.1007/s10924-022-02649-1.
Kumar V, Alam MN, Park SS. Robust magneto-rheological elastomers performance for composites based on iron oxide and carbon black in silicone rubber. J Polym Res. 2022;29:251.
Acknowledgements
The authors greatly acknowledge the financial assistance from KSCSTE, Government of Kerala, India (Order No.566/2017/KSCSTE).
Funding
Funding was provided by Kerala State Council for Science, Technology and Environment, 566/2017 KSCSTE, M. T. Ramesan
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this article have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parvathi, K., Verma, M. & Ramesan, M.T. Enhanced thermal, electrical and mechanical properties of nickel oxide reinforced chlorinated natural rubber/poly (indole) blend nanocomposites. J Therm Anal Calorim 148, 10139–10149 (2023). https://doi.org/10.1007/s10973-023-12358-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12358-z