Abstract
In this study, the combustion characteristics and kinetics of various mixtures of both raw and torrefied rose pulp and red pine sawdust with each other and with Elbistan lignite were investigated in the context of lignocellulosic biomasses’ potential use as fuel. Ignition temperatures, peak temperatures, burnout temperatures, and comprehensive combustion indexes of the fuel mixtures were found to rise with the torrefaction process. This finding indicates that the fuel/combustion performance of the waste biomass can be improved by the torrefaction process. Moreover, the combustion behavior of rose processing waste and pine sawdust has been significantly improved by adding lignite to the samples to be torrefied. Average activation energies of raw pine sawdust, rose processing waste, and Elbistan lignite were found to be 178, 187, and 91 kJ mol–1, respectively. However, the activation energies of both raw samples and their mixtures with lignite as well as each other decreased with the torrefaction process. Furthermore, a synergistic effect was also observed during the combustion of the mixtures of both raw and torrefied biomass with Elbistan lignite.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The lignite reserves of Turkey are estimated to be 16.3 billion tons; while lignite’s share in Turkey’s total primary energy consumption is about 27% [1]. Imports account for about 75% of Turkey’s energy demand [2], making the country heavily dependent on fossil fuel imports. Therefore, it is crucial for Turkey to use domestic lignite reserves to cover a larger portion of its energy needs. Yet, only 1% of lignite reserves in Turkey have a calorific value above 4000 kcal/kg. Against this background, burning these low-quality coals in combination with biomass represents a more stable means of biomass disposal, all the while generating comparatively cleaner energy with existing resources [3]. On the other hand, biomass is usually considered a secondary fuel, due to a number of reasons such as seasonality and storage issues. When woody biomass is used in combustion systems and is blended with coal, certain economic and technical problems such as low grindability, low energy density, and high transportation cost arise [4, 5]. To overcome these problems, torrefaction—a thermal pre-treatment method—is applied on biomass. Torrefaction is the heat treatment of biomass at certain temperatures (250–350 °C) in order to improve its fuel properties and combustion characteristics [73]. The torrefaction process converts most of the raw biomass into a solid fuel that is relatively odorless and smokeless. Moreover, with torrefaction, biomass turns into a dense, hydrophobic, non-biodegradable and energy-dense fuel that can be stored for longer periods [6,7,8]. Most crucially, with the help of torrefaction, the share of biomass in co-fired plants can be increased up to 60–70% and thus GHG emissions into atmosphere can be significantly reduced [9].
Turkey is one of the most important production centers of both rose concrete and rose oil worldwide [16]. Approximately 80% of rose oil and rose concrete production in Turkey takes place in Isparta [17]. The amount of distillation waste produced in an average year is approximately 26,000 tons [18]. Uncontrolled disposal of this waste increases the pollution burden on surface and groundwater in Isparta and the surrounding provinces, leading to various environmental problems such as odor pollution and aesthetic problems [18]. On the other hand, Turkish red pine (Pinus brutia Ten.) is the most wide-spread tree species in Turkey, covering an area of 5,610,215 hectares [19]. One should also note that, in 2019, nearly 850,000 m3 of wood residues from forest products were produced in Turkey [20].
In the light of these figures, it is understood that Turkey has a considerable potential in energy recovery from biomass. Against this background, the combustion kinetics and combustion characteristics of such biomass containing high amounts of volatile organic materials will play a crucial part in designing efficient sustainable co-pyrolysis and co-combustion processes [21]. In this context, thermal decomposition stands out as an important factor during combustion and co-combustion processes, whereas thermogravimetric (TG) analysis is useful for analyzing the kinetics of thermal decomposition of biomass, as well as the combustion/co-combustion characteristics of biomass, lignite, and their blends. TGA is noted as a useful tool for investigating the synergy between biomass and lignite samples during co-combustion processes [21].
Although there are various studies on both combustion and co-combustion kinetics and properties of torrefied lignocellulosic biomass samples including chars made from raw and torrefied willow [10, 11], raw and torrefied duckweed [12], torrefied Norwegian birch and spruce [13, 14], raw and torrefied willow [15], raw and torrefied Canadian yellow poplar, Colombian sugarcane bagasse and their chars [9], and more (corn stalk, olive tree pruning, vine pruning) [5], no studies so far have been found in the literature on the thermal kinetic constants or co-combustion characteristics of both raw and torrefied rose pulp. Furthermore, the majority of the studies in the literature are mostly focused on char production and combustion kinetics, along with combustion characteristics of the chars produced. The studies on the synergistic effect arising with the co-combustion of lignite and torrefied biomass, on the other hand, are rare. Therefore, the present study aims to evaluate the fuel characteristics of both rose pulp and wood sawdust samples and their blends with low quality Turkish lignite, followed by an investigation of their potential for use as fuel, and how their fuel properties can be improved with the torrefaction process. Another goal is to investigate the synergy between raw/torrefied biomass and lignite in blends thereof, and to determine the activation energy of the samples, as one of the most important pieces of data required for the design of combustion systems.
Material and methods
Material and thermal treatment
In this study, rose pulp and red pine sawdust samples were obtained, respectively, from a local rose oil factory and a sawmill plant in Isparta. Lignite samples to represent local Turkish lignite were taken from Afşin-Elbistan lignite Works. Since both rose pulp (68% ± 5.14%) and Elbistan lignite (55% ± 8.24%) contain substantial amounts of moisture, they were first dried at room temperature and then in an oven at 70 °C. Then, collected samples were crushed using a ball mill (pine sawdust was not extra milled because it was originally obtained in dust form) and sieved in the 300–850 µm range for thermogravimetric analysis (TGA).
The proximate and elemental analysis results of the samples used for the TG analysis are given in Table 1. ASTM D-871–82 and ASTM E-872–82 standards were applied, respectively, for the determination of the moisture and volatile matter (VM) content of the biomass samples [74]. Ash (A) contents of the woody biomass and rose pulp samples were determined using ASTM D-1102–84 and ASTM E-1755–01 standards, respectively [74, 75]. The ASTM D3172-13 standard was also used for the proximate analysis of lignite samples [76]. The fixed carbon (FC) content of both biomass and lignite samples was calculated using Eq. (1) [22].
Empirical correlations developed in the literature for raw [Eq. (2)] and torrefied [Eq. (3)] biomass were used to calculate the higher heating values (HHV) of the samples [23].
where VM and FC, respectively, represent the volatile matter and fixed carbon content of the sample.
In order to calculate the HHV of the lignite sample, Eq. (4) using the elemental content of Elbistan lignite was applied [24];
Previous studies revealed that the sulfur contents of lignite samples are higher than those of other biomass samples. Demirbaş et al. (2008) examined 20 Turkish lignites collected from 20 distinct regions of Turkey and found that the HHV values of the studied samples varied between approximately 5398 kcal/kg and 7022 kcal/kg [25]. Even though Elbistan lignite has almost the lowest calorific value among all Turkish lignites, its calorific value was found to be significantly higher than those of the biomass samples analyzed (Table 1). The total sulfur and ash contents of the Elbistan lignite used in this study were also found to be very close to those of the lignites examined in Demirbaş’s (2008) study. Moreover, in the literature, it has been stated that the carbon and nitrogen contents of local Turkish lignites vary from 61 to 72.6%, and from 1.5 to 2.6%, respectively [25]. However, the present study found the carbon (40.51%) and nitrogen (0.68%) contents of Elbistan lignite to be lower than other local Turkish lignites analyzed in the literature [26].
For the torrefaction of the biomass samples, 10 g of samples was placed in 50-mL airtight crucibles. Then, the crucibles were subjected to thermal treatment in an ash furnace. Preliminary experiments to identify optimum torrefaction conditions of rose pulp and sawdust samples were performed in our previous study [27]. During the torrefaction process, biomass begins to decompose at around 150–200 °C [28]. Beyond 300 °C, a significant amount depolymerization is observed [29]. Three different temperatures (250, 270, and 290 °C) and three different holding times (15, 30, and 60 min) were tried. Since the highest energy density was obtained at 270 °C with 60 min holding time for both rose pulp and sawdust samples, [27] these were assumed to be the optimum torrefaction conditions for the purposes of the present study.
Thermogravimetric analysis and applied kinetic model
In this study, 14 different mixtures were prepared using raw pine sawdust, rose pulp and Elbistan lignite with different rates of combination (Table 2). A further set of 14 blends was prepared using torrefied biomass samples at various rates. TG analyses were performed for each blend, using PerkinElmer-Pyris-1 model TG at four different heating rates, 10 °C min−1, 20 °C min−1, 30 °C min−1, and 40 °C min−1 under 40 mL min−1 dry air flow at a temperature range of 25 °C to 1000 °C in temperature. The repeatability of the TG analysis was also checked in the study, and the process was found to be repeatable at a temperature range of approximately ± 2 °C.
The fundamental rate equation expresses the rate of conversion –dα/dt– as a function of the reactant concentration (α) and the rate constant (k), at a constant temperature (T):
where
where mO is the initial mass of the reactant, mt is the mass at time t, m∞ is the final mass. k (s − 1) is usually given by the Arrhenius equation as,
where A is the pre-exponential constant (min-1), Ea is the activation energy (kJ mol–1), T is the temperature (K), and R is the universal gas constant (8.314 kJ mol–1). The combination of Eqs. (5) and (7) gives;
For non-isothermal TGA, if linear heating rate is assumed as β = dT/dt, the reaction rate in Eq. 8 gives;
Equations (8) and (9) are used for the calculation of thermal kinetics from thermogravimetric data. In this study, the model-free Flynn–Wall–Ozawa method was used for the calculation of activation energies of the blends [30]. Model-free methods perform an important function in terms of calculating the activation energies of biomass samples [31]. The most important advantages of model-free methods are that they are easy to use and that they prevent problems arising from kinetic model selection. With the Flynn–Wall–Ozawa method, the activation energy was calculated by using temperatures corresponding to specific conversion values at different heating rates for an independent model [32]. This method is defined by Eq. (10) [30],
where β is the heating rate and A is the pre-exponential factor. Ea is the activation energy, g(α) is a function of the conversion, and R is the gas constant. Since log (β) is plotted vs. 1/T at any conversion rate for different heating rates, the slope of the straight line will give 0.4567 Ea/R. Therefore, Ea is calculated based on the slope of this line.
The comprehensive combustion characteristics index (S) was also calculated using the formula below [33],
In the equation, (dW/dt)max and (dW/dt)mean stand for maximum and average burning rates, respectively. Ti and Tb are the ignition and burnout temperatures of the samples, respectively. High S values indicate high combustion properties for the fuels [34].
Results and discussion
Analyzing combustion characteristics and activation energies of the raw blends
Combustion characteristics of the raw blends
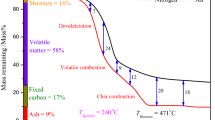
The TG/DTG curves of the selected blends are presented in Fig. 1. DTG is the first derivative of the TG curve, and it leads to preliminary results based on the oxidative thermal decomposition of the samples. Moreover, the height of the DTG curves also shows the rate of mass decomposition of the sample in the specified temperature ranges [35]. Previous studies noted roughly three regions in lignite-biomass blends [21]. The present study also identified three regions on the TG curves of the blends. These regions were determined based on the approximate starting and ending points of the DTG curves, which showed the thermal decomposition of organic matter/volatile substances or the loss of water in the samples [36]. The first region on the DTG curve is associated with the loss of moisture in the sample. The second region, where the highest mass loss occurred and the main oxidation started, appeared due to the oxidation and removal of volatile substances. Thermal decomposition of hemicellulose and cellulose occurred in this region as hemicellulose decomposes in the 200–300 °C temperature range. Then, decomposition of cellulose occurs between 300 and 450 °C [37]. The last region occurred due to the oxidation of the remaining char after the volatiles were removed from the sample. Lignin breaks down over a broad temperature range (250–500 °C). Therefore, oxidative breakdown of lignin in the samples also occurred in this region [27]. Furthermore, the decomposition of biomass is more prevalent in the blends, compared to the case with lignite. On the other hand, in the last region, the decomposition of lignite in addition to that of the residual biomass is observed as a prominent mechanism [21].
The ignition, peak, burnout temperatures, maximum DTG value and the comprehensive combustion characteristic index (S) were calculated for each blend (Table A.1). Ignition temperature is an important property of fuels as a certain amount of combustion is required in a gasifier to provide the energy required for drying and pyrolysis, and ultimately for the endothermic gasification reaction. Therefore, it is important to know the ignition properties of the fuels. When the temperature reaches a certain value—the ignition temperature—the rate of heat production matches or exceeds the rate of heat loss [38]. Peak temperature (PT) and burnout temperature (BT) are the typical temperatures that can be determined by looking at the DTG curves. The peak temperature on the DTG curves occurred where the rate of mass loss peaked and serves as a shorthand for the flammability of the samples: low PT indicates easier combustion. On the other hand, the burnout temperature indicates the temperature at which the sample oxidation is completed [36].
According to the figures in Table A.1, the ignition temperatures of R100 and P100 were found to be 198 ± 21 °C and 220 ± 13 °C, respectively. Fuels with higher volatile matter content can ignite more easily and complete their burning in a shorter time [39]. In the study, the ignition temperatures of the blends usually increased as the lignite content in the blends increased. The average ignition temperatures of R98L2, R96L4, and R90L10 blends were 210 ± 13 °C, 214 ± 12 °C, and 218 ± 12 °C, respectively. Similarly, the average ignition temperatures of P98L2, P96L4, and P90L10 blends also increased with the addition of lignite and were found to be 225 ± 7 °C, 230 ± 12 °C, and 235 ± 11 °C, respectively. Since the volatile matter content of both pine and rose pulp was significantly higher than that of lignite (Table 1), the ignition temperatures of the blends increased as the lignite content in the blends increased. A similar situation was also with for the biomass and lignite blends, and average ignition temperatures of P49R49L2, P48R48L4, and P45R45L10 samples were recorded, respectively, as 219 ± 14 °C, 220 ± 14 °C, and 223 ± 16 °C. Looking at these figures, one can forcefully argue that both raw pine sawdust and rose pulp samples were easier to ignite compared to their blends with lignite.
The average peak temperatures in the second region where the main decomposition occurred were 374 ± 16 °C and 343 ± 8 °C for P100 and R100 samples, respectively. It is also noteworthy that, in the second region the degradation of hemicellulose and cellulose occurred. The average peak temperatures for R98L2, R96L4, and R90L10 blends were 343 ± 10 °C, 346 ± 13 °C, and 347 ± 11 °C, respectively. On the other hand, the average peak temperatures for P98L2 (377 ± 7 °C), P96L4 (378 ± 12 °C), and P90L10 (379 ± 11 °C) fuel blends showed a significant increase compared to the P100 sample (374 ± 16 °C), but did not exhibit a significant increase overall, parallel to the increase in the percentage of lignite in the blend. A noticeable increasing trend was not observed in the main thermal decomposition regions of the blends, probably due to low percentage of lignite in the blends. The average peak temperatures of the blends consisting of rose and pine were 348 ± 11 °C, 362 ± 8 °C, and 371 ± 7 °C for P25R75, P50R50, and P75R25, respectively. Therefore, it can be concluded that higher thermal decomposition temperatures were obtained as the content of pine increased in the blends.
The average burnout temperatures of the P100 and R100 samples were found to be 692 ± 76 °C and 866 ± 109 °C, respectively (see Table A.1). With the addition of lignite, the burnout temperatures of the blends containing pine increased significantly and the average burnout temperatures of the P98L2, P96L4, and P90L10 blends reached 804 ± 121 °C, 834 ± 112 °C, and 824 ± 101 °C, respectively. Even though the burnout temperatures of blends increased with addition of the lignite, no systematic trend was observed. Similar findings were also observed during the co-combustion of biomass/coal blends. Similarly, higher or lower burnout temperatures were reported in the literature, depending on the fuel properties and blending ratios [77, 78].
Maximum DTG values were found to be − 23 ± 11% min–1 and − 12 ± 7% min–1 for the P100 and R100 samples, respectively. The maximum DTG levels decreased with the addition of lignite into the fuel blends. In other words, in this study, raw pine samples were found to be the most reactive and the easiest to burn woody fuel. The increased lignite content of the blends could cause less volatile matter formation in the oxidative environment. Accordingly, average maximum DTG values for R98L2, R96L4, and R90L10 blends were found to be − 11 ± 5% min–1, − 11 ± 6% min–1, and − 9 ± 5% min–1, respectively. Average maximum DTG values for P98L2, P96L4, and P90L10 blends were found to be − 22 ± 10% min–1, − 22 ± 11% min–1, and − 20 ± 9% min–1, respectively. The addition of 2% and 4% lignite by mass produced did not lead to significantly altered maximum DTG values. Although the maximum DTG is also considered to be directly related to the reactivity of the sample, the temperature at which the maximum DTG is achieved is generally considered inversely proportional to the reactivity and combustion of the sample [40]. The average maximum DTGs of the fuel blends containing both pine and rose (P25R75 (− 11 ± 6% min–1), P50R50 (− 21 ± 14% min–1), and P75R25 (− 19 ± 15% min–1)) were calculated, leading to the conclusion that the reactivity of the blends with equal amounts of rose and pine wastes was higher than those of the other two blends.
S represents the reactivity of coal combustion during the oxidation reaction. The fuels with higher S values exhibit better performance during combustion [22], as higher S values are associated with lower ignition and burnout temperatures and higher mass loss rates [41]. Therefore, coal with the highest S value is easier to ignite [22]. In this study, average S values for R98L2, R96L4, and R90L10 blends were found to be 7.41E-07 ± 5.22E-07, 6.47E-07 ± 4.76E-07, and 6.2E-07 ± 4.58E-07, respectively. Average S values for P98L2, P96L4, and P90L10 fuel blends were found to be 1.51E-06 ± 1.07E-06, 1.42E-06 ± 1.07E-06, and 1.15E-06 ± 7.53E-07, respectively. It was observed that S values decreased as more lignite was added to both pine and rose pulp blends. As the volatile content of the samples was reduced, lower S values were obtained for the samples containing lignite, compared to the raw samples. All blends containing lignite exhibited a better combustion performance compared to lignite itself. The average S values of P25R75, P50R50, and P75R25 samples containing both rose and pine were 8.14E-07 ± 6.16E-07, 2.39E-06 ± 2.43E-06, and 1.7E-06 ± 1.89E-06, respectively. A glance at the bigger picture involving the S values of all blends reveals that the best combustion performance was obtained with P75R25 blend.
The flammability of the samples increased significantly with increasing heating rates. Table A.1 shows that ignition, peak, and burnout temperatures are also shifting to higher ranges as the heating rate increases, given the higher efficiency of transfer occurs at lower heating rates [42]. In the light of the results presented so far, one can forcefully argue that higher thermal decomposition temperatures are observed for all samples, with increases in heating rates due to heat transfer limitations.
Activation energies of the raw blends
In the study, the Flynn–Wall–Ozawa method was used to calculate the activation energies of raw blends. The average activation energies of the blends are presented in Table 3. The activation energies of the blends varied from 101 kJ mol–1 (R90L10) to 187 kJ mol–1 (R100). According to data presented in Table 3, the activation energies of both raw pine and raw rose samples and blends thereof decreased with the addition of lignite. The highest activation energies were recorded with raw rose and pine samples. No study examining the thermal kinetic constants and combustion characteristics of rose pulp was found in the literature so far. However, the Ea values obtained for the biomass blends in this study were found to be similar to the values noted in the literature for other lignocellulose biomasses and their blends with coal, including wood (96–147 kJ mol–1) [43], pine wood (91.8 to 175.8 kJ mol–1) [44], wood chips, nutshells (139–155 kJ mol–1) [45], Turkish Beypazari Lignite and their blends (90.9–215.3 kJ mol–1) [46], branched millet and beet root (43.6–133.3 kJ mol–1) [47], pine sawdust, coal, and their blends (143.1–167.7 kJ mol–1) [34].
Analysis of combustion characteristics and activation energies of the torrefied blends
Combustion characteristics of the torrefied blends
The TG/DTG curves of the selected torrefied blends are given in Fig. 2. In the study, the average ignition temperatures of the torrefied TP100 and TR100 samples were found to be 228 ± 7 °C and 230 ± 8 °C, respectively (Table A2). Therefore, the ignition temperatures of the torrefied pine and rose samples increased about 8 °C and 32 °C, respectively, when compared against their raw form. Because of the degradation of organic matter (e.g., hemicellulose) in the biomass, the ratio of the lignin content rich in carbon increases during torrefaction [48]. Agricultural residues have relatively higher hemicellulose content than that of woody biomass [49, 50]. Such higher hemicellulose content of the rose pulp sample compared to the pine-based sample is most probably the reason why the carbon content of the rose pulp sample increased further after the torrefaction, and higher ignition temperatures were observed for rose pulp samples compared to pine samples. A similar trend was also observed with the addition of lignite into the torrefied rose pulp samples (see Table A2). Accordingly, higher ignition temperatures were observed for TR98L2 (244 ± 11 °C), TR96L4 (254 ± 6 °C), and TR90L10 (258 ± 5 °C) blends compared to the case with torrefied pine and lignite blends –TP98L2 (227 ± 8 °C), TP96L4 (235 ± 9 °C), and TP90L10 (238 ± 3 °C). Therefore, the highest ignition temperature was that of TR90L10, followed by lignite (259 ± 14 °C). Again, a similar trend was observed for TP49R49L2, TP48R48L4, and TP45R45L10 samples, the ignition temperatures of which were recorded as 235 ± 8 °C, 239 ± 7 °C, and 243 ± 8 °C, respectively. On the other hand, the average ignition temperature of fuels consisting of both pine and rose decreased with the increase in the content of pine in the blends, with ignition temperatures of the TP25R75, TP50R50, and TP75R25 blends being 236 ± 4 °C, 236 ± 92 °C, and 231 ± 47 °C, respectively. Against this background, it can be concluded that it is easier to ignite the raw blends compared to their torrefied forms, because fuels with high volatile matter content are easier to ignite and take a shorter time to burn [39]. As seen in Tables A1 and A2, since lignite was poorer in terms of volatile matter compared to both raw pine and rose pulp, the ignition temperatures have increased in the blends of both raw and torrefied biomass with lignite.
In this study, the peak temperatures of torrefied blends were also found to be slightly higher than those of their raw forms, probably due to the carbonization of pine and rose pulp with torrefaction [4]. The mean peak temperatures in the second region were recorded as 377 ± 9 °C and 344 ± 7 °C for the TP100 and TR100 samples, respectively. The average peak temperatures of TR98L2, TR96L4, and TR90L10 blends, in turn, were, respectively, 344 ± 10 °C, 347 ± 8 °C, and 351 ± 11 °C. The average peak temperatures of TP98L2, TP96L4, and TP90L10 blends were recorded as 378 ± 15 °C, 382 ± 13 °C, and 383 ± 14 °C, respectively. Therefore, like the case with raw blends, higher peak temperatures were observed with the addition of lignite into the torrefied biomass. Table A2 shows that the peak temperatures in the third region where char combustion occurred were generally more affected by the torrefaction process compared to measured peak temperatures in the second region. The result of the torrefaction process for this region was higher peak temperatures.
Average burnout temperatures were found to be 805 ± 134 °C and 882 ± 98 °C for TP100 and TR100, respectively. The burnout temperatures increase with the addition of lignite to the torrefied pine and the average values for TP98L2, TP96L4, and TP90L10 samples reached 806 ± 83 °C, 825 ± 154 °C, and 839 ± 68 °C, respectively. The burnout temperatures of torrefied blends containing rose pulp also increased with the addition of lignite, with average values being measured as 872 ± 8 °C, 890 ± 54 °C, and 893 ± 90 °C for TR98L2, TR96L4, and TR90L10 blends, respectively. It has been observed that the burnout temperatures of the torrefied samples have shifted to higher temperatures with the torrefaction process, compared to their raw forms. Similar findings were also reached for both biomass and lignite-biomass blends. Consequently, due to devolatilization and depolymerization [23], higher ignition and burnout temperatures were observed with torrefaction of blends, compared to their raw counterparts.
The maximum DTG value of the Elbistan lignite (2.62 ± 1% min–1), which is suitable for pulverized combustion and which has a low calorific value, was found to be the lowest DTG value when compared with other blends. In the study, torrefaction led to an increase in the maximum DTG values of the blends in general, and the maximum DTG values of the torrefied TP100 and TR100 samples were found to be − 29.81 ± 13% min–1 and − 11.80 ± 6% min–1, respectively. Furthermore, the reactivity of the torrefied pine samples was found to be higher than that of rose pulp samples. This point is also supported by the lower ignition, peak and burnout temperatures of torrefied pine samples compared to those of the torrefied rose pulp samples [51]. Average maximum DTG values for TR98L2, TR96L4, and TR90L10 blends were − 12.40 ± 7% min–1, − 12.72 ± 7% min–1, and − 11.79 ± 6% min–1, respectively. The average maximum DTG values for P98L2, P96L4, and P90L10 blends were found to be − 26.57 ± 13% min–1, − 26.84 ± 13% min–1, and − 24.90 ± 910% min–1, respectively. Thus, the addition of 2% and 4% lignite by mass led to DTG values of the torrefied blends as well as raw blends approaching maximum. Moreover, the pine-lignite blends were more reactive compared to rose pulp and lignite blends and that the highest reactivity was observed with the blends with the highest pine content.
The lowest S value measured in the study was that of lignite (1.01E-07). When the S values of the torrefied blends were examined, it was seen that the comprehensive combustion index has improved with the torrefaction process. Moreover, S values were found to decrease with the addition of lignite into the torrefied biomass, with mean S values for TR98L2, TR96L4, and TR90L10 blends being 7.85E-07 ± 7.40E-07, 7.05E-07 ± 6.46E-07, and 6.99E-07 ± 6.23E-07, respectively. Mean S values for TP98L2, TP96L4, and TP90L10 blends, again, were found to be 1.52E-06 ± 1.51E-06, 1.45E-06 ± 1.41E-06, and 1.21E-06 ± 1.15E-07, respectively. Therefore, inverse correlation exists between S values and the amount of lignite added into torrefied blends. Moreover, all torrefied blends with lignite had a better combustion performance compared to lignite itself. The respective S values for TP25R75, TP50R50, and TP75R25 samples were also found to be 8.16E-07 ± 6.83E-07, 1.7E-06 ± 2.41E-06, and 2.17E-06 ± 2.85E-06. Accordingly, it can be concluded that higher S values were obtained with increase of torrefied pine content in the blends.
A review of Table A2 reveals that the peak, ignition, burnout temperatures and maximum DTGs were increased by the torrefaction process, following a trend comparable to the one observed in previous studies [33, 52]. The fuel performance of the blends during combustion has been improved as a result of the torrefaction process. At the same time, adding lignite to the torrefied samples significantly improved the burning behavior of rose pulp and pine, compared to their raw forms.
Activation energies of the torrefied blends
The activation energies of the torrefied materials were also calculated and are presented in Table 4. The average activation energy of Elbistan lignite (91 kJ mol–1) was found to be close to the calculated activation energies of other low quality Turkish lignites, namely Orhaneli (84.74 kJ mol–1), Tunçbilek (34.6 kJ mol–1), Elbistan (108.6 kJ mol–1), and Soma (25.3 kJ mol–1) [53]. The average activation energies of the blends, on the other hand, were found to be between 172 kJ mol–1 (TP100) and 103 kJ mol–1 (TP25R75). As shown in the table, all thermally treated blends with the exception of TR90L10 saw a significant decrease in their activation energies compared to the activation energy levels of their raw forms. Moreover, the addition of lignite into both raw and torrefied blends led to an observed decrease in the activation energies of the blends. Similar results are reported in the literature [40, 42, 54]. The fall in the activation energies of the blends containing torrefied biomass is due to dehydration and decarboxylation of the biomass during the torrefaction process [55].
In the literature, synergy and additivity were observed between various biomass and lignite samples during the co-combustion studies [32, 34, 39, 56,57,58,59,60,61,62,63,64,65,66,67,68]. However, the studies on synergy in the co-combustion of coal/lignite and torrefied biomass are still in their infancy. Although a number of studies suggest a synergy between torrefied biomass and lignite during co-combustion [69, 70], Mi et al. (2016) found that there was no synergy between torrefied bamboo, torrefied wood and their blends under the oxidative atmosphere [41]. Rizkiana et al. (2020) also found no interaction between coal and biomass on solid yield during co-torrefaction [71]. Therefore, more studies should be performed to understand the effect of synergy on thermal decomposition of various torrefied biomass and lignite/coal samples during the co-combustion process, to successfully design and operate thermo-chemical processes.
In the present study, to understand the combustion of raw/torrefied biomass with the addition of lignite or other biomass, first of all, DTG curves of the blends were calculated using the formula [69];
where x1 and x2 represent mass fractions of the pine/rose pulp/lignite in the blend and (dw/dt)1 and (dw/dt)2 represent the corresponding mass loss rates (% min–1). Experimental and calculated DTG curves for both selected raw and torrefied blends are given in Fig. 3. As indicated in the figure, the experimental and calculated mass loss figures did not match, indicating synergy between raw/torrefied biomass and lignite.
Secondly, the average activation energies of the blends were also plotted against mass% of the biomass in the blends [27]. A synergistic effect was observed for both raw rose pulp/lignite and torrefied rose pulp/lignite blends (Fig. 4). Furthermore, the synergistic effect was found to be stronger for raw rose pulp/lignite blends (R2 = 0.488) compared to torrefied rose pulp/lignite blends (R2 = 0.8371). As in the case with rose pulp samples, the synergistic effect was also observed for raw pine/lignite and torrefied pine/lignite blends. However, in this case the effect was stronger with the torrefied pine/lignite blends (R2 = 0.5916) compared to raw pine/lignite blends (R2 = 0.8242) (Fig. 5). Decomposition of agricultural residues is much faster compared to that of woody biomass, because of the higher hemicellulose content of the former [49, 50]. Different synergistic effects were observed during the decomposition of agricultural versus woody residues, probably due to different proportions of hemicellulose, cellulose and lignin content thereof [72].
And finally, to investigate the effect of blending on the co-combustion process for all blends, the average activation energies of both raw and torrefied blends were calculated using the formula [69];
where mb and mL refer to the mass% of the biomass and lignite in the blends, respectively. Eb and Ec represent the activation energies (kJ mol–1) of the biomass and lignite. Nearly all of the experimental activation energies of raw and torrefied blends (Fig. 6) were found to be higher than the corresponding calculated activation energies. Therefore, a synergy was observed for both raw biomass/lignite and torrefied biomass/lignite blends in the co-combustion process [69]. Moreover, with increasing lignite content in the blends, synergistic effects became stronger for both raw and torrefied blends. Besides, in addition to biomass/lignite blends, the synergistic effect was also observed for raw and torrefied rose pulp/pine blends during the co-combustion process.
Conclusions
In the study, raw pine and rose pulp blends were found to have the lowest ignition and burnout temperatures among all. Therefore, the raw blends were identified as the most reactive fuel blends. The peak temperatures, ignition temperatures, final combustion temperatures and comprehensive combustion performances of the blends examined also increased with torrefaction. As a result, the fuel/combustion performance of raw biomass was observed to be improved by the torrefaction process. At the same time, by adding lignite to the torrefied biomass, the burning behaviors of both rose pulp and pine were significantly improved compared to their raw forms. Therefore, the results of this study can be useful in the development of thermochemical systems utilizing raw/torrefied wastes of pine and rose pulp. Moreover, the findings of the study also emphasize the potential of blending waste biomass with low quality local lignite during the disposal of waste, in the context of environmentally friendly energy production.
The average activation energies of raw pine, rose pulp, and Elbistan lignite were calculated to be 178, 187, and 91 kJ mol–1, respectively. With torrefaction, average activation energies of pine and rose pulp samples decreased to 172 and 148 kJ mol–1, respectively. The activation energies of the raw pine/lignite and torrefied pine/lignite blends varied between 133–174 kJ mol–1 and 118–149 kJ mol–1, respectively. The activation energies of the raw rose pulp/lignite and torrefied rose pulp/lignite blends varied between 101–164 kJ mol–1 and 120–136 kJ mol–1, respectively. Moreover, the activation energies of pine/rose pulp blends also decreased as the amount of rose pulp in the blend increased. It has also been observed that there was a synergistic effect during the co-combustion of both raw and torrefied biomass with lignite. Since the kinetic parameters of raw/torrefied biomass and lignite or their blends are important for the proper design and operation of thermochemical systems, it is recommended to design co-combustion systems taking such synergetic effects between raw/torrefied biomass and lignite into account. Against this background, further studies are required to examine the effects of synergy during the thermal conversion of various biomass/lignite blends, especially with reference to the design and operation parameters of co-processing systems.
References
Eurocoal. 2020. https://euracoal.eu/info/country-profiles/turkey/. Accessed 5 Feb 2021.
Godron P, Cebeci ME, Tör OB, Saygın D, In: Increasing the Share of Renewables in Turkey’s Power System: Options for Transmission Expansion and Flexibility, 2018. https://www.shura.org.tr/wp-content/uploads/2018/12/SHURA_Increasing-the-Share-of-Renewables-in-Turkeys-Power System_Report.pdf. Accessed 2 Feb 2021.
Atay OA. Yağ Gülü Damıtma Atıkları, Kızılçam Kabuğu ve Linyit Kömür Tozundan Elde Edilen Peletlerin Baca Gazı Emisyonlarının Belirlenmesi. Ziraat Fakültesi Dergisi. 2016;13(2):1–9.
Bada SO, Falcon RMS, Falcon LM. Investigation of combustion and co-combustion characteristics of raw and thermal treated bamboo with thermal gravimetric analysis. Thermochim Acta. 2014. https://doi.org/10.1016/j.tca.2014.05.021.
Toptas A, Yildirim Y, Duman G, Yanik J. Combustion behavior of different kinds of torrefied biomass and their blends with lignite. Bioresour Technol. 2015. https://doi.org/10.1016/j.biortech.2014.11.072.
Dhungana A, Basu P, Dutta A. Effects of reactor design on the torrefaction of biomass. J Energy Resour Technol. 2012. https://doi.org/10.1115/1.4007484.
International Biomass Torrefaction Council. 2019. http://ibtc.bioenergyeurope.org/torrefaction/. Accessed 12 Oct 2019.
Eseyin AE, Steele PH, Pittman CU Jr. Current trends in the production and applications of torrefied wood/biomass-A review. BioResources. 2015;10(4):8812–58.
Granados DA, Basu P, Nhuchhen DR, Chejne F. Investigation into torrefaction kinetics of biomass and combustion behaviors of raw, torrefied and char samples. Biofuels. 2019. https://doi.org/10.1080/17597269.2018.1558837.
Fisher EM, Dupont C, Darvell LL, Commandre JM, Saddawi A, et al. Combustion and gasification characteristics of chars from raw and torrefied biomass. Bioresour Technol Elsevier. 2012. https://doi.org/10.1016/j.biortech.2012.05.109.
Jones JM, Bridgeman TG, Darvell LI, Gudka B, Saddawi A, Williams A. Combustion properties of torrefied willow compared with bituminous coals. Fuel Process Technol. 2012. https://doi.org/10.1016/j.fuproc.2012.03.010.
Zhang S, Chen T, Li W, Dong Q, Xiong Y. Physicochemical properties and combustion behavior of duckweed during wet torrefaction. Bioresour Technol. 2016. https://doi.org/10.1016/j.biortech.2016.07.086.
Broström M, Nordin A, Pommer L, Branca C, Di Blasi C. Influence of torrefaction on the devolatilization and oxidation kinetics of wood. J Anal Appl Pyrolysis. 2012. https://doi.org/10.1016/j.jaap.2012.03.011.
Tapasvi D, Khalil R, Skreiberg Ø, Tran KQ, Grønli M. Torrefaction of Norwegian birch and spruce: an experimental study using macro-TGA. Energy Fuels. 2012. https://doi.org/10.1021/ef300993q.
Kopczyński M, Plis A, Zuwała J. Thermogravimetric and kinetic analysis of raw and torrefied biomass combustion. Chem Process Eng. 2015. https://doi.org/10.1515/cpe-2015-0014.
Aydinli M, Tutaş M. Production of rose absolute from rose concrete. Flavour Fragr J. 2003. https://doi.org/10.1002/ffj.1138.
Demircan V. Isparta İlinde Gülün Üretim Girdileri, Maliyeti ve Karlılığının Belirlenmesi, Süleyman Demirel Üniversitesi. Fen Bilimleri Enstitüsü Dergisi. 2005;9:3.
Tosun İ. Gül işleme posasının evsel katı atıklarla birlikte kompostlaşabilirliği, Yıldız Teknik Üniversitesi, Unpublished PhD thesis, 2003.
Türkiye Ormancılığı. 2019. ISBN: 978–975–93478–4–0, Kuban Matbaacılık Yayıncılık. Ankara.
FAOSTAT. 2021. http://www.fao.org/faostat/en/#home. Accessed 4 Feb 2021.
Hameed Z, Naqvi SR, Naqvi M, Ali I, Taqvi SAA, Gao N, Hussain SA, Hussain S. A comprehensive review on thermal coconversion of biomass, sludge, coal, and their blends using thermogravimetric analysis. J Chem. 2020. https://doi.org/10.1155/2020/5024369.
Zhang Q, Fang J, Meng Z, Chen C, Qin Z. Thermogravimetric analysis of soot combustion in the presence of ash and soluble organic fraction. RSC Adv. 2020. https://doi.org/10.1039/D0RA06384C.
Nhuchhen DR, Basu P, Acharya B. A comprehensive review on biomass torrefaction. Int J Renew Energ Biofuels. 2014. https://doi.org/10.5171/2014.506376.
Mason DM, Gandhi K. Formulas for calculating the heating value of coal and coal char: development, tests, and uses (No. CONF-800814–25). Institute of Gas Technology, Chicago, IL (USA), 1980.
Demirbaş A. Relationships proximate analysis results and higher heating values of lignites. Energ Source Part A. 2008. https://doi.org/10.1080/10916460701462846.
Sis H. Evaluation of combustion characteristics of different size Elbistan lignite by using TG/DTG and DTA. J Therm Anal Calorim. 2007. https://doi.org/10.1007/s10973-005-7447-4.
Yurdakul S. Determination of co-combustion properties and thermal kinetics of poultry litter/coal blends using thermogravimetry. Renew Energ. 2016. https://doi.org/10.1016/j.renene.2015.12.034.
Chen WH, Lin BJ, Lin YY, Chu YS, Ubando AT, Show PL, Ong HC, Chang JS, Ho SH, Culaba AB, Pétrissans A. Progress in biomass torrefaction: Principles, applications and challenges. Prog Energy Combust Sci. 2021. https://doi.org/10.1016/j.pecs.2020.100887.
Anca-Couce A. Reaction mechanisms and multi-scale modelling of lignocellulosic biomass pyrolysis. Prog Energy Combust Sci. 2016. https://doi.org/10.1016/j.pecs.2015.10.002.
Poletto M. Assessment of the thermal behavior of lignins from softwood and hardwood species. Maderas Cienc Tecnol. 2017. https://doi.org/10.4067/S0718-221X2017005000006.
Yao F, Wu Q, Lei Y, Guo W, Xu Y. Thermal decomposition kinetics of natural fibers: Activation energy with dynamic thermogravimetric analysis. Polym Degrad Stabil. 2008. https://doi.org/10.1016/j.polymdegradstab.2007.10.012.
Liu Z, Jiang Z, Fei B. Thermal decomposition characteristics of Chinese fir. BioResources. 2013;8(4):5014–24.
Qing W, Hao X, Hongpeng L, Chunxia J, Jingru B. Thermogravimetric analysis of the combustion characteristics of oil shale semi-coke/biomass blends. Oil Shale. 2011. https://doi.org/10.3176/oil.2011.2.03.
Wang G, Zhang J, Shao J, et al. Thermal behavior and kinetic analysis of co-combustion of waste biomass/low rank coal blends. Energy Convers Manage. 2016. https://doi.org/10.1016/j.enconman.2016.07.045.
Bahreini M, Movahedi M, Peyvandi M, Nematollahi F, Tehrani HS. Thermodynamics and kinetic analysis of carbon nanofibers as nanozymes. Nanotechnol Sci Appl. 2019. https://doi.org/10.2147/NSA.S208310.
Sonibare OO, Ehinola OA, Egashira R, KeanGiap L. An investigation into the thermal decomposition of Nigerian Coal. J Appl Sci. 2005;5:104–7.
Pecha MB, Arbelaez JIM, Garcia-Perez M, Chejne F, Ciesielski PN. Progress in understanding the four dominant intra-particle phenomena of lignocellulose pyrolysis: chemical reactions, heat transfer, mass transfer, and phase change. Green Chem. 2019. https://doi.org/10.1039/C9GC00585D.
Basu P. Biomass gasification, pyrolysis and torrefaction: practical design and theory. Academic press, 2018.
Muthuraman M, Namioka T, Yoshikawa K. A comparison of co-combustion characteristics of coal with wood and Hydrothermally treated municipal solid waste. Bioresour Technol. 2010. https://doi.org/10.1016/j.biortech.2009.11.060.
Gil MV, Riaza J, Álvarez L, Pevida C, Pis JJ, Rubiera F. Oxy-fuel combustion kinetics and morphology of coal chars obtained in N2 and CO2 atmospheres in an entrained flow reactor. Appl Energy. 2012. https://doi.org/10.1016/j.apenergy.2011.09.017.
Mi B, Liu Z, Hu W, Wei P, Jiang Z, Fei B. Investigating pyrolysis and combustion characteristics of torrefied bamboo, torrefied wood and their blends. Bioresour Technol. 2016. https://doi.org/10.1016/j.biortech.2016.02.087.
Idris SS, Abd Rahman N, Ismail K. Combustion characteristics of Malaysian oil palm biomass, sub-bituminous coal and their respective blends via thermogravimetric analysis (TGA). Bioresour Technol. 2012. https://doi.org/10.1016/j.biortech.2012.07.065.
Schniewind AP. Concise Encyclopedia of Wood and Wood Based Materials. 1st ed. Elmsford: Pergamon Press; 1989.
Zakrzewski R. Pyrolysis kinetics of wood comparison of iso and polythermal thermogravimetric methods. Electron J Pol Agric Univ. 2003;6:2.
Vhathvarothai N, Ness J, Yu QJ. An investigation of thermal behaviour of biomass and coal during copyrolysis using thermogravimetric analysis. Int J Energy Res. 2014. https://doi.org/10.1002/er.3120.
Kocabaş-Ataklı ZÖ, Okyay-Öner F, Yürüm Y. Combustion characteristics of Turkish hazelnut shell biomass, lignite coal, and their respective blends via thermogravimetric analysis. J Therm Anal Calorim. 2015. https://doi.org/10.1007/s10973-014-4348-4.
Liu X, Chen M, Wei Y. Kinetics based on two-stage scheme for co-combustion of herbaceous biomass and bituminous coal. Fuel. 2015. https://doi.org/10.1016/j.fuel.2014.11.085.
Manouchehrinejad M, Mani S. Torrefaction after pelletization (TAP): Analysis of torrefied pellet quality and co-products. Biomass Bioenerg. 2018. https://doi.org/10.1016/j.biombioe.2018.08.015.
Bergman PC, Boersma AR, Zwart RWR, Kiel JHA. Torrefaction for biomass co-firing in existing coal-fired power stations. Energy Research Centre of the Netherlands. 2005. ECN-C-05-013.
Zhang Y, Geng P, Liu R. Synergistic combination of biomass torrefaction and co-gasification: reactivity studies. Bioresour Technol. 2017. https://doi.org/10.1016/j.biortech.2017.08.197.
Rahib Y, Elorf A, Sarh B, Ezahri M, Rahib Y, Bonnamy S. Experimental analysis on thermal characteristics of argan nut shell (ANS) biomass as a green energy resource. Int J Renew Energ Res. 2019;9(4):1606–15.
Park SW, Jang CH, Baek KR, Yang JK. Torrefaction and low-temperature carbonization of woody biomass: Evaluation of fuel characteristics of the products. Energy. 2012. https://doi.org/10.1016/j.energy.2012.07.024.
Özbaş KE, Kök MV. Effect of heating rate on thermal properties and kinetics of raw and cleaned coal samples. Energy Sources. 2003. https://doi.org/10.1080/00908310290142091.
Otero M, Gómez X, García AI, Morán A. Effects of sewage sludge blending on the coal combustion: a thermogravimetric assessment. Chemosphere. 2007. https://doi.org/10.1016/j.chemosphere.2007.05.077.
Chowdhury ZZ, Pal K, Johan RB, Dabdawb WAY, Ali ME, Rafique RF. Comparative evaluation of physiochemical properties of a solid fuel derived from Adansonia digitata trunk using torrefaction. BioResources. 2017;2:3816–33.
André RN, Pinto F, Franco C, Dias M, Gulyurtlu I, Matos MAA, Cabrita I. Fluidised bed co-gasification of coal and olive oil industry wastes. Fuel. 2005. https://doi.org/10.1016/j.fuel.2005.02.018.
Aznar MP, Caballero MA, Sancho JA, Francés E. Plastic waste elimination by co-gasification with coal and biomass in fluidized bed with air in pilot plant. Fuel Process Technol. 2006. https://doi.org/10.1016/j.fuproc.2005.09.006.
Xiang-guo L, Bao-guo M, Li X, Zhen-wu H, Xin-gang W. Thermogravimetric analysis of the co-combustion of the blends with high ash coal and waste tyres. Thermochim Acta. 2006;441:79–83.
Haykiri-Acma H, Yaman S. Effect of co-combustion on the burnout of lignite/biomass blends: a Turkish case study. Waste Manage. 2008. https://doi.org/10.1016/j.wasman.2007.08.028.
Fitzpatrick EM, Kubacki ML, Jones JM, Pourkashanian M, Ross AB, Williams A, Kubica K. The mechanism of the formation of soot and other pollutants during the co-firing of coal and pine wood in a fixed bed combustor. Fuel. 2009. https://doi.org/10.1016/j.fuel.2009.02.037.
Kazagic A, Smajevic I. Synergy effects of co-firing wooden biomass with Bosnian coal. Energy. 2009. https://doi.org/10.1016/j.energy.2008.10.007.
Sahu SG, Sarkar P, Chakraborty N, Adak AK. Thermogravimetric assessment of combustion characteristics of blends of a coal with different biomass chars. Fuel Process Technol. 2010. https://doi.org/10.1016/j.fuproc.2009.12.001.
Yuzbasi NS, Selçuk N. Air and oxy-fuel combustion characteristics of biomass/lignite blends in TGA-FTIR. Fuel Process Technol. 2011;92(5):1101–8.
Riaza J, Gil MV, Álvarez L, Pevida C, Pis JJ, Rubiera F. Oxy-fuel combustion of coal and biomass blends. Energy. 2012. https://doi.org/10.1016/j.energy.2012.02.057.
Farrow TS, Sun C, Snape CE. Impact of biomass char on coal char burn-out under air and oxy-fuel conditions. Fuel. 2013. https://doi.org/10.1016/j.fuel.2012.07.073.
Vamvuka D, Tsamourgeli V, Zaharaki D, Komnitsas K. Potential of poor lignite and Biomass blends in energy production. Energ Sources Part A. 2016. https://doi.org/10.1080/15567036.2015.1014980.
Wei Y, Chen M, Niu S, et al. Evaluation on oxy-fuel co-combustion behavior of Chinese lignite and eucalyptus bark. J Ther Anal Calorim. 2016. https://doi.org/10.1007/s10973-015-5050-x.
Zhang R, Lei K, Ye B, Cao J, Liu D. Combustion characteristics and synergy behaviors of biomass and coal blending in oxy-fuel conditions: A single particle co-combustion method. Sci China Technol Sci. 2018. https://doi.org/10.1007/s11431-018-9214-9.
Liu HP, Liang WX, Qin H, Wang Q. Synergy in co-combustion of oil shale semi-coke with torrefied cornstalk. App Therm Eng. 2016. https://doi.org/10.1016/j.applthermaleng.2016.08.125.
Caliskan Sarikaya A, Haykiri Acma H, Yaman S. Synergistic Interactions During Cocombustion of Lignite, Biomass, and Their Chars. J Energy Resour Technol. 2019. https://doi.org/10.1115/1.4044057.
Rizkiana J, Zahra ACA, Wulandari W, Saputra WH, Andrayukti R, Sianipar AM, Sasongko D. Effects of coal and biomass types towards the quality of hybrid coal produced via co-torrefaction. In IOP Conf Ser Mater Sci Eng. 2020. https://doi.org/10.1088/1757-899X/823/1/012028.
Shao H, Zhao H, Xie J, Qi J, Shupe TF. Agricultural and forest residues towards renewable chemicals and materials using microwave liquefaction. Int J Polym Sci. 2019. https://doi.org/10.1155/2019/7231263.
Szwaja S, Magdziarz A, Zajemska M, Poskart A. A torrefaction of Sida hermaphrodita to improve fuel properties. Advanced analysis of torrefied products. Renew Energy. 2019. https://doi.org/10.1016/j.renene.2019.04.055.
Álvarez-Álvarez P, Pizarro C, Barrio-Anta M, Cámara-Obregón A, Bueno JLM, Álvarez A, Gutiérrez I, Burslem DF. Evaluation of tree species for biomass energy production in Northwest Spain. Forests. 2018. https://doi.org/10.3390/f9040160.
Balat M. Pyrolysis of cherry laurel (Prunus Laurocerasus L.) seed in the presence of sodium carbonate. Energy Explor Exploit. 2016. https://doi.org/10.1177/0144598715623682.
Ndecky A, Tavares PW, Senghor A, Kane M, Ndiath H, Youm I. Proximate analysis of alternatives cooking solides fuels in sub saharan by using astm standards. Int J Clean Coal Energy. 2022. https://doi.org/10.4236/ijcce.2022.111001.
Gil MV, Casal D, Pevida C, Pis JJ, Rubiera F. Thermal behaviour and kinetics of coal/biomass blends during co-combustion. Bioresour Technol. 2010. https://doi.org/10.1016/j.biortech.2010.02.008.
Haykiri-Acma H, Yaman S, Kucukbayrak S. Co-combustion of low rank coal/waste biomass blends using dry air or oxygen. Appl Therm Eng. 2013. https://doi.org/10.1016/j.applthermaleng.2012.06.028.
Funding
This study was supported by the Scientific and Technological Research Council of Turkey (TÜBİTAK), Grant No.: 118Y247.
Author information
Authors and Affiliations
Contributions
SA was involved in the methodology, formal analysis, and software. MC contributed to writing—reviewing and editing. SY helped in the conceptualization, project administration, supervision, data curation, and writing—original draft preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Armakan, S., Civan, M. & Yurdakul, S. Determining co-combustion characteristics, kinetics and synergy behaviors of raw and torrefied forms of two distinct types of biomass and their blends with lignite. J Therm Anal Calorim 147, 12855–12869 (2022). https://doi.org/10.1007/s10973-022-11432-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11432-2