Abstract
As a special engineering plastics with excellent comprehensive properties, polyarylether (PAE) has been widely used in automobile manufacturing, aerospace, electronic communications, mechanical manufacturing and other fields. In industry, PAE is obtained by polymerization reaction. However, a number of accidents were caused by polymerization reactions. Therefore, a series of experiments were carried out to investigate the thermal hazard of PAE polymerization process to prevent the polymerization accidents. First, the reaction calorimeter (RC1e) was used to measure the heat released in the reaction process to obtain the thermodynamic parameters. The RC1e results showed that the adiabatic temperature rise (ΔTad) of polymerization was 97.99 °C, the maximum temperature attained by synthesis reaction was 127.99 °C and the maximum temperature technical for reasons was 110.60 °C. Then, the pyrolysis characteristics of the PAE in air and nitrogen atmosphere at different heating rates were scanned via thermogravimetry, and the apparent activation energy was calculated by Starink method. The PAE showed two significant mass loss peaks in the air atmosphere, but only one in the nitrogen atmosphere. The pyrolysis of PAE in the air atmosphere was more thoroughly compared to that in the nitrogen atmosphere. Finally, the risk class of thermal runaway of polymerization was evaluated according to the relevant criteria, the severity was “class 2,” and the risk class was “class 3.” These results were the foundation for preventive measures to reduce the potential risk during polymerization of PAE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The chemical industry is closely related to human life; however, the chemical industry has the characteristics of high temperature and high pressure, and it is flammable and explosive, toxic and harmful. The industry involves many dangerous chemicals, which have led to a large number of fire and explosion accidents, causing casualties and economic losses. These accidents were mainly caused by the thermal runaway reactions [1,2,3]. And there were some polymerization processes also causing the thermal runaway accidents. Lin [4] has stated that a significant number of incidents were related to styrene polymerization. Saada [5] studied the runaway reaction incidents for the last 25 years (1988–2013) and showed that most of the incidents occurred in polymerization and decomposition processes. Therefore, it is significant to study the polymerization process and the potential danger of the products to minimize the accidents.
Scholars have developed and improved the research methods and system of thermal hazard assessment for runaway reaction process [6,7,8]. Gygax [9] first proposed the concept of maximum temperature attained by synthesis reaction (MTSR) and cooling failure scenario. Then the assessment method based on the uncontrolled scenario was further expanded, and its possibility and severity were introduced into the risk assessment. On this basis, the risk of the reaction heat was divided into five classes, and finally the systematic process of the risk assessment of the uncontrolled chemical reaction was formed [10]. In order to obtain the heat released in the reaction process, the researchers used a variety of instruments to measure, among which the reaction calorimeter (RC1e) was the most widely used [11, 12]. The reaction calorimeter was utilized to investigate the risk of some complex reactions and MTSR [13]. Four reactions were chosen to take experiments with RC1 (from Mettler Toledo) and with DRC (from SETARAM) in isoperibolic mode [14]. The differential reaction calorimeter was coupled with a volumetric hydrogen measurement, allowing a simultaneous thermodynamic and kinetic to study the mechanism of hydrogen generation by hydrolysis of sodium borohydride catalyzed by Co2B nanoparticles generated in situ [15]. A novel theoretical blueprint was proposed to evaluate the ΔTad (t) from the transient heat flow data generated in a reaction calorimeter [16]. Zhang [17] analyzed the exothermic oxidation of 3-methylpyridine with hydrogen peroxide by Reaction Calorimeter in semi-batch operation and used risk matrix to assess the risk of the reaction. Ben [18] measured the thermal kinetic parameters of free radical solution polymerization by reaction calorimeter. Hua [19] obtained the thermal properties of the oxidation reaction by the Reaction Calorimeter and proposed the inherent safer designs (ISD) according to the risk assessment results to increase the level of safety of chemical industry technique. At the same time, many scholars have used different instruments and methods to study the thermal hazard of materials or products involved in the reaction process [20,21,22,23]. The differential scanning calorimetry (DSC), thermal activity monitor III (TMAIII), vent sizing package 2 (VSP2) were used to assess thermal risk of tert-butylperoxy-2-ethylhexyl carbonate [24]. The uncontrolled behavior of styrene bulk polymerization was studied by DSC and obtained the severity grading based on the severity evaluation criteria of runaway reaction [25]. Thermal hazard of 2,2′-azobis (2-methylpropionate) (AIBME) was investigated by DSC, simultaneous thermogravimetric analyzer (STA) and accelerating rate calorimeter (ARC) and the specific safety parameters such as time to maximum rate under adiabatic condition (TMRad) and self-accelerating decomposition temperature (SADT) were also calculated [26].
Polyarylether (PAE) is a kind of high-performance thermoplastic, which has been widely used in automotive manufacturing, aerospace, electronic communication, mechanical manufacturing and other fields because of its high thermal deformation resistance, good mechanical properties and inherent fire resistance. The polymerization process of PAE is first introduced from abroad, the reaction time is long, and no reaction safety risk assessment has been done, so that the thermodynamic data of reaction are not comprehensive and there are unknown hazards. In order to avoid the risk in the reaction process, the reaction risk assessment must be carried out for this type of process. Therefore, in this study, the isothermal reaction calorimeter experiment was carried out to obtain the relevant thermal data of the polymerization process, and then the pyrolysis characteristics of the product under different atmosphere were investigated by the thermogravimetry analyzer. The kinetic was calculated by Starink method to better understand its pyrolysis behaviour. At last, the hazard of polymerization process was assessed. According to relevant data and risk class, some suggestions and measures were put forward to reduce the process risk.
Research methods
Sample
The chemical reagents used in the experiment included 2,6-dimethylphenol (≥ 92.0%), HBr (≥ 48%), Cu2O (≥ 95%), toluene (≥ 99.7%), oxygen (≥ 99%).
RC1e experiment
RC1e (reaction calorimeter) is an advanced fully automated laboratory reaction calorimeter developed by Swiss company Ciba-Geigy and commercialized by Swiss company Mettler in 1986.
RC1e is composed of reactor device, temperature control device, electronic control device and PC software. It can carry out intermittent and semi-intercalated reactions. The thermal information such as reaction temperature, jacket temperature, pressure and heat flow can be obtained, then the heat release of the reaction, the specific heat capacity of the mixture in the reactor can also be acquired. The RC1e used in this paper is equipped with a 2-L glass atmospheric pressure reactor, a stainless steel metal probe thermometer and a glass 25 W calibrated heater probe. Before the experiment, the reactor should be kept dry. This experiment was a small-scale experiment carried out by scaling down the original process reaction parameters in proportion, and the steps were as follows. Initially, monomer solution (prepared with 2,6-dimethylphenol and toluene in advance, with a concentration of 50%) was added to the reactor, and then the stirring was started, with the stirring speed of 750 r min−1. After the above materials were put into the reactor, the catalyst (Cu2O + HBr) was added. Then the temperature was set to 30 °C. Ultimately, the nitrogen continuously flowed above the liquid level in the reactor at the rate of 0.5 L min−1, oxygen flowed below the liquid level in the reactor at the rate of 0.3 L min−1 for 45 min. Then the temperature was raised to 50 °C and kept for 60 min.
Thermogravimetric experiment
When the RC1e experiment was done, the PAE product was gotten through a series of treatments, such as extracting and drying the products obtained from RC1e reaction. Then the thermogravimetric experiment was carried out on the treated products. In this paper, a thermogravimetry (Pyris 1 TG, PerkinElmer, Inc., Massachusetts, USA) was carried out TG experiment. The specific test process was as follows: 9.0 ± 0.5 mg of the sample was weighed and placed in a 70-μL standard alumina crucible at different heating rates of 5.0, 10.0, 15.0, 20.0 °C min−1 in air and nitrogen atmosphere, respectively. The temperature range for TG was from 30.0 to 800.0 °C.
Dynamic calculation method
Under non-isothermal reaction conditions, many methods such as Ozawa method, Kissinger method and Starink method have been used to calculate the apparent activation energy. Among these reported methods, Kissinger method was widely used to calculate apparent activation energy due to its accuracy. However, Kissinger method was a single step dynamic method, so only a single dynamic value can be calculated. On the contrary, the Starink method not only integrated three integral models of Kissinger, Ozawa and Bosswell, but can also get the change of Ea with the conversion rate [27, 28]. And Starink method was easily applicable and there was a possibility to reliably obtain the Ea even if the reaction mechanism is uncertain [29]. So this paper used the method to calculate kinetic parameters. The formula is as follows [30] :
where β is heating rate, K min−1; T is temperature, K; Ea is apparent activation energy, kJ mol−1; R is ideal gas constant, 8.314 J mol−1 K−1; C is constant.
The apparent activation energy can be obtained by linear fitting of ln(β/T1.8) − 1/T and obtaining the slope.
Parameters for assessing the risk of reaction
Adiabatic temperature rise (ΔT ad)
The adiabatic temperature rise is an important data for safety assessment. If the reactants are not completely transformed after cooling failure, these untreated materials will continue to react in an uncontrolled state and cause the temperature to rise under adiabatic conditions [31]. The adiabatic temperature rise is calculated as follows:
where ΔTad is adiabatic temperature rise, K; ΔH is reaction enthalpy, J mol−1; Cp is specific heat capacity, J K−1 mol−1.
Maximum temperature attained by synthesis reaction (MTSR)
The maximum temperature attained by synthesis reaction (MTSR) is another important parameter to evaluate the risk of reaction. When the exothermic reaction is in the condition of cooling failure and heat exchange out of control, the temperature of the system will rise in an approximately adiabatic condition due to the heat accumulation in the reaction system. When the reactant accumulation reached maximum, the maximum temperature that the system can reach is called the maximum temperature attained by synthesis reaction [32]. MTSR is related to the accumulation degree of reactant. The greater the accumulation degree of reactant is, the higher the MSTR when the reaction is out of control, which is expressed as [32]:
where Tcf is cooling failure temperature, °C; Tr is reaction temperature, °C; X is heat accumulation degree, %; ΔTad is adiabatic temperature rise, °C.
Maximum temperature technical for reasons (MTT)
MTT represents the technical limit of the reactor, depending on the process and equipment. In an open system (process operated under atmospheric pressure) the MTT is typically the boiling temperature of the reaction mass. In a closed system, the temperature corresponding to the maximum allowable pressure of reaction vessel could be the MTT [33].
Temperature at which time to maximum rate is 24 h (T D24)
Under adiabatic condition, the time required for runaway reaction to reach the maximum reaction rate is called time to maximum rate under adiabatic conditions (TMRad). The temperature at which TMRad is equal to 24 h is TD24. TD24 is used as a quantitative criterion to evaluate the probability of thermal runaway of a process that involves an exothermic chemical reaction. The temperature for which the TMRad falls below 24 h is considered critical.
Results and discussion
RC1e experiment
RC1e was used to measure the temperature and the change of heat flow during the polymerization reaction process. The temperature and heat flow during the reaction process are shown in Fig. 1.
In Fig. 1, the Tj represents the jacket temperature change curve, the Tr represents the reactor temperature change curve, and the qr − hf represents the heat flow change curve. It can be seen that there are two stages of reaction: exothermic and endothermic. After reaction started (at about 1.25 h), the jacket temperature decreased sharply by approximately 20 °C to maintain the reactor temperature at the set temperature of 30 °C, the heat flow curve showed exothermic, and the heat release rate fluctuated between 0 and 90 W. After the temperature was set rise to 50 °C, the jacket temperature increased to maintain the reactor temperature at the set point, the heat flow curve showed endothermic, and the heat release rate fluctuated between 0 and 10 W. Then by integrating the heat flow curve, the total exothermic amount of polymerization was 145.15 kJ. According to Eq. (2), the adiabatic temperature rise ΔTad can be calculated to be 97.99 °C.
. Since the curve of oxygen feeding mass with time could not be obtained in the experiment, the heat accumulation degree (X) was taken to 100% for strict consideration. So based on Eq. (3) and (4), the maximum temperature attained by synthesis reaction (MTSR) was 127.99 °C.
TG experiment
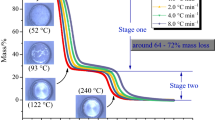
The thermal decomposition curves of PAE at different heating rates in air and nitrogen atmosphere are shown in Figs. 2–5. It can be seen from Figs. 2 and 3 that the thermal decomposition of PAE in nitrogen atmosphere was not complete, and there were residues after the pyrolysis. The pyrolysis process can be divided into three stages. The first and second stages were in 100–180 °C and 200–300 °C, respectively. The mass rate of these two stages was not obvious. The third stage that occurred after 400 °C was identified as the main pyrolysis of PAE. The initial temperature of decomposition was 400 °C, the end temperature of decomposition was 500 °C, and the maximum mass loss rate was about 1.8% °C−1. From Figs. 4 and 5, it can be seen that the thermal decomposition of PAE in air atmosphere was relatively completed with little residues. The pyrolysis process can be divided into four stages. The first and second stages were also in 100–180 °C and 200–300 °C, respectively. The mass loss rates of the third and fourth stages were the most obvious, which were the main pyrolysis stages. The third stage of initial temperature of decomposition was 420 °C, the end temperature was 500 °C, and the maximum mass loss rate was about 1.4% °C−1. The fourth stage of initial temperature of decomposition was 500 °C, the end temperature of decomposition was about 600–700 °C, and the maximum mass loss rate was about 0.7% °C−1.
In order to better compare the pyrolysis characteristics of PAE in different conditions, the TG/DTG curves in air and nitrogen atmosphere with heating rate of 10 °C min−1 are shown in Figs. 6 and 7 to analyze the pyrolysis characteristics of PAE in different atmospheres. And the TG/DTG curves in nitrogen atmosphere with different heating rate, as shown in Figs. 2 and 3, were selected to further study the decomposition characteristics of PAE at different heating rates. On the one hand, in different atmosphere conditions, it can be observed that the decomposition of PAE in nitrogen atmosphere was not completed and there were still residues after pyrolysis, but in air atmosphere the reaction was almost completed. The pyrolysis process of PAE can be divided into three stages in nitrogen atmosphere, and the three stages were consistent with that of air atmosphere. However, there was a fourth stage in the air. So we can know that this may be due to the presence of oxygen in the air and the redox reaction between the residual carbon and oxygen. And the first and second stages of mess loss of PAE were the decomposition of a few remaining raw materials, and the main pyrolysis process was the third stage. On the other hand, it can be seen from Figs. 2 and 3 that the curves of different heating rates in the same atmosphere were basically similar, and the effects of heating rate mainly lie in the peak value of mass loss rate, the temperature that reaching the peak value and the total mass loss rate. With the increase in the heating rate, the peak value of the mass loss rate decreased, the temperature that reached the peak value moved to the high temperature direction, and the total mass loss rate decreased.
Kinetic analysis
In order to have a better understanding of the pyrolysis process of PAE, kinetic calculation was carried out according to the data obtained from TG experiments. The apparent activation energy can be calculated based on Eq. (1). Ea as a function of selected α, across the range of 0.1–0.9 in air atmosphere and 0.1–0.65 in nitrogen atmosphere. The fitting results are shown in Figs. 8 and 9. The correlation coefficient of almost all fitting curves was above 0.9, which indicated that there was a high fitting degree. Figure 10 shows the variation of apparent activation energy at a series of conversion degree. As can be seen, the values of Ea in the nitrogen and air were low and closed at first. This is because at the beginning of the experiment, the decomposition of a few residual raw materials required a smaller activation energy. With the proceeding of decomposition, it required a higher activation energy. And in the air, the Ea of the reaction first increased to the maximum when the conversion was 40% and then gradually decreased. However, the Ea of the reaction in nitrogen increased at the conversion of 20% and then basically remained unchanged. As seen, only one significant decomposition stage of PAE in nitrogen is emerged in Fig. 2 or Fig. 3 and a steady variation of Ea is also shown in Fig. 10, which proved that pyrolysis of PAE in nitrogen was a single reaction. On the contrary, two significant decomposition stages of PAE in air are observed in Fig. 4 or Fig. 5, and the fluctuation of Ea is also shown in Fig. 10, which indicated that pyrolysis of PAE in air might be a two-stage or multistage reaction.
Severity assessment of reaction
According to “Guidelines for Safety Risk Assessment of Fine Chemical Reactions (Trial)” [30], reaction severity refers to the extent to which uncontrolled energy release may cause damage under uncontrolled conditions. Many of the reactions are exothermic. When the reaction was out of control, the temperature of the system would rise and this may cause the secondary decomposition of some components in the system, resulting in the continuous rise of the temperature and pressure of the system. When the temperature or pressure reaches the threshold of the reaction vessel, an explosion will occur with severe consequences. The more the temperature in the runaway system rises, the higher the severity of the explosion. Therefore, in order to assess the reaction severity, the adiabatic temperature rise was used as a parameter to measure the reaction severity. The assessment criteria are shown in Table 1.
The adiabatic temperature rise (ΔTad) of PAE is 97.99 °C, so the production process severity of PAE is class 2, if the out-of-control reaction occurs, it will have the consequences of the factory’s short-term damage. So the cooling system should be effective to avoid accidents due to heat accumulation.
Risk assessment of reaction
The risk degree of reaction refers to the risk degree of process reaction itself. The greater the risk degree of reaction, the greater the severity of accident caused by reaction out of control. Temperature is the evaluation standard of process risk assessment. Four important temperature parameters were considered, namely the process temperature Tp, maximum temperature technical for reasons MTT, the temperature TD24 and MTSR. Tp was 30.00 °C, MTSR was 127.99 °C. MTT was the highest technical temperature of the reaction vessel 110.60 °C in this paper. Before conducting TG experiment, we dried the sample at 150 °C for up to seven days, and the sample did not react. It can be inferred that MTSR was less than TD24. The four temperatures of the reaction are shown in Table 2, and the assessment criteria are shown in Table 3. In class 1 and class 2, the loss of control of the main reaction will not trigger the secondary reaction and will not reach the technical limit of the equipment. The reaction risk in class 1 is low, and the reaction in class 2 has potential decomposition risk. In class 3, the technical limit is reached and can be used as a safety barrier, but the secondary reaction will not be triggered, the reaction in class 3 has flushing and decomposition risk. In class 4, the secondary reaction may be triggered, but the technical limit of the equipment can be used as a barrier and the reaction has high flushing and decomposition risk and potential explosion risk. In class 5, the second-order reaction is triggered. The technical barrier will be reached, and in most cases, the runaway speed is too fast for the safety barrier to work, so the reaction in class 5 has high explosion risk [32]. It can be seen that the risk degree of PAE polymerization process was class 3, and there was the risk of flushing and decomposition. According to the evaluation class, in order to reduce the reaction risk, it is suggested that in addition to the conventional automatic control system, centralized monitoring and automatic adjustment of the main reaction parameters should be carried out. The alarm and interlock control that deviate from the normal value should be set. On the basis of setting relief facilities such as bursting disc and safety valve, control facilities such as emergency cut-off, emergency stop reaction, emergency cooling and cooling should also be set.
Conclusions
In this work, the process hazard of polymerization was studied by RC1e and the thermal decomposition characteristics of polyarylether (PAE) were studied by TG experiments. The kinetic parameters were calculated by using Starink method. Then the process severity and risk were estimated.
RC1e experiment showed that there were exothermic and endothermic stages in the process, so the heat accumulation was relatively low. By calculation, the adiabatic temperature rise of the reaction was 97.987 °C, the enthalpy of polymerization was 145.15 kJ, and the maximum temperature attained by synthesis reaction was 127.98 °C. Assessment results of process severity (class 2) and risk (class 3) can provide the basis for establishing corresponding control measures for the safe production process of PAE.
According to the results of TG, it can be known that the thermal decomposition characteristics of PAE in different atmospheres and different heating rates. It showed that the decomposition in air was more thorough than in nitrogen. The decomposition process can be divided into three and four stages in different atmospheres, respectively. But there were only one and two significant decomposition peaks in nitrogen and air, respectively. Combined with the variation of activation energy, it can be seen that the decomposition of PAE in nitrogen may be a single-stage reaction, while that in air may be a two-stage or multistage reaction. As a result, this study can provide information concerning the avoidance of thermal accidents during transportation and storage.
Abbreviations
- β :
-
Heating rate (K min−1)
- C :
-
Constant
- C p :
-
Specific heat capacity (J K−1 mol −1)
- E a :
-
The apparent activation energy (kJ·mol−1)
- ΔH :
-
Reaction enthalpy (J mol−1)
- MTT :
-
Maximum temperature for technical reasons (°C)
- MTSR :
-
Maximum temperature attained by synthesis reaction (°C)
- T :
-
Temperature (°C)
- ΔT ad :
-
Adiabatic temperature rise (°C)
- T cf :
-
Cooling failure temperature (°C)
- TMRad :
-
Time to maximum rate under adiabatic decomposition conditions (°C)
- T D24 :
-
Temperature at which time to maximum rate is 24 h (°C)
- T r :
-
Reaction temperature (°C)
- T p :
-
Process temperature (°C)
- R :
-
Ideal gas constant (8.314 J mol−1 K−1)
- X :
-
Heat accumulation degree (%)
References
Mihailidou EK, Antoniadis KD, Assael MJ. The 319 major industrial accidents since 1917. Int Rev Chem Eng. 2012;4:529–40.
André R, Giordano M, Mathona C, Naumann R. A new reaction calorimeter and calorimetric tools for safety testing at laboratory scale. Thermochim Acta. 2003;405:43–50.
Sun Q, Jiang L, Li M, Sun J. Assessment on thermal hazards of reactive chemicals in industry: state of the art and perspectives. Prog Energy Combust Sci. 2020;78:100832.
Lin Z, Wen Z, Maria IP, Mannan MS, Mustafa A. Probing into styrene polymerization runaway hazards: effects of the monomer mass fraction. ACS Omega. 2019;4:8136–45.
Saada R, Patel D, Saha B. Causes and consequences of thermal runaway incidents-will they ever be avoided? Process Saf Environ Prot. 2015;97:109–15.
Hugo P, Steinbach J. A comparison of the limits of safe operation of a SBR and a CSTR. Chem Eng Sci. 1986;41:1081–7.
Maestri F, Rota R. Temperature diagrams for preventing decomposition or side reactions in liquid–liquid semibatch reactors. Chem Eng Sci. 2006;61:3068–78.
Steensma M, Westerter R. Thermally safe operation of a cooled semi-batch reactor. Slow liquid–liquid reactions. Chem Eng Sci. 1988;43:2125–32.
Gygax R. Chemical reaction engineering for safety. Chem Eng Sci. 1988;8:1759–71.
Stoessel F. Thermal safety of chemical processes: risk assessment and process design. 1st ed. Weinheim: Wiley; 2008.
Am Ende DJ, Clifford PJ, Northrup DL. The role of reaction calorimetry in the development and scale-up of aromatic nitrations. Thermochim Acta. 1996;289(2):143–54.
Clark JD, Shah AS, Peterson JC, Grogan FM, Camden SK. Application of reaction calorimetry toward understanding the large scale chemistry of ethyl diazoacetate. Thermochim Acta. 2001;367:75–84.
Lerena P, Wehner W, Weber H, Stoessel F. Assessment of hazards linked to accumulation in semi-batch reactors. Thermochim Acta. 1996;289:127–42.
Nogent H, Le Tacon X. The differential reaction calorimeter: examples of use. J Loss Prev Process Ind. 2003;16(2):133–9.
Garron A, Swierczynski D, Bennici S, Auroux A. New insights into the mechanism of H2 generation through NaBH4 hydrolysis on Co-based nanocatalysts studied by differential reaction calorimetry. Int J Hydrog Energy. 2009;34:1185–99.
Das PK. Theoretical estimation of adiabatic temperature rise from the heat flow data obtained from a reaction calorimeter. Thermochim Acta. 2012;530:17–24.
Zhang L, Yu WD, Pan XH, Fang JJ, Hua M, Chen FM, Jiang JC. Thermal hazard assessment for synthesis of 3-methylpyridine-N-oxide. J Loss Prev Process Ind. 2015;35:316–20.
Ben TI, Balland L, Bensahla N, Nordine M. Thermokinetic parameter determination of methacrylates radical polymerization by using real-time reaction calorimetry. J Therm Anal Calorim. 2017;130:2341–9.
Hua M, Liang X, Wei CY, Zhang L, Pan X, Jiang J, Ni L, Jiang J. Inherent safer design for chemical process of 1,4-dioldiacetate-2-butene oxidized by ozone. Chem Eng Commun. 2019. https://doi.org/10.1080/00986445.2019.1657420.
Fila K, Gargol M, Goliszek M, Podkościelna B. Synthesis of epoxy resins derivatives of naphthalene-2,7-diol and their cross-linked products. J Therm Anal Calorim. 2019;138:4349–58.
Alonso A, Lázaro M, Lázaro P, Lázaro D, Alvear D. LLDPE kinetic properties estimation combining thermogravimetry and differential scanning calorimetry as optimization targets. J Therm Anal Calorim. 2019;138:2703–13.
Tseng JM, Wu TC, Hsieh TF, Huang PJ, Lin CP. The thermal hazard evaluation of 1,1-di(tert-butylperoxy) cyclohexane by DSC using non-isothermal and isothermal-kinetic simulations. J Loss Prev Process Ind. 2012;25:703–8.
Yu AD, Cao CR, Pan XH, Shu CM, Wang WJ. Solid thermal explosion of autocatalytic material based on nonisothermal experiments: multistage evaluations for 2,2′-azobis(2-methylpropionitrile) and 1,1′-azobis (cyclohexanecarbonitrile). Process Saf Prog. 2019;38:10–9.
Wei CY, Lin WC, Pan XH, Shu CM, Hua M, Jiang HC, Jiang JC. Thermal risk assessment of tert-butylperoxy-2-ethylhexyl carbonate for storage and transport. J Therm Anal Calorim. 2019;138:2891–900.
Wang WJ, Fang JJ, Pan XH, Hua M, Jiang JJ, Ni L, Jiang JC. Thermal research on the uncontrolled behavior of styrene bulk polymerization. J Loss Prev Process Ind. 2019;57:239–44.
Liu SH, Zhan XB, Lu YM, Xu ZL, Wu T. Thermal hazard evaluation of AIBME by micro-calorimetric technique coupled with kinetic investigation. J Therm Anal Calorim. 2020;141:1443–52.
Zhang T, Wang J, Derradji M, Ramdani N, Wang H, Lin ZW, Liu W. Synthesis, curing kinetics and thermal properties of a novel self-promoted fluorene-based bisphthalonitrile monomer. Thermochim Acta. 2015;602:22–9.
Yu X, Shang Z, Zhang K. Thermally stable polybenzoxazines via tetrahydrophthalimide-functional monobenzoxazines: synthesis, characterization and thermally activated polymerization kinetics. Thermochim Acta. 2019;675:29–37.
Liang XM, Cheng YC, Lin WC, Tung PH, Huang HQ, Pan X, Shu CM, Jiang J. Analysis and characterisation of 1-butyl-3-methylimidazolium hexafluorophosphate as a humectant of nitrocellulose. J Mol Liq. 2020;303:112617.
Jiang HC, Lin WC, Hua M, Pan XH, Shu CM, Jiang JC. Analysis of thermal stability and pyrolysis kinetic of dibutyl phosphate-based ionic liquid through thermogravimetry, gas chromatography/mass spectrometry, and Fourier transform infrared spectrometry. J Therm Anal Calorim. 2019;138:489–99.
https://www.mem.gov.cn/gk/gwgg/agwzlfl/yj_01/201701/t20170112_242237.shtml.
Jiang JC, Jiang W, Ni L, Zhang WX, Zou MY, Shen SL, Pan Y. The modified Stoessel criticality diagram for process safety assessment. Process Saf Environ Prot. 2019;129:112–8.
Nanchen A, Steinkrauss M, Stoessel F. Utilisation of the criticality classes within TRAS410. Forsch Ingenieurwes. 2009;73(1):3–10.
Acknowledgements
The authors are grateful for the financial support of the National Key Research and Development Program (2016YFC0801500), the Major Program of the National Natural Science Foundation of China (21436006, 51874181, 51834007, 51804167), the Major Projects of the Natural Science Research for Colleges in Jiangsu Province (17KJA620002), the Priority Academic Program Development of the Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, J., Yu, AD., Suetor, C.G. et al. Risk assessment of polyarylether polymerization process. J Therm Anal Calorim 144, 295–303 (2021). https://doi.org/10.1007/s10973-020-10084-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10084-4