Abstract
The objective of the present study is to scrutinize the influence of a binary blend of diesel–safflower oil biodiesel and ternary blends of diesel–biodiesel–pentanol on performance, emission and combustion characteristics of a diesel power generator. The test fuels were prepared on volume basis by splash blending and named as follows: B20, B20P5, B20P10, B20P15, and B20P20. The tests were carried out on a single-cylinder, four-stroke, naturally aspirated, and direct-injection diesel engine at four engine loads with a constant engine speed of 3000 rpm. According to the results, ternary blends vaguely reduced BTE while increased BSFC up to 13.90% as compared to diesel. In addition, an increase in pentanol concentration has a considerable effect on the decrease in NOX emissions. It is noted that the addition of pentanol to diesel–biodiesel blend caused to lower emissions (CO, HC, and smoke), whereas CO2 emission increased noticeably thanks to the more complete combustion due to the excess oxygen content. Reviewing combustion analysis results, pentanol addition led to decrease heat release rate and lower ignition delay up to 15% blend ratio compared to diesel. Based on the present study, pentanol can be evaluated as a promising type of higher alcohol for the compression ignition engines in the near future.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The energy crisis is mainly related to the supply and demand for energy, and it is an indicator of the core of any country’s economy. The requisition for energy is unlikely to deteriorate due to consistent economic growth and high urbanization rates in developing countries [1]. As a power source for the transportation sector and industrial applications has remarkably utilized fossil-based fuels for a long time [2], the continuous and uncontrolled consumption of fossil-based fuels as a basic energy source has led to motivating the researchers to monitor for alternative and renewable fuels. In addition, the depletion of natural resources and an alarming increase in the pollution level of the environment have necessitated the use of alternative fuels all over the world [3].
Biodiesel fuels have been of great interest in recent years since they have provided both energy and environmental benefits and can be blended with mineral diesel fuel at any proportions. Biodiesel is a mixture of fatty acid mono-alkyl esters. It can be produced from wider feedstocks by different methods such as transesterification, micro-emulsion, dilution, and pyrolysis. On the other hand, rapeseed, soybean, and palm oils have been considerably preferred as edible raw materials for the production of biodiesel worldwide [4]. It has a significant potential for reducing dependence on petroleum-based fossil fuels and is a renewable, environmentally friendly, non-toxic, non-explosive, and free of sulfur fuel. One of the most important properties of biodiesel fuels has lower exhaust emission results [5]. It has been noted that the compression ignition (CI) engines have not been fueled with neat biodiesel up to 100% as an alternative fuel without any engine modifications due to high viscosity and density [6]. However, the opposite outcomes have been presented that pure biodiesel can be utilized in the diesel engines without any major engine alterations [7].
Safflower (Carthamus tinctorius L.), which is a significant industrial plant, is named as “aspir” or “haspir” in Turkey. It is also called as pseudo-saffron around the world. It is highly resistant to arid conditions [8, 9]. Safflower production in Turkey ranks fifth in the world [10]. Although safflower has a crucial potential as an edible oil source for Turkey, it has not been consumed by humans at the desired level. Therefore, it can be evaluated in different areas. The plant length of the safflower can be reached up to 30–100 cm; meanwhile, its root length may be 100–150 cm depending on the characteristics of the soil. The oil contents of the seed with and without the shell have been found to be at 20–25% and 45%, respectively [11].
According to the article title, abstract, and keywords searching in Scopus, which is the largest abstract and citation database of peer-reviewed literature, only 78 papers have been found in the last 10 years. When these results were taken into account, it may be obviously noted that safflower oil has not been shown enough interest in the world. Thus, further studies related to the safflower oil biodiesel can be conducted by researchers in order to fulfill this gap in the literature.
Correspondingly, the research papers related to the safflower oil biodiesel usage in the diesel engine were taken into consideration. Safflower oil biodiesel was blended with diesel fuel; however, the addition of various additives such as bioethanol and n-butanol into the biodiesel–diesel fuel blends was also investigated. In general, pure safflower oil biodiesel demonstrated lower unburned hydrocarbon (HC), carbon monoxide (CO), particulate matter (PM), carbon dioxide (CO2) although its nitrogen oxides (NOX) were noticeably high as compared to the diesel fuel. Also, engine power, torque, brake mean effective pressure (BMEP), and brake thermal efficiency (BTE) were diminished at different ratios [12,13,14,15].
Apart from biodiesels, the engines may be also operated with alcohol-blended fuels. At the beginning of the twenty-first century, the usage of diesel and short- or long-chain alcohol (methanol, ethanol, butanol, etc.) blends has emerged as a typical dual fuel mode [16]. However, the price of alcohols is higher than that of petroleum-based diesel fuels. Thereby, the use of alcohols in the CI engines was limited in the past. In recent years, the investigations have been conducted that the alcohols can play an important role for replacing of petroleum-based fuels in the engines owing to the fact that they will be run out in the near future. Accordingly, the legal arrangements have been implemented in order to promote the usage of alcohols in the engines and they have been also supported by various incentives [17].
Higher alcohols produced from renewable sources have potentially superior properties than lower alcohols due to their better fuel properties. Pentanol has a five-carbon chemical chain and can be easily blended with both biodiesel and diesel fuels. Furthermore, it can help environmental apprehensions and energy security issues. Among the higher alcohols, pentanol (having five carbons) is a long-chain alcohol and is produced from renewable feedstocks by the biomass fermentation process. Pentanol has higher energy content and cetane number compared to the other alcohols. It was indicated that pentanol may be used up to 45% by adding to the diesel fuel [18, 19]. However, Campos-Fernández et al. [20] recommended a 25% pentanol–75% diesel fuel blends for the applications. The formula of pentanol is C5H12O, and it means that pentanol has five carbons, 12 hydrogens, and one oxygen molecule in the chemical structure.
Background of research work
Although numerous works were reported with diesel–biodiesel–methanol/ethanol/butanol fuel blends, very few investigations have been carried out with the addition of pentanol into the biodiesel–diesel fuel blends as an additive for fueling CI engines. Some of them were summarized as follows: Yesilyurt [21] investigated the performance, emissions, and combustion characteristics of a single-cylinder, four-stroke, water-cooled, naturally aspirated, direct-injection (DI) diesel engine operated with yellow mustard oil biodiesel–diesel–1-butanol and yellow mustard oil biodiesel–diesel-n-pentanol and compared with the diesel fuel at different engine speeds. They prepared the test fuels with 5% and 10% alcohols on a volume basis. They obtained that the usage of alcohols led to increase in BSFC, while the brake power and engine torque decreased according to the increase in alcohol concentration. In addition, alcohol-treated fuels improved the emissions especially smoke opacity and NOX. They concluded that n-pentanol-blended fuels illustrated better results in terms of performance and emissions than that of 1-butanol-treated fuel blends. Zhu et al. [22] added volumetrically 10%, 20%, and 30% n-pentanol into the waste cooking oil (WCO) biodiesel. They then tested these fuel samples in four-cylinder, four-stroke, water-cooled, naturally aspirated, DI diesel engine so as to compare the combustion and exhaust emission behaviors with diesel fuel. Reviewing the experimental result, they determined that the crank angle (CA) moved away from the top dead center at which the start of combustion (SOC) and the maximum heat release occurred, and the cylinder pressure and the rate of heat release increased by increasing the rate of pentanol in the blended fuels. As compared to diesel and biodiesel fuels, HC and CO emissions increased and NOX emissions reduced up to 20% alcohol blends when using fuel blends. Imdadul et al. [23] produced tamanu oil biodiesel via transesterification method and blended with diesel fuel and pentanol in 10%, 15%, and 20% ratios. Afterward, the test fuels were performed in a single-cylinder, four-stroke, naturally aspirated, water-cooled, 8.8 kW, DI diesel engine and compared the performance and emission results with B20 fuel. With the addition of pentanol, compared to B20 fuel, the fuel consumption diminished by 8.7%, the BTE increased by 15%, the brake power increased by 10.4%, the NO emission increased by 4.4%, and the smoke, CO, HC, and CO2 emissions were reduced by 21.2%, 33.1%, 43.45%, and 2.5%, respectively. They also indicated that the addition of pentanol increased the maximum cylinder pressure, improved combustion, and delayed the SOC. In other study performed by Imdadul et al. [24], the tamanu oil biodiesel (BD) was blended with diesel fuel (DF) at 15% and 20%; then, n-butanol (B) and 1-pentanol (P) were added these fuels at ratios of 15 and 20%. The fuel samples (70% DF + 15% BD + 15% B, 70% DF + 15% BD + 15% P, %60 DF + %20 BD + %20 B, and %60 DF + %20 BD + %20 P) were tested in a single-cylinder, four-stroke, naturally aspirated, water-cooled, 8.8 kW, DI diesel engine and compared the engine performance and exhaust emission characteristics with B15 and B20 fuel blends. They expressed that the addition of alcohol decreased BSFC, increased brake power, increased NO and CO2 emissions, and reduced CO and HC emissions compared to B15 and B20 fuels. Atmanli [25] obtained 50% BD 50%, 40% DF + 40% BD + 20% Pr, 40% DF + 40% BD + 20% B, and 40% DF + 40% BD + 20% P with using WCO biodiesel, diesel fuel, propanol (Pr), n-butanol (B), and 1-pentanol. In order to investigate the effects of different alcohols on the engine performance and exhaust emissions, the tests were carried out in the four-cylinder, four-stroke, IDI, naturally aspirated, 12 kW, air-cooled, Onan DJC model at 1800 rpm and different loads (1, 3, 6 and 9 kW). They concluded that the addition of n-butanol and 1-pentanol decreased the BSFC by 0.89% and 0.95%, respectively, and increased the BTE by 5.58% and 4.94%, whereas the addition of propanol increased the BSFC by 5.28% and decreased the BTE by 0.95% compared to the B50 fuel. Propanol, n-butanol, and 1-pentanol decreased CO2 emissions by 39.95%, 38.83, and 12.60%, respectively, and reduced NOX emissions by 15.05%, 19.27, and 27.44%, respectively; meanwhile, HC emissions were obtained low in other fuels except 1-pentanol. Wei et al. [26] analyzed that the addition of pentanol into the diesel fuel may noticeably reduce the particulate emissions and slightly increase NOX emissions. The comprehensive review about the utilization of higher alcohols (from propanol (three carbons) to phytol (20-carbons)) in the diesel engine applications was done by Kumar and Saravanan [18]. They reported that the CI engines can be fueled with higher alcohols or blends with diesel fuel instead of neat diesel fuel and it seemed to be successful in general because higher alcohols enhance the efficiency and reduce the regulated emissions. Li et al. [19] investigated the impact of pentanol addition to diesel and biodiesel fuels at different percentages on the combustion and exhaust emission characteristics of a single-cylinder, four-stroke, and DI diesel engine running on various engine loads and 1600 rpm of constant engine speed conditions. Consequently, the fuel containing 40% diesel–30% biodiesel–30% pentanol blend showed better properties based on the combustion and emission results as well as the economic performance. Nanthagopal et al. [27] compared the influence of 1-butanol and 1-pentanol addition to the biodiesel synthesized from Calophyllum inophyllum oil on the engine performance and emission characteristics under various engine loads. As a result of the experiments, the biodiesel–higher alcohols have led to be higher brake-specific fuel consumption (BSFC) and lower BTE due to the lower heating value of alcohols than that of diesel and biodiesel fuels. CO, HC, and smoke opacity decreased, and at the same time, NOX reduced because of the cooling effect of the alcohols for fuel blends. Devarajan et al. [28] observed significant reductions in NOX, HC, CO, and smoke emissions of a diesel engine running on cashew nut shell biodiesel and the pentanol blends. Babu and Anand [29] tested the biodiesel/diesel/n-pentanol or n-hexanol blends in the diesel engine without any alterations on the engine. As a result, it has been stated that the addition of higher alcohol into the biodiesel–diesel fuel blends caused to improve the engine performance and combustion behaviors. The best blend ratio was found to be at 85% biodiesel–5% diesel–10% pentanol when the CO, HC, and filter smoke number were taken into consideration. Dhanasekaran et al. [30] studied the impact of n-pentanol-WCO biodiesel–diesel fuel ternary blends on the exhaust emission, engine performance, and combustion characteristics in a stationary DI diesel engine applying and not applying exhaust gas recirculation (EGR) process. Accordingly, lower NOX and smoke emissions were observed with ternary blends; however, CO and HC were high. As compared to the B50 fuel, the addition of n-pentanol improved the BSFC results. Yılmaz and Atmanli [31] experimentally evaluated a diesel power generator fueled with diesel–biodiesel–1-pentanol blends at three engine loads (0, 1.5, and 3 kW) with constant engine speed (2000 rpm). Ternary fuel blends caused to increase BSFC while reduced BTE in comparison with the diesel fuel. CO, HC, and NOX emissions of pentanol blends increased, but combustion efficiency was depleted owing to the cooling impact of higher alcohols. Yılmaz et al. [32] conducted an experiment with the blends of 80% biodiesel and 20% propanol, n-butanol, and 1-pentanol. As compared to the B100 fuel, the improvements were achieved with those of blends. Zhang and Balasubramanian [33] blended 10% and 20% n-butanol and n-pentanol with biodiesel and the diesel engine operated with these fuel blends under three different engine loads. Those blends demonstrated decreasing in polycyclic aromatic hydrocarbons (PAHs). In order to increase the cetane number of test fuels, Imdadul et al. [34] added a cetane improver into the alcohol–diesel–biodiesel because of lower cetane number of alcohols. Test fuels were 75% diesel–20% palm biodiesel, and 80% diesel–10% palm biodiesel contain 5% and 10% pentanol and 1000–2000 ppm ethyl hexyl nitrate. It has been stated that a higher cetane number caused to be higher HC and CO, but less BSFC and NOX emissions.
Novelty and objective of the present study
From the intensive review of available technical literature, numerous investigations have been carried out on the CI engines so as to detect both the engine performance and exhaust emissions of ternary blends of biodiesel–diesel–alcohols. On the other hand, there are limited numbers of studies scrutinizing the combustion characteristics of these ternary fuel blends in the literature. Also, enough published paper of work has not been generated on the application of higher alcohols especially pentanol as an additive in CI engines discussed in the above section. However, it could be inferred that pentanol can be utilized in the CI engines as an oxygenated additive with biodiesel and diesel fuels. In general, pentanol has been blended with diesel and biodiesel fuels up to 20% on a volume basis for the preparation of ternary blends. Furthermore, the addition of pentanol to the biodiesel fuel was found to be a viable resource to ensure the fuel properties. From the recent technical-scientific literature review, safflower (Carthamus tinctorius L.) oil is found to be a largely suitable raw material that can be taken into consideration as an alternative source of renewable energy to produce biodiesel fuel, especially for oil-importing countries all over the world such as Turkey. In other words, the biodiesel fuel produced from the safflower oil, which is not consumed by people in our country as food, can be beneficial to decline the dependency of fossil fuel. The safflower oil biodiesel’s fuel characteristics can be enhanced by mixing with pentanol higher alcohol in order to use in the CI engine application for the further effective implementation. However, the researches related to the addition of pentanol into the safflower oil biodiesel or safflower oil biodiesel–diesel fuel blends have not been found in the literature to the best of the authors’ knowledge. Therefore, in the present experimental research work, a systematic methodology has been exhibited to utilize the pentanol with safflower oil biodiesel/diesel fuel blends for CI engine applications. In this context, the main purpose of the present experimental study is to investigate the effects of diesel–safflower oil biodiesel–pentanol blends on the engine performance, combustion characteristics, and exhaust emissions of a diesel engine. With this strategic conception, biodiesel–diesel fuel (20% safflower oil biodiesel) was blended with pentanol (five carbons) at ratios of 5%, 10%, 15%, and 20% (by volume) in order to obtain B20P5, B20P10, B20P15, and B20P20 test fuels that can be subsequently led to a novel alternative fuel blend pattern which is observed to be the research gap. The biodiesel was produced from safflower oil via the transesterification method by using NaOH as a catalyst and methanol as an alcohol. The fuel samples were performed in a single-cylinder, four-stroke, naturally aspirated, air-cooled, DI diesel engine under 3000 rpm constant engine speed but four different engine loads (500, 750, 1000, and 1250 W). All of the engine trials were performed at a constant injection timing of 31°bTDC under a constant injection pressure of 200 bars. The experimental results were compared to both baseline diesel fuel and B20 and discussed with other related researches.
Materials and methods
Material and Reagents
The materials and reagents were selected on the basis of convenience and ease of supply. In this study, the safflower oil was used as a raw material in the biodiesel production and it was purchased from a commercial company located in Kırıkkale, Turkey. The commercially available petroleum-based diesel fuel was obtained from a local station in Yozgat, Turkey, and the fuel properties meet the EN 590 standard. Methanol (99.8%) was used as an alcohol in the biodiesel production, and it was procured from Isolab (Wertheim, Germany). Sodium hydroxide (NaOH) pellet (99%) was preferred as a catalyst in the reaction, and it was purchased from Merck Chemical Company (Darmstadt, Germany). Ethanol (> 99.5%) and reference standards of the fatty acid methyl esters (> 99%) were supplied from Sigma-Aldrich Chemical Company (St. Louis, Missouri, USA). Potassium hydroxide (KOH) solution (0.1 N) and phenolphthalein indicator were bought from Norateks Chemical Company (Istanbul, Turkey). Diethyl ether (> 99.5%) was supplied from Isolab (Wertheim, Germany). Finally, pentanol (> 98%), five-carbon straight chain alcohol, was supplied from Tekkim Laboratory Chemicals (Bursa, Turkey). All of the chemicals were utilized as a received form not to apply purifications because of analytical reagent grade. The qualitative filter paper (125 mm) was procured from S&H Labware (Ankara, Turkey).
Analysis of fatty acid profile
The fatty acid profile of safflower oil and safflower oil biodiesel was analyzed with the help of Shimadzu brand QP2010 model (Kyoto, Japan) Gas Chromatograph (GC) system equipped with DB-5MS capillary column (30 m × 0.32 mm × 0.25 µm). Firstly, 1 μL of the sample was injected into the system and the temperature of the column was increased up to 70 °C for 1 min after the injection process. The temperature was initially increased to 120 °C with the heating ramp of 20 °C min−1 and held for 2 min. The column temperature was reached to 180 °C with 10 °C min−1 for 3 min. In the end, it was risen up to 240 °C with applying 5 °C min−1 for 10 min. In addition, the fuel properties of safflower oil biodiesel like linolenic acid methyl ester and polyunsaturated (≥ 4 double bonds) methyl esters were obtained by using GC–MS system. Table 1 shows the comparison of fatty acid profiles of safflower oil and its biodiesel with different feedstocks used in the production of biodiesel. Moreover, the GC analysis graphs of the safflower oil and safflower oil biodiesel are illustrated in Appendix A, Supplementary data file.
Biodiesel production from safflower oil
As mentioned above, in the present study, the safflower oil biodiesel was produced via transesterification method because it is the most used method in the literature. However, transesterification reaction was affected by various parameters like water content, free fatty acid (FFA), alcohol type and ratio, catalyst type and concentration, reaction temperature and time, etc. The reasons for the selected parameters’ levels were explained in the next paragraphs comprehensively.
First of all, the safflower oil was heated up to 130 °C for 2 h in order to eliminate the water possibility contained in the oil. In addition, the safflower oil was passed through a qualitative filter paper before using in the production of biodiesel to avoid undesirable impurities in the oil.
The most important parameters negatively affecting the transesterification reaction are the water content and the amount of FFA in the oil. The influence of the water content on the transesterification process is higher than that of FFA in terms of reducing the yield of biodiesel. The higher water content in the oil can lead to being an incomplete reaction, form saponification, and decrease the efficiency of ester. Additionally, saponification reduces the yield of biodiesel owing to the difficulties of glycerol separation. Ma and Hanna [41], Zullaikah et al. [42], Helwani et al. [43], and Atadashi et al. [44] indicated that the water content of the oil should not exceed 0.06% (600 ppm). The higher FFA content in the feedstock caused to reduce the yield of biodiesel because of consuming more catalyst in the reaction and forming saponification. Therefore, a lot of researchers have recommended the two-step process including esterification and transesterification instead of the direct transesterification process.
In general, suitable FFA content is less than 1% [45,46,47]. Some of the important physicochemical properties of safflower oil were analyzed and are tabulated in Table 2. Furthermore, the comparison of the physicochemical properties of safflower oil with different feedstocks used in the production of biodiesel is presented in Table 2. As can be seen, it has lower water content (< 0.05 mass%) and FFA (0.71%) values. Thus, it can be noted that only single-step transesterification process was carried out to obtain biodiesel from safflower oil. In other words, a pre-treatment stage was not performed in the present study.
The biodiesel production was carried out in a 2-L reaction flask supported with a magnetic stirrer, reflux condenser, and thermometer. In the transesterification reaction, NaOH and methanol were used as a catalyst and reactant due to the giving high efficiency in the production of biodiesel. They were verified from the literature. Rashid et al. [54] performed biodiesel production from sunflower oil via transesterification method by using methanol and four types of catalysts (KOH, NaOH, KOCH3, and NaOCH3), and the optimum catalyst was found to be as NaOH. Another experimental study was conducted by Atapour et al. [55], who optimized the biodiesel production from WCO according to the yield of the product. They also indicated that the optimum catalyst was NaOH. When we evaluated the usage of alcohol in the biodiesel production process, generally short-chain alcohols such as methanol, ethanol, and propanol were preferred due to the reaction activity [56, 57].Sánchez et al. [57], for instance, investigated the impact of different alcohols (methanol, ethanol, 2-propanol, and n-butanol) on the Jatropha oil biodiesel yield. It can be concluded that methanol gave the maximum yield in the transesterification reaction followed by ethanol, n-butanol, and 2-propanol. Similar results were detected from Meneghetti et al. [58] and Rashid et al. [59]. Consequently, methanol is short-chain alcohol, easy to produce, and inexpensive and reacts with triglycerides better than other alcohols [56].
Firstly, 0.6 mass% of NaOH and 6:1 of methanol to oil molar ratio were mixed in the sealed glass bottle at the room temperature (~ 20 °C) until all of the catalysts were dissolved in the alcohol in order to obtain methoxide. The temperature of the safflower oil was increased up to 60 °C by helping heating magnetic stirrer (Scilogex brand MS7-H550-Pro model) and kept as constant at this temperature. The reason for the selected reaction temperature is the boiling point of the methanol. Thus, the loss of alcohol was blocked both using a low temperature and reflux condenser. During the transesterification process mixing, intensity was kept constantly at 600 rpm. When the temperature of the oil reached the reaction temperature, the methoxide mixture was poured into the oil and reaction time was started. After 60 min, which is the optimum reaction time giving the highest biodiesel yield, the mixture was sent to a separation funnel and settled for 8 h in order to sink the glycerol. The crude glycerol was gathered from methyl ester. Then, crude biodiesel was again put into the reactor and heated up to 75 °C to evaporate the trapped alcohol. Afterward, the crude biodiesel was cooled to 55 °C for purification process and washed by warm distilled water until the wastewater became clear. The wastewater was removed by helping the separation funnel. Finally, the washed biodiesel was dried at 130 °C for 2 h in order to remove the excess water in the biodiesel. In the end, the produced biodiesel was filtered with filter paper.
Unfortunately, it was observed that the production cost of safflower oil biodiesel was 1.5 times higher than the price of the diesel fuel. The safflower oil biodiesel was evaluated for further investigations. The flowchart of the biodiesel production from safflower oil is also briefly shown in Fig. 1.
Binary and ternary fuel blends preparation
For this experiment, safflower oil biodiesel, diesel fuel, and pentanol were used to obtain test fuels for testing phase. The test fuels were prepared on a volume basis using calibrated glass beaker (± 0.5 mL). The splash blending technique, which is the least expensive one, was applied because it is most preferred method. The pentanol was blended along with safflower oil biodiesel and diesel fuel in different concentrations keeping the amount of biodiesel as 20% in all blends. The fuel blends were generated using 5%, 10%, 15%, and 20% of pentanol mixed with 75%, 70%, 65%, and 60% of diesel fuel, respectively. In addition, B20 (%80 diesel fuel–20% safflower oil biodiesel) of a binary blend has also been prepared. The experimental results of the ternary fuel blends were compared both neat diesel fuel and a binary blend of B20. The test fuels were stored in a sealed glass bottle and in a dark place at the room temperature for 24 h prior to the testing stage. Accordingly, no phase separation was observed in the blends. All of the prepared fuels and abbreviations are given in Table 3.
Physical and chemical properties of the test fuels
In order to measure the FFA content of the safflower oil and acid value of its biodiesel product, the acid–base titration method was used. The FFA was found by using the following equation [60]:
where v and b are the volumes in mL of the titration solution and blank, respectively. m is the mass of the oil sample, and N is the normality of the titration solution.
Some of the properties were estimated using suggested equations in the literature. The saponification number (SN), iodine value (IV), and higher heating value (HHV) of the safflower oil biodiesel, for instance, were calculated by using the following empirical equation [13, 61]:
where Ai is the proportions of each component in the fatty acids, and D is the number of double bonds and MMi molecular mass of each component.
Degree of unsaturation (DU) was obtained from the mono- and polyunsaturated fatty acid component of the sample, and the equation was given as follows [35, 40]:
Long-chain saturated factor (LCSF) was detected from the saturated fatty acid profile and their melting points (MPn). For this, it was determined by the following equation [35, 40]:
In the end, oxidation stability (OS) was predicted by using Eq. 7 [13]:
In addition, the Kay’s mixing rule technique was applied in order to obtain the physicochemical properties of biodiesel–diesel and biodiesel–diesel–pentanol blends. The general formula of the Kay’s mixing rule was presented as follows:
where y is the estimated value of the property, c is the blending ratio, and ϕ is the respective property of available data.
Experimental
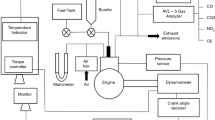
In the present work, the engine experiments were carried out on a single-cylinder, four-stroke, air-cooled, naturally aspirated, DI diesel engine generator. The schematic view of the experimental setup is illustrated in Fig. 2. The technical specifications of the diesel engine and generator are presented in Table 4.
The exhaust emission values and smoke opacity were measured by using an Italo plus-spin type having digital displaying exhaust emission device. The measuring probes are placed to the tailpipe of the engine in order to get exhaust gas samples. Prior to the experimentation, the exhaust gas sensors were calibrated with standard gases so as to eliminate the errors. The technical specification of the exhaust gas analyzer is presented in Table 5. A K-type thermocouple connected to a digital display unit was used to decipher the exhaust gas temperature (EGT). The fuel consumption was obtained with the help of an electronic precision scale and a stopwatch in comparison with the mass of fuel initial and final each 15 min trial at each load conditions for each of the test fuels.
The in-cylinder pressure was recorded at different engine loads for each test fuels with the help of a fiber optic pressure transducer (Optrand brand H32294-Q model) having measurement range between 0 and 3000 psi using 1.80 mV psi−1. The crank angle was measured using an incremental optic rotary encoder (Opkon brand PRI 50 model) mounted to the crankshaft of the test engine. The technical specifications of the pressure sensor and encoder are tabulated in Table 6.
Test methodology
Prior to the engine trials, the calibrations of the test equipment were supplied. Then, the pretests were performed so as to obtain the operating conditions of the test engine. Afterward, the stabilization time of the engine was found and the engine was ensured to stabilize for all test fuels before the engine experiments. All of the problems emerged in the pretests were resolved; then, the main test process was started. The engine was operated with neat diesel fuel for at least 15 min, and the engine was warmed up, thereby avoiding from cold-start effects. In addition, all of the records were read when the engine was stabilized. After, the ternary fuel tanks containing pentanol were placed for testing. The engine tests were performed at constant engine speed of 3000 rpm and under four different engine loads (500, 750, 1000, and 1250 W) for each fuel in order to investigate the effects of pentanol addition into the diesel–biodiesel fuel blend on the engine performance, exhaust emissions, and combustion characteristics of a CI engine. The loads were provided by a series of electrical resistance elements. During the engine tests, the ambient temperature was measured to be as 25 °C. The pressures inside the cylinder were detected in each work cycle up to 720°CA for each 1°CA intervals at various engine loads. The in-cylinder pressure data were monitoring and transferred to the computer thanks to the digital oscilloscope (Rigol brand DS1064B model). Moreover, the average in-cylinder pressures of the test fuels were calculated by applying a suitable filtering technique to eliminate the noise and errors and considering at least 20 cycles. Also, the coefficient of variations (COV) was calculated with respect to the combustion results and presented in this work. The heat release rate (HRR) was calculated by using the following equation according to the first law of thermodynamics. However, the heat losses of the wall were not taken into consideration.
where dQnet (J) indicates the energy amount passing through the cylinder wall and combustion chamber wall at the end of combustion, θ (°) is the crank angle, γ denotes the constant polytrophic exponent and is considered as 1.35, P (Pa) refers to the cylinder pressure, and V (m3) is the cylinder volume.
The start of combustion (SOC) can be identified as the point at which the heat release plot crosses over the zero transition [62]. The start of injection (SOI) is defined as the crank angle at which the injector opens the injector pressure set at 200 bar. The ignition delay (ID) is the difference between SOI and SOC. The diesel engine was operated directly without any major modifications. It was not observed difficulties during the operations of the diesel engine fueled with test fuels except B20P20 fuel blends which contain a high concentration of pentanol. In the end, experiments were realized three times due to reducing the errors.
Indicated mean effective pressure (IMEP) values are not dependent on the cylinder number, the volume of the cylinder, and engine speed factors; therefore, IMEP may be used an efficient parameter for observation of the engine efficiency. IMEP is calculated by division of net work to the displacement volume. IMEP can be obtained with Eq. 10:
where Wnet and Vstroke refer to the net work and swept volume of the cylinder, respectively. Wnet was calculated using Eq. 11.
Uncertainty analysis
The determination of errors during the experimental measurements and the calculation of various performance parameters is very important for accuracy. The occurrence of errors and uncertainties was influenced by various parameters such as the selection of equipment, calibration, relative status, environment, reading, observation, and test planning. The uncertainty analysis is required to prove the accuracy of the test results obtained from the experiments. The method recommended by Holman [63] was implemented to determine the uncertainties. The equation was given as follows:
where R is the dependent factor and it is a function of independent variables x1, x2, x3,…, xn, wR is the uncertainty value of the results, and w1, w2, …, wn are the uncertainties of the independent variables.
The uncertainty results of each type of equipment were calculated by using Eq. 12 and are presented in Table 7. As a result, the uncertainties of mass fuel consumption, BSFC, BSEC, and BTE were found to be at ± 0.83%, ± 1.39%, ± 1.43%, and ± 0.57%, respectively. As known, the acceptable range for the uncertainty is below ± 5%. In this regard, the overall uncertainty of the system was within the acceptable limits.
Analysis of the cycle-to-cycle variations
It can be noted by considering the differences between certain numbers of consecutive cycles in which an engine operates regularly. For all test fuels, the engine stability was found by examining the variation in the IMEP according to the cyclic differences. Cyclical differences may be observed when the in-cylinder pressure is measured for more than one thermodynamic cycle. The most commonly used expression in the analysis of cycle-to-cycle differences is the coefficient of variation (COV). This is due to the fact that the cycle-to-cycle pressure change is mainly caused by a cycle-to-cycle change in the combustion process. The COV for IMEP can be calculated by using Eq. 13 [64].
where COV (%) is the coefficient of variation, \(\overline{\text{imep}}\) indicates the average IMEP occurred in a number of cycles, and \(\sigma_{\text{imep}}\) defines the standard deviation. \(\overline{\text{imep}}\) and \(\sigma_{\text{imep}}\) can be computed by the following equations [65]:
Results and discussion
The physicochemical properties of safflower oil biodiesel, diesel fuel, and pentanol
Table 8 shows some physical and chemical properties of pure safflower oil biodiesel, conventional diesel fuel, and pentanol. As can be seen in Table 8, most of the fuel characteristics of the safflower oil biodiesel may generally satisfy the both ASTM D6751 and EN 14214 standards. Moreover, the elemental analysis was carried out using ICP-MS device and results of safflower seed oil biodiesel are tabulated in Appendix B section.
The influence of pentanol addition into the biodiesel–diesel fuel blend on the basic fuel properties
The basic fuel properties of ternary blends were predicted according to the given equations as mentioned above and listed in Table 9. It can be observed that the density, kinematic viscosity, calorific value, and cetane number of diesel–biodiesel–pentanol ternary blends were decreased with an increase in the proportion of alcohol. However, the oxygen amount was increased in the fuel samples due to the pentanol and biodiesel additions, because biodiesel and pentanol contain more oxygen in the structure and, therefore, improve the combustion process. Consequently, it can be noted that binary blend and all of the ternary blends could be operated directly in diesel engines without engine modifications.
Engine performance and exhaust emissions characteristics
The engine performance and exhaust emission characteristics like the variations in mass fuel consumption, brake-specific fuel consumption (BSFC), brake thermal efficiency (BTE), exhaust gas temperature (EGT), CO, CO2, HC, NOX, and smoke opacity emissions in accordance with different engine loads of 500, 750, 1000, and 1250 W at a constant engine speed of 3000 rpm were studied for the ternary blends of safflower oil biodiesel–pentanol–diesel fuel and compared to both a binary blend of B20 and diesel fuel. Moreover, the test results acquired in the present experimental study for biodiesel–higher alcohol–diesel fuel blends were compared with works of the literature concerned of the biodiesel–diesel fuel blends treated with the higher alcohols and discussed elaborately with regard to the engine performance and exhaust emission behaviors.
The fuel consumption and brake-specific fuel consumption (BSFC)
Figure 3 illustrates the brake-specific fuel consumption (BSFC) for all of the test fuels with respect to the engine loads. As can be seen in Fig. 3, a binary blend of B20 possessed a higher BSFC than that of diesel fuel at all engine load conditions. However, the highest ratio was observed at a lower engine load of 500 W with 8.02%. The average increment in B20 fuel blend was 4.80% in BSFC as compared to the diesel fuel. This can be explained considering Table 8 owing to the lower calorific value of safflower oil biodiesel than diesel fuel. Similar trends were obtained with pentanol addition to the biodiesel–diesel fuel blend. The higher alcohol of pentanol addition to the biodiesel–diesel fuel blend increased the BSFC values for all engine loads.
As compared to the diesel fuel, the BSFC of the ternary blends increased within the ranges of 7.19%–13.90% on average. Increasing the ratio of pentanol in the mixture further increased the BSFC. There is no doubt that the largest BSFC was obtained 19.50% with B20P20 at engine load of 750 W. The BSFC of B20P5, B20P10, B20P15, and B20P20 was risen averagely 2.66%, 3.68%, 5.86%, and 9.66%, respectively, compared to B20. The first reason for higher BSFC is lower heating value of pentanol than both safflower oil biodiesel and diesel fuel. As shown in Table 8, the safflower oil biodiesel and diesel fuel had higher heating values in order of 21.56% and 9.91% than that of pentanol. As compared to biodiesel and diesel fuel, the pentanol has lower heat of combustion; therefore, BSFC of ternary blends was observed to be higher. Pentanol has 18.18% oxygen (by mass) in its atomic structure. The other reason for increasing the BSFC is the oxygen content of the fuel, because it is well known that the calorific value is depended on the oxygen content and decreases with respect to increasing the oxygen content in the fuel. It causes a larger BSFC value, and the engine needs to use more fuel to produce the same engine power output while operating with alcohol blends [66, 67].
The similar results have been demonstrated by many researchers. Yilmaz and Atmanli [31], for instance, observed that the pentanol addition to the biodiesel–diesel fuel blends increased BSFC values within the ranges of 5.27%–8.61% in comparison with the neat diesel fuel. In another investigation, Atmanli [25] also reported that D40BD40P20 blend presented lower BSFC than that of D50B50 blend. Yesilyurt [21] confirms that the higher alcohols of butanol and pentanol additions into the biodiesel–diesel fuel blends caused to increase the BSFC between 0.77% and 8.07%. Imdadul et al. [23] determined the lower BSFC results with D60B20P20 as compared to D80B20. Campos-Fernández et al. [20] and Wei et al. [26] indicated the increasing ratio of pentanol led to an increase in the BSFC values of pentanol–diesel fuel blends. In addition, similar findings for biodiesel–pentanol–diesel fuel blends were exhibited by other researchers [19, 33].
The mass fuel consumptions of the test fuels are represented in Fig. 4. The ternary blends of biodiesel–pentanol–diesel fuel blends showed higher mass fuel consumption than that of diesel fuel and B20. The largest mass fuel consumption was observed B20P20 fuel blend at higher engine load of 1250 W. Moreover, the increase in pentanol concentration in the blend led to increase the mass fuel consumption owing to the lower calorific value of pentanol. It caused to decrease the calorific value of the blends and an increase in the mass fuel consumption.
Brake thermal efficiency (BTE)
The brake thermal efficiency (BTE) can be described as the value of the output work divided by the input energy amount in the internal combustion engines. The BTE of CI engines is affected by air–fuel ratio, fuel properties, combustion process, and compression ratio [68]. The variation in BTE as a function of the engine load is given in Fig. 5. It can be well known that the BTE is inversely proportional to the BSFC. In general, BTE of the test engine increased up to 750 W of the engine load; then, it began to decrease when further increase owing to the fact that the larger quantity of fuel injected at the higher engine load led to accumulate fuel in the cylinder and the possibility of incomplete combustion [29].
According to the results, the maximum BTE was observed with diesel fuel. However, the BTE values of ternary blends showed almost similar results at higher engine loads. BTE values of all the test fuels were found to be between 15.49 and 22.75%. The maximum BTE values of the diesel fuel, B20, and B20P5 were found to be at 22.75%, 21.82%, and 20.96% under 750 W engine load condition, respectively, while the maximum BTE of B20P10, B20P15, and B20P20 which contain higher amount of pentanol as an additive was obtained to be in order of 20.95%, 20.87%, and 20.28% at 1000 W. Accordingly, it can be concluded that the pentanol addition to the safflower oil biodiesel–diesel fuel blend had no significant influence on the variation in the BTE under the higher engine loads. This case was also reported by Wei et al. [26] and found only a small difference in BTE with the increase in pentanol proportion as compared to the diesel fuel at all engine loads. Campos-Fernández et al. [20] indicated that there is no significant change in BTE statistically with regard to the pentanol addition in diesel fuel blends.
However, the opposite observations were found in the literature. In Yilmaz and Atmanli [31], for instance, the pentanol addition to the biodiesel–diesel fuel blend demonstrated a negative impact on the BTE value and the average reductions of 5%, 10%, and 20% pentanol-added fuels were within the range of 3.73%–1.76%. Dhanasekaran et al. [30] presented that the BTE of 50% diesel fuel–30% WCO–20% pentanol blend was comparable to the neat diesel fuel because the efficiency of the combustion improved due to the higher oxygen content of pentanol.
Exhaust gas temperature (EGT)
Exhaust gas temperature (EGT) is one of the substantial factors that influence the exhaust emission characteristics of a CI engine. In general, EGT is depended on the fuel properties such as cetane number, kinematic viscosity, density, and calorific value and engine parameters like injection timing and pressure, compression ratio, etc. [69]. The variations in EGT for diesel fuel, a binary blend of biodiesel–diesel fuel, and ternary blends of biodiesel–pentanol–diesel fuel under various engine load conditions are illustrated in Fig. 6. As can be seen in Fig. 6, the increase in engine load led to increasing the EGT linearly. This is a reason that the quantity of the fuel sprayed into the combustion chamber rises with respect to the engine load increases and it caused to higher temperature inside the cylinder [70]. The average EGT values of diesel fuel, B20, B20P5, B20P10, B20P15, and B20P20 were found to be at 242 °C, 255.8 °C, 235.8 °C, 228 °C, 218.8 °C, and 209.4 °C, respectively.
The largest EGT values may be obtained throughout the better combustion process [71]. As compared to the diesel fuel, a binary blend of B20 showed higher EGT at each engine load. This can be clarified as follows. The cetane number of biodiesel is higher than that of diesel fuel as shown in Table 8; therefore, the combustion begins earlier well the ignition delay period shortens. The combustion process has continued at work time due to the high boiling point compounds in the biodiesel chemical structure. Also, the high oxygen content of biodiesel fuel compared to the diesel fuel improves combustion and increased EGT values. Devan and Mahalakshmi [72] and Panwar et al. [73] also reported that high viscosity of biodiesel–diesel fuel blends led to become poor combustion behaviors. Thus, higher EGT could be occurred because of increasing the duration of the combustion.
The higher alcohol addition into the biodiesel–diesel fuel blend caused to decrease EGT values remarkably in comparison with both biodiesel and neat diesel fuel results. In addition, EGT diminished due to the increase in the rate of pentanol in the blends. The alcohols absorb more heat from the environment in order to evaporate because of the high latent heat of evaporation of alcohols. It led to decreasing EGT of ternary fuel blends. Also, EGT is a function of ignition delay and is an indicator of the combustion end temperature. The large oxygen content and low calorific value of fuel blends caused to the reduction in the EGT. The alcohols having more oxygen molecules improve the rate of the combustion, and EGT descends. Similar reductions in EGT were observed by many researchers [74, 75]. Cheung et al. [76] observed decrement in EGT for alcohol-blended fuels due to the lower calorific value and higher latent heat of evaporation of alcohol. They also indicated that lower EGT caused to decrease NOX emissions. This subject will be explained in more detail in the following section. However, dissimilar results were detected by Atmanli [25], Yilmaz and Atmanli [31], and Yasin et al. [77]. They claimed that the higher oxygen content of alcohol led to become a higher EGT.
NOX emissions
It is well known that the air contains approximately 78% nitrogen and it may not react in normal conditions. Due to the larger temperature inside the combustion chamber, nitrogen can react with oxygen molecule and nitrogen oxides emission can occur. The nitrogen oxides (NOX) are made up of minor amount of NO2 and higher amount of NO. In many cases, the other oxides of nitrogen such as N2O, N2O5, and NO3 are not considered. Moreover, NOX emissions are categorized as harmful and undesired product. For this reason, one of the most substantial issues for the reduction in NOX emissions is to understand the mechanism of NOX formation. In the literature, there are different types of mechanisms that describe the NOX formation throughout the combustion of diesel fuel. These are known as Zeldovich (thermal), Fenimore (prompt), N2O pathway, fuel-bound nitrogen, and the NNH. Among them, the first two mechanisms (Zeldovich and Fenimore) have been accepted for diesel combustion as well as biodiesel combustion [78]. Based on the recent literature, the general pathway of the Zeldovich mechanism is given as follows.
Fenimore or prompt NOX mechanism is summarized in the following reactions:
As widely recognized, the thermal NOX formation is basically influenced by two factors as follows: (1) high charge temperature and (2) high oxygen content [79]. The change in NOX emissions of diesel fuel, B20, B20P5, B20P10, B20P15, and B20P20 as a function of engine load is exemplified in Fig. 7. As mentioned above, the NOX emissions increase perceptibly over 1900 K of the temperature inside the cylinder. At the same time, NOX also rises when the residence time of the blends lengthens under elevated temperature [80].
The NOX emissions results were evaluated, and all of the test fuels showed an increase with regard to the engine load because the increasing engine load caused to increase the temperature consistently in the cylinder. This can be verified with EGT results as shown in Fig. 6. Besides this, for better understanding, the correlation of the final NOX concentration with the EGT for each of the cases tested is illustrated in Fig. 8. Accordingly, NOX concentrations for all the tested fuel samples have increased linearly with the increase in EGT values. As observed, minimum R2 value was found to be at 0.91544. It is well known that R2 must be close to 1. It means that the linear model can be preferred to fit the present data accurately.
The ternary fuel blends showed a decrease in NOX emissions, while B20 fuel blend increases as compared to the neat diesel fuel. In addition, the decrement in NOX emissions was observed more according to the pentanol addition into the safflower oil biodiesel–diesel fuel blend. B20 fuel exhibited an increase of 15.38% on average for NOX emissions. This is because that there are many high temperature regions in the cylinder due to the oxygen molecules. As presented in Table 8, biodiesel has higher oxygen content than that of diesel fuel in the chemical structure. Therefore, NOX emissions of biodiesel fuel may be increased significantly. The results were well agreed with the investigations conducted by many researchers. For instance, Ozcelik et al. [81] found that the NOX emissions of B7 and pure biodiesel were to be higher at ratios of 17.6% and 58.8% than that of diesel fuel. Mofijur et al. [82] found almost similar results with the findings in the present study. They observed that B10 and B20 fuel blends released in order of 8.46% and 18.56% higher NO emission than diesel fuel. How et al. [83] showed that the largest increment in NOX emissions was observed to be at 12% for B50 fuel blend as compared to the baseline diesel fuel.
Another reason for increasing NOX emission using biodiesel fuel was clarified by Rashed et al. [84]. They indicated that the higher cetane number of biodiesel decreases the ignition delay period and thus advances combustion. On the other hand, it can be a questionable issue for the formation of NOX emissions. Although this case was also verified by different authors in the literature, indeed, many researchers [85, 86] claimed that the high cetane number led to decreasing NOX emissions. Additionally, this argument was discussed comprehensively by Xue et al. [87]. The present study demonstrated that if the cetane number increases, NOX emission will be reduced. However, a few studies [88,89,90] showed that using biodiesel fuel led to become a reduction in NOX emissions.
When the effects of pentanol addition to the safflower oil biodiesel–diesel fuel blend on the formation of NOX emissions were evaluated, it can be seen that the NOX emissions were reduced consistently. It is noteworthy that the NOX emissions for B20P5, B20P10, B20P15, and B20P20 decreased averagely 3.16%, 11.85%, 21.58%, and 31.44%, respectively, as compared to B20 fuel blend. This is because the temperature of the residual gases inside the cylinder reduces owing to the high oxygen content, low calorific value, and high latent heat of evaporation of alcohols. Thereby, it causes cooling effect and nitrogen and oxygen atoms cannot be reacted. Consequently, the NOX formation was decreased. Furthermore, lower density and viscosity values of alcohols than biodiesel and diesel fuels also affect directly the end temperature of the combustion process. The experimental results were in accordance with most of the other researchers’ studies conducted by higher alcohols. In Celik et al. [91], for instance, the combustion temperature in the cylinder reduces because the alcohols have the cooling impact due to the latent heat of evaporation degree. For this reason, NOX emission was decreased significantly. Kumar and Saravanan [18] presented the latent heat of evaporations of diesel fuel and pentanol as 308 kJ kg−1 and 270–375 kJ kg−1, respectively. Mahalingam et al. [92] resulted that the 10% and 20% pentanol addition to the biodiesel occurred in the reduction in NOX emissions at a ratios of 3.3% and 3.9%, respectively, at all engine load conditions as compared to pure biodiesel. Nanthagopal et al. [27] also declared that the pentanol in the biodiesel caused to decrease NOX emissions from 10% to 23% compared to B100 fuel. It can be concluded that the pentanol addition to the biodiesel–diesel fuel blends is a suitable, easy, and inexpensive way to reduce the NOX emissions. On the other hand, the opposite view was also highlighted in the literature. Yilmaz and Atmanli [31] represented that the NOX emissions of D75B20Pen5, D70B20Pen10, and D60B20Pen20 were observed to be at an increase of 30.22%, 36.87%, and 29.13%, respectively, in comparison with the baseline diesel fuel. In addition, Li et al. [19] found that the NOX emissions reduced in the exhaust gas at low engine load condition when using pentanol–diesel–biodiesel fuel blends.
HC emission
The deviation of HC emissions of the test fuels with respect to the engine load and pentanol addition is illustrated in Fig. 9. HC emissions can form mainly incomplete combustion process and slower oxidation reactions. The reasons may be drawn as follows: (1) very poor or rich air–fuel ratios in the cylinder, (2) heat loss to the cold regions around cylinders, and (3) flame quenching in these areas [93].
HC emissions also show similar trends with the CO emissions. It can be considered that both CO emissions and HC emissions were obtained lower than that of neat diesel fuel. The maximum HC emissions were observed at the highest engine load of 1250 W. In addition, the pentanol addition into the biodiesel–diesel fuel led to decrease in the HC emission significantly. As compared to the baseline diesel fuel, the average reduction in HC emission for B20 was found to be between 1.51% and 4.48%. This is because the excess oxygen content in the biodiesel fuel as presented in Table 8. Li et al. [19] argued that the oxygenated fuel blends showed lower HC emissions than that of diesel fuel over most of the engine load condition. Although the addition of alcohol to the biodiesel has a cooling effect, the decrease in HC emissions can be considered as better atomization of the fuel spray. The viscosity of biodiesel diesel blends which is higher than diesel caused to becoming the poor atomization, less homogenous mixtures, and uneven distribution small portions of fuel across the combustion chamber. The high latent heat of evaporation of alcohol leads to cooling effect inside the combustion chamber. The lower density and viscosity values of alcohol have led to improving the characteristics of the fuel spray, and thus, the better fuel–air mixture can be formed inside the combustion chamber, and it enhances the combustion in the cylinder. The result obtained for the present study was well satisfied with the findings from other researchers performed by alcohol-treated fuels [31, 94, 95].
Based on the literature survey, HC emissions of ternary blends including higher alcohol were higher than diesel fuel [96, 97]. However, it is clearly seen from Fig. 9 that the pentanol addition to biodiesel–diesel fuel blends decreased the HC emission. HC emissions of B20P5, B20P10, B20P15, and B20P20 reduced in an average of 5.25%, 8.01%, 13.33%, and 30.47%, respectively, compared to diesel fuel. At the same time, they were observed in order of 2.65%, 5.31%, 10.56%, and 27.47%, respectively, in comparison with the B20 fuel blend. Atmanli [25] highlighted that the pentanol was the most efficient alcohol to decrease the HC emissions among butanol and propanol. Mahalingam et al. [92] reported that the adding 10% and 20% of pentanol to the pure biodiesel executed in 2.1% and 3.6% decrement in HC emissions, respectively, as compared to B100 fuel all of the engine loads. Pentanol addition improves the combustion efficiency and encourages the combustion. Thus, HC emission of fuels was decreased slightly. Another reason for the reduction in HC emission was clarified by El-Seesy et al. [98], who investigated the ternary blends of jojoba oil biodiesel–n-butanol–diesel fuel, and as a result, they found that the n-butanol in the blends caused to decrease HC emissions due to the low viscosity and density values of ternary blends. It may improve the efficiency of the atomization. These reductions are also consistent with the other investigations conducted by different researchers [11, 28, 99].
CO2 emission
The most significant contributor to global warming all over the world is the CO2 emissions which cause greenhouse impact in the atmosphere [100]. It can also play a major role in the critical public health problem and the formation of ozone [101]. But, some of the researchers [102] highlighted that all of the CO2 gases emitted by the combustion of biodiesel fuel have been captured and utilized by the plant along with the photosynthesis which is a vital process for the plant. The CO2 emission observation is a key factor that can give an opinion related to the complete combustion in the cylinder of the diesel engine. Therefore, the more oxygen molecule in the cylinder may cause to generate more complete combustion. If enough oxygen molecules are attainable in the cylinder, CO will convert to CO2 emission because of the fact that one of the most important parameters to oxidize is hydroxyl radical OH [23].
The CO2 emission values for all test fuels under constant engine speed of 3000 rpm and different engine loads are depicted in Fig. 10. As shown in Fig. 10, the maximum CO2 values were determined at the highest engine speed. In addition, the increase in the engine load caused to increase simultaneously the CO2 emission at each test fuel. The CO2 emissions of both binary and ternary blends were higher than diesel fuel because of the excess oxygen content in their structures resulting in complete combustion. The mean CO2 emissions of diesel fuel, B20, B20P5, B20P10, B20P15, and B20P20 were found to be at 7.19%, 7.70%, 8.30%, 8.59%, 9.39%, and 9.90%, respectively. The maximum CO2 emission of 13.15 vol% has appeared for B20P20 fuel blend at the engine load of 1250 W, which was 38.72% higher than diesel fuel and 21.87% higher than B20 fuel blend. The lower density and kinematic viscosity of the ternary blends could improve the evaporation of the fuel; as a result, CO2 emissions were increased consistently. The almost same trends in the CO2 emission were presented by different authors, and some of them were summarized as follows: Hulwan and Joshi [103] investigated that the CO2 emission of fuel samples involves 20% and 30% ethanol in the biodiesel–diesel fuel blend and was increased under the low engine load operating conditions. This is mainly due to the fact that the consumption of fuel was ascended in order to generate the same power. They also indicated that the variations in the injection timing from 21° to 13° CA bTDC could not influence the CO2 emission results at each engine load condition. Imdadul et al. [23] experimented that the ternary blends of biodiesel–diesel–pentanol demonstrated a statistically significant increment in the CO2 emissions as compared to the diesel fuel. Imdadul et al. [24] also reported that the butanol and pentanol additions into the Alexandrian laurel biodiesel–diesel fuel blends led to becoming lower CO2 emission results compared to the biodiesel–diesel fuel blends. The reason is mainly due to the fact that those alcohols ascended the percentage of peroxyl and hydrogen peroxide (H2O2) radicals that affected the conversion of the carbon monoxide process remarkably. Babu and Anand [29] observed higher CO2 emission in the exhaust gas when using used frying oil biodiesel–alcohol–diesel fuel blends owing to the complete combustion process in the chamber. Moreover, they found the maximum CO2 emission with D5B85P10 fuel blend that was in the order of 0.85% and 1.1 vol% higher than that of biodiesel and diesel fuels. Sureshkumar et al. [104] claimed that Pongamia pinnata methyl ester released higher CO2 emission than diesel fuel at larger engine load because the high cetane number of biodiesel might lead to the auto-ignition.
On the other hand, the literature survey showed that the opposite experimental results were also presented by different researchers. Yesilyurt [21] stated the n-pentanol and 1-butanol additions to the biodiesel–diesel fuel blends, and lower CO2 emissions were observed than that of neat diesel fuel because of having fewer carbon atoms of alcohols in the composition. The compatible results were found by Randazzo and Sodré [105], who reported that the increase in ethanol proportion in the biodiesel–diesel fuel blend caused to reduce the CO2 emissions owing to the low C/H ratio in the lower alcohol molecule. Alptekin et al. [106] highlighted that the CO2 emission of the test fuel consists of 60% diesel fuel–20% waste oil biodiesel–20% bioethanol reduced up to 7.1% in contrast to the diesel fuel under the engine load of 600 Nm due to the low C/H ratio of bioethanol. Akar [107] also observed a decreasing trend in the CO2 emission by using butanol as an additive to the biodiesel–diesel fuel blends. Oliveira et al. [108] experimented the performance, emissions, and combustion characteristics of a diesel engine operating with biodiesel–diesel–ethanol blends by fixing biodiesel portion to 7% and different ethanol concentrations. They observed a trend of descending in the CO2 emissions with the increasing ethanol ratio.
CO emission
It is well known that the carbon monoxide has not a color, odor, and taste. Its density is slightly higher than that of atmospheric air. In addition, CO has a greatly toxic property that influences human health negatively in nature [109]. Moreover, a small amount of CO may lead to breathlessness and headaches [95]. The various parameters like engine speed, fuel type, injection pressure, air–fuel ratio, and injection timing are able to influence the formation of CO emission in the internal combustion engines [110].
The CO emission values of each test fuel versus engine load are illustrated in Fig. 11. When Fig. 11 evaluated according to the engine load, the CO emissions of the test fuels were observed and appeared to be low values at the lowest and medium engine loads. However, the highest engine load led to becoming the largest CO emission for each fuel samples. CO emissions of B20, B20P5, B20P10, B20P15, and B20P20 were descended averagely by 0.19%, 5.66%, 11.57%, 26.81%, and 31.61%, respectively, in contrast to the petroleum-based diesel fuel results.
It can be easily seen that the pentanol addition to the diesel–biodiesel fuel blend reduced the CO emissions due to the oxygen content of alcohol in the structure. CO emission indicates the chemical energy to be lost through the exhaust gases. Furthermore, CO emission in the exhaust gases may conclude the incomplete combustion process in the cylinder of the CI engine. This is mainly caused by inadequate oxygen concentration in the combustion chamber. The more oxygen molecule in the medium can form CO2 emission instead of forming CO emission. Therefore, the oxygen amount is an important factor so as not to release CO emission among the exhaust gases. Besides biodiesel fuels, the alcohols have much more oxygen in the chemical bonds than diesel fuel. As a result, it cannot be hard to say that the small amount of CO emission will be emitted when using these oxygenated fuels in the diesel engine. It is also estimated that the excess amount of oxygen will be combined with carbon atoms and cause CO2 emission in the exhaust gases. Moreover, alcohols possess lower carbon atoms in their structures, and therefore, less CO emission will be released. When the results of the present work were analyzed, B20 fuel blend showed lesser CO emission and also alcohols added fuel blends exhibited even lower CO emission than that of diesel fuel. Cetane number of fuel is another reason in order to reduce the CO emission. This is because of the high cetane number as presented in Table 8. Biodiesel fuels have a higher cetane number than that of diesel fuel; thus, it improves the combustion in the chamber. Ajav et al. [111] acquired that the CO emissions decreased from 36.8% to 62.5% with the help of 5–20% ethanol addition into the diesel fuel in contrast to the diesel fuel. Choi et al. [112] recommended a split injection method instead of a single injection on the diesel engine in order to observe more reduction in the level of CO emission. In comparison with the diesel fuel, the decreasing trends in the CO emissions when the diesel engine running on biodiesel–pentanol–diesel fuel blends and biodiesel–butanol–diesel fuel blends were detected by Li et al. [19] and Akar [107], respectively. Yesilyurt [21] remarked that CO emissions of B2P5, B2P10, B20P5, and B20P10 descended between 19.3% and 32.40% on average. Furthermore, a similar reduction in CO emissions for butanol–diesel–micro-algae oil biodiesel fuel blends was presented by Tuccar et al. [113]. Imdadul et al. [23] found that CO emissions were decreased when using the pentanol addition to the biodiesel–diesel fuel blends.
Another reason for the reduction in CO emissions for pentanol additive fuel blends is a result of improved combustion. Pentanol behaves as an oxygen buffer which releases throughout combustion. Further, an additional reason is owing to lesser viscosity of pentanol additive fuels. Less viscous fuel aids healthier evaporation of fuel and results in better combustion. The viscosity of fuel blends is reduced by pentanol addition when compared to biodiesel fuel. The fuel with lesser viscosity improves the rate of mixing with air which in turn reduces the un-burnt HC emissions [114]. This result is in line with the experimental trial by Atmanli [25]. Consequently, the mixing rate of fuel is enhanced and provides improved combustion and lower HC emission [115]. Besides, the rate of vaporization of fuel blends increases with an increase in pentanol content. More rapid vaporization increases the mixing rate of air–fuel mixture and aids improved combustion and reduces the ignition delay and HC emissions [116]. The rate of vaporization of fuel increases with an increase in pentanol content. Rapid vaporization reduces the ignition delay and HC emissions [117,118,119].
The reason for lower CO emissions for pentanol-blended fuels is due to improved combustion in the cylinder [65]. Hydroxyl group and n-pentanol oxygen atoms get bonded during the combustion and result in the lower formation of soot by slowing down the soot formation and increase the oxygen availability [120, 121]. Because biodiesel and alcohol fuels contain oxygen in their molecular structure, it requires lesser oxygen for complete combustion. As the percentage of alcohol increases in the biodiesel–diesel–alcohol blends, the CO emission reduces [122]. The lower density of pentanol than diesel and pentanol evaporates easily into the cylinder, thus decreasing the spray atomization length. This effect helps the blending process and decreases CO formation [123]. Ternary blends with a high ratio of pentanol contents improve the fuel–air-mixing process, particularly in the fuel-rich region of the combustion chamber by providing high O2. Mixing pentanol with diesel fuel leads to a leaning effect on the ternary blends because of the low stoichiometric air–fuel ratio of pentanol, thus lowering CO emissions. The addition of fuel-bound O2 in the blends ensures CO oxidation even in locally fuel-rich zones, thus helping reduce CO emissions [19]. The droplet diameter of the fuel spray plays a key role in the heating, evaporation, and ignition in the burning chamber [23]. Increasing the droplet diameter in the fuel spray leads to a decrease in the evaporation rate and an increase in ignition delay and HC formation. In the present study, the addition of pentanol to the fuel blends helps to improve the atomization of the fuel spray and this phenomenon leads to a reduction in HC and CO emissions via better combustion.
On the other hand, the opposite outcomes in CO emission could be seen with using the binary blends of biodiesel–alcohol or diesel fuel–alcohol and ternary blends of various types of alcohol–biodiesel–diesel fuel when the recent literature was reviewed. For instance, Wei et al. [26] reported that CO emissions ascended when the CI engine fueled with diesel/pentanol blends, and its effect was considerable at the lower loads of engine. Randazzo and Sodré [105] noted that CO emission results were directly proportional to the ethanol quantity in the B20 fuel blend. Zhu et al. [22] noticed that CO emissions of a diesel engine fueled with WCO methyl ester–n-pentanol blends were found to be larger than both biodiesel and diesel fuels. According to Atmanli [25], the increasing trend of CO emissions when using higher alcohols as additives might be as a result of the evaporation and ignition behaviors of fuel samples having smaller cetane number levels. Yilmaz and Atmanli [31] also concluded that CO emissions of D75B20P5, D70B20P10, and D60B20P20 were increased averagely by 6.44%, 18.93%, and 29.34%, respectively, in contrast to D80B20 fuel blend.
Smoke opacity
One of the basic problems in the CI engines is smoke opacity. Smoke is an undesirable end combustion product in the diesel engine, and it reveals the incomplete combustion process of the fuel [91]. In the present study, the measurement of smoke emissions was performed for all test fuels with the help of a smoke meter. The variations in smoke opacity for diesel fuel, safflower oil biodiesel–diesel blend, and safflower oil biodiesel–diesel–pentanol ternary blends with respect to the different engine loads are presented in Fig. 12. The smoke opacity values of all test fuels were increased slightly according to the increase in the engine load. Moreover, the smoke opacity was appeared visually lower than that of petroleum-based diesel fuel at each engine load operating condition. The average smoke opacities of diesel fuel and B20 were found to be in order of 74.38% and 73.48%.
The incomplete combustion of the hydrocarbon and carbon particles in the blends led to form smoke opacity in the exhaust [124]. Ren et al. [125] indicated that the reduction in smoke opacity is remarkably depended on the oxygen content of the fuel. Another reason for the decrement in the smoke opacity is the lower sulfur content and impurities in the biodiesel [23]. Most of the experimental results conducted by various researchers [126,127,128] highlighted the similar decreasing trend in the CI engine fueled with biodiesel–diesel fuel blends. Chauhan et al. [129], for instance, obtained lower smoke opacity results by using Jatropha methyl ester and its blends than that of diesel fuel. This is possible to be occurred better combustion in the cylinder owing to the higher cetane index and oxygen content of biodiesel. Aliyu et al. [130] examined that the smoke opacity values of croton megalocarpus methyl ester–diesel blends were found to be lower than that of baseline diesel fuel. Jeyakumar and Narayanasamy [131] found the percentage reduction in smoke emission for 20% used cooking oil methyl ester/80% diesel fuel blend by 15.5% as compared to diesel fuel. In contrast, dissimilar results were observed by Hebbal et al. [132], who investigated high smoke opacity for deccan hemp seed oil biodiesel–diesel blends because of the low flame temperature and poor mixing process in the cylinder.
The smoke opacity of ternary blends of B20P5, B20P10, B20P15, and B20P20 was measured as 72.85%, 71.03%, 69.6%, and 68.63%, respectively. It is noteworthy that it can be observed that the percentage increase in pentanol in the blends led to an even decreasing trend in the smoke opacity. As compared to diesel fuel, the reduction ratios of the smoke opacity were calculated between 1.20% and 3.40% for B20P5, between 3.19% and 6.11% for B20P10, between 4.92% 7.75% for B20P15, and between 6.25% and 8.15% for B20P20. This is possibly because of the oxygen content of the pentanol as shown in Table 8. In addition, the viscosity and density values of the ternary blends of diesel–biodiesel–pentanol are lower than of B20 fuel; thereby, they caused to enhance the smoke opacity values in contrast to B20 fuel blend. Imdadul et al. [23] added higher alcohol (pentanol) to the Calophyllum inophyllum oil biodiesel–diesel fuel blends up to the ratio of 20%; as a result, the smoke opacity was substantially reduced at level of 0.0006 < p < 0.001. Rakopoulos [133] showed that the smoke opacity decreased owing to the addition of 20% n-butanol to the cottonseed oil biodiesel. Emiroglu and Sen [134] also reported that the reduction in smoke opacity was observed when adding biodiesel and alcohols into the diesel fuel because of the lower C/H ratios of additives and higher oxygen content.
Trade-off study
First of all, it should be noted that the reduction in smoke opacity due to any alteration of a parameter caused to increase NOX emissions or vice versa. This is a frustrating problem for the diesel engine designers with the mentioned smoke opacity–NOX emission trade-off [135]. For this reason, the trade-off analysis was conducted for the smoke opacity–NOX emissions and BSFC–NOX emissions. The relationship between smoke opacity and NOX emissions is presented in Fig. 13. Interestingly, there is no trade-off between smoke opacity and NOX emissions for ternary blends as compared to B20 fuel blend under all engine load conditions owing to the fact that the pentanol addition to the biodiesel–diesel fuel blends caused to reduce NOX emission and smoke opacity values at each of the engine loads. The probable reason for this trend can be explained by the oxygen content of the pentanol because it might be led to a cooling effect in the combustion chamber. It makes sense that the NOX would decrease by decreasing engine load and using pentanol–diesel–biodiesel fuel blends because the peak temperature is decreasing. Similarly, the CO would increase due to worse oxidation, because it is an intermediate, a product of incomplete combustion; better combustion causes the CO to be continued to oxidize CO2. However, smoke oxidation would also be enhanced under these conditions. Normally, NOX increases in oxidizing environments and decreases in reducing environments (fuel-rich), and the smoke concentration shows the opposite trend. Smoke and NOX should not be showing the same trend unless there is another reason for this behavior. However, this situation may be due to low calorific value and high latent heat of vaporization of pentanol that reduces in-cylinder temperatures leading to less thermal NOX formation. The addition of pentanol with lower viscosity and high volatility could improve the atomization quality of diesel–biodiesel blends, and the higher oxygen content in pentanol could reduce soot emission [19] Alcohols, served as a fuel additive, can reduce the viscosity and surface tension, improve atomization, and provide additional oxygen content to blended fuel and thus have the potential to simultaneously reduce the emissions of both NOX and particulate matter (PM) [22]. The decrease in particle number concentration after adding pentanol to biodiesel can be explained from several perspectives. Firstly, because of the lower cetane number and lower viscosity of pentanol, there is a longer period for the fuel/air mixing, leading to a more thorough mixture. Secondly, pentanol with a single embedded oxygen atom is more effective in the reduction in soot precursors than the two oxygen atoms in biodiesel as R (C=O)O R′. Because the CH3O(CO) radical is decomposed primarily to CH3 + CO2, rather than CO + CH3O, leading to the bonding of the two oxygen atoms to a single carbon atom, these two oxygen atoms are less efficient in removing carbon atoms from the reactive medium [22]. Thirdly, the addition of pentanol can increase the concentrations of OH by the reaction of methyl radical with HO2 radical (CH3 + HO2 = CH3O + OH), which could promote the oxidation of soot precursors. Compared to diesel fuel, the higher oxygen content and the absence of aromatics in biodiesel could lead to a reduction in the total number concentration and the size of the particles. For biodiesel–pentanol blends, on the one hand, the higher oxygen content, the lower boiling, viscosity, and the longer ignition delay period lead to more fuel burned in the premixed mode and less fuel burned in the diffusion mode [136]. Hydroxyl group in the fuel and the oxygen atoms present in n-pentanol get bonded during the phase of combustion and lower the formation of soot [120, 121]. The oxygen atoms bonded to the hydroxyl group of n-pentanol reduce soot formation inhibiting smoke precursors. In addition, the low C/H ratio of n-butanol also reduces soot formation [137]. The oxygen content of pentanol contributes to improved combustion resulting in the reduction in smoke [138]. Smoke depends on the air during combustion. Since biofuels have more inherent oxygen, combustion is complete causing lesser smoke emissions [139, 140]. This is caused by surplus oxygen available in pentanol which develops the combustion. Further, the presence of oxygen atoms in pentanol gets bonded toward the hydroxyl group in the fuel and lessens the smoke emissions [116, 139]. In the literature, there are many studies where both NOX and soot emission decreased together [29, 119, 120, 133].
Although Rakopoulos et al. [135] observed similar trends by using vegetable oil and its biodiesel in blends with 10% and 20% of diethyl ether or n-butanol, Imdadul et al. [23] indicated that there was a trade-off scenario between smoke opacity and NOX emission when the diesel engine fueled with Calophyllum inophyllum biodiesel includes different proportions pentanol such as 10 vol%, 15 vol%, and 20 vol%.
As mentioned above, most of the experimental results have presented the disappointing and inconvenient cases in the smoke opacity and NOX emissions trade-off. But, there is no precise information related to the interactions between CO and HC emission whenever any change in a parameter under the constant engine load operating conditions with petroleum-based diesel fuel. Nevertheless, many studies conducted by different authors with lower or higher alcohols in the fuel blends have reported that the HC emission increases, while CO emission decreases. In this case, it should be necessary to investigate corresponding CO–HC emissions diagrams. The trade-off analysis results of CO and HC emissions for each of the test fuels at different engine loads are illustrated in Fig. 14. The idea of drawing this diagram is almost identical to Fig. 13. Obviously, both CO and HC emissions were slightly decreased considering the pentanol-added fuel samples.
The correlation between BSFC and NOX emissions for each of the test fuels at different engine loads is represented in Fig. 15. It can be pointed out that most of the fuel samples showed a contemporaneous augmentation in both BSFC and NOX emissions in contrast to the petroleum-based diesel fuel. Therewithal, the optimum engine load could find to be at 500 W and 750 W when the interaction between BSFC and NOX emission was evaluated. Although B20 fuel blend exhibited the maximum values of NOX emissions at all engine loads, the BSFC values were slightly high as compared with diesel fuel. Interestingly, the ternary blends of pentanol–biodiesel–diesel fuel showed the highest BSFC results compared to both diesel fuel and B20 fuel blend. On the other hand, modified blends of pentanol demonstrated even lower NOX emissions than that of B20 fuel thanks to increasing the alcohol percentage in the blend. Consequently, there is a trade-off scenario between BSFC and NOX emission of test fuels.
Combustion characteristics
In this section, the combustion characteristics such as in-cylinder pressure, ignition delay (ID), peak pressure, and heat release rate (HRR) of baseline diesel fuel, B20, B20P5, B20P10, B20P15, and B20P20 were investigated and discussed with the recent literature in the point of the better understanding aspects of the fuel samples.
In-cylinder pressure
One of the most important parameters is the cylinder pressure inside the combustion chamber in order to understand the combustion behavior in diesel engine [141]. The alteration in the pressure inside the cylinder with respect to the crank angle (CA) for each test fuels under four different engine loads (500 W, 750 W, 1000 W, and 1250 W) can be portrayed in Fig. 16. Reviewing the combustion characteristics of the fuels, they have shown similar trends and B20 fuel blend shows the highest in-cylinder pressure averagely as compared to the diesel fuel and other tested fuel samples. This is because of the more complete combustion as compared to the other test fuels. The biodiesel has more oxygen content in the chemical structure than the diesel fuel; therefore, the biodiesel–diesel fuel blend shows better combustion efficiency, which is similar to the results of Ashok et al. [97]. Although the pentanol has much more oxygen content, the calorific value was lower than that of biodiesel and diesel fuel. The average in-cylinder pressure of the pentanol-treated fuels was generally decreased due to the lower calorific value of the alcohol.
For clear observation, the peak pressure across the engine loads is also illustrated in Fig. 17. At the highest engine load of 1250 W, the peak pressures of D100, B20, B20P5, B20P10, B20P15, and B20P20 were found to be at 74.85 bar, 76.48 bar, 75.93 bar, 77.65 bar, 75.65 bar, and 74.94 bar, respectively. When the pressure profiles inside the cylinder were rigorously analyzed, it was indicated that the maximum in-cylinder pressures for all of the test fuels were advanced with the increase in engine load to be approximately 10–11°CA for diesel fuel, 10–12°CA for B20, 11–13°CA for B20P5, 11–12°CA for B20P10, 11–13°CA for B20P15, and 10–12°CA for B20P20 from the top dead center considering Fig. 16. This statement is similar to the results of Tse et al. [142] and Qi et al. [143]. This is due to the cetane number of the alcohol because it is lower than both diesel fuel and biodiesel. Furthermore, the start of combustion was delayed owing to the lower cetane number of pentanol. Thereby, more fuel was combusted in the phase of premixed. As a result, the higher gas pressure inside the cylinder was monitored with the ternary fuel blends. The second influential factor for the in-cylinder pressure is the latent heat of evaporation. It can be noted that the latent heat of evaporation has led to an increase in the ignition delay period. This issue was discussed in the ignition delay section. However, it is useful to say that the latent heat of evaporation of pentanol is higher than that of diesel fuel, as shown in Table 8. It can bring about declining the temperature of the cylinder and hence augment the ignition delay. It can be verified from Fig. 6. The EGT values of the alcohol-treated test fuels were lower than that of diesel fuel. Another reason was also presented by Anbarasu et al. [144], who specified that the lower density and viscosity values of alcohols have led to improve the characteristics of spray; thus, more better fuel–air mixture may be formed inside the chamber of the combustion and higher cylinder pressure was observed.
Heat release rate (HRR)
Heat release rate (HRR) which is a significant combustion indicator can be identified as the combustion phases such as like premixed combustion, rapid combustion, controlled combustion, and period after burning [97]. It also supplies an opinion concerning the total heat released with regard to the CA of the engine. In the present study, all of the experimental trials for diesel, diesel–biodiesel blend, and diesel–biodiesel–pentanol blends were realized under the injection timing of 31° before top dead center. The alteration of the net heat release rate with respect to the CA for all test fuel samples is shown in Fig. 18. Based on the HRR graphs, the heat release progressively enlarges as the CA increases after that again decreases and further augments. On the other hand, it can be noticed that after the fuel injected inside the combustion chamber, the HRR values have become negative at the beginning of the combustion process due to the cooling effect.
The maximum HRR results of the tested fuel are also presented in Fig. 19. The combustion analysis results of diesel fuel showed higher HRR as compared to the biodiesel blend fuel because of the higher calorific value of diesel and were found to be at 27.87 J deg−1 and 26.06 J deg−1 for diesel and B20, respectively, under the maximum engine load condition. Another reason for decreasing the HRR values for biodiesel was explained by Balasubramanian and Purushothaman [145]. They observed that the maximum HRR for neem oil biodiesel (43.1 J deg−1) and corn oil biodiesel (42.8 J deg−1) was lower than that of diesel fuel (51.4 J deg−1) due to the combustion characteristics associated with both biodiesel. At the same time, the average maximum HRR values of B20P5, B20P10, B20P15, and B20P20 were found to be at 19.82 J deg−1, 18.94 J deg−1, 19.54 J deg−1, and 16.10 J deg−1, respectively. In addition, it could be concluded that the biodiesel combustion begins earlier than that of conventional diesel fuel due to the higher cetane number of safflower oil biodiesel. Addition of pentanol to the biodiesel–diesel fuel blend results in a lower cetane number than that of both diesel and biodiesel. Therefore, it leads to a larger ignition delay period and therewith postpones combustion starting. Moreover, it can be inferred that the calorific values of the fuel blends were lower because of pentanol addition. Lujaji et al. [146] tested the croton oil–diesel–butanol blends and reported that the deterioration in HRR was owing to the lower cetane number of the fuel blends; thus, ignition delay was increased. Similarly, Ashok et al. [97] observed that the increments in the alcohol content in the ternary fuel blends have caused to decrease in HRR.
Ignition delay (ID)
Another important combustion parameter is the ignition delay (ID) period, and it is principally depended on the cetane number of fuels [147]. In the present work, ID was computed by the difference between injection timing of the fuel and the start of the combustion in which the heat release starts to increase during the combustion process as mentioned above. ID period of the tested fuel samples is illustrated in Fig. 20. As seen, ID was comparatively lower at higher engine loads than the lower load. This is due to the temperatures of the cylinder and residual gas. The ID values of diesel, B20, B20P5, B20P10, B20P15, and B20P20 were found to be at 14.60 deg, 5.34 deg, 8.08 deg, 10.05 deg, 10.46 deg, and 20.49 deg, respectively, under the maximum engine load condition. The ID period of the biodiesel was observed lesser than that of diesel fuel owing to the higher cetane number of biodiesel. The ID is inversely proportional to the cetane number [148]. But, addition of pentanol into the biodiesel–diesel blend has led to increase the ID gradually. It can be noted that the cetane number of pentanol is lower than both diesel fuel and biodiesel, as shown in Table 8. Therefore, pentanol addition caused to delay the SOC of the process of the combustion. In addition, the ID period was increased with the increase in alcohol proportion. However, the ID period of the fuel up to 15% pentanol concentration was lower than that of diesel fuel. The maximum ID was monitored with the high-concentration pentanol-treated fuel of B20P20. During the experimental work, the engine was not properly operated with B20P20. This is due to the lower cetane number of pentanol. The injection timing of the engine was influenced the ID of B20P20. Another reason for affecting the ID period is the latent heat of evaporation. It also retards the combustion initiation. The longer ID was reported for the ternary fuel blends of diesel–biodiesel–higher alcohol by Babu and Anand [29] and Ashok et al. [97].
Cycle-to-cycle variation results
In the present study, the coefficient of variations (COV) of CI operating with tested fuels was estimated under the four different engine loads using Eq. 13. It has been found that the use of all tested alternative fuels has not impaired engine stability and the COV values were found to be lower than 10%. Furthermore, it was found sufficient for the reproducibility of the results for 20 consecutive cycles. The use of biodiesel has reduced the cyclic variations due to the fact that biodiesel has improved the combustion efficiency due to its high cetane number and high oxygen content [149]. It could be verified when looked Table 8. Besides that, up to 15% pentanol addition has not affected the engine stability. The cetane number of pentanol is 20 which is much lower than that of diesel and biodiesel fuels. Heywood [64] stated that the COV of the engine stability has been generally affected if it exceeds 10%. Ibrahim and Bari [150] showed that when the COV exceeds 5%, the engine stability begins to deteriorate. In contrast, Gharehghani et al. [151] stated that the limit value of the COV for the IMEP was 2.5%. Ibrahim [65] tested butanol–WCO biodiesel–diesel fuel blends in a diesel engine and as a result, the COV was found to be less than 5% and stated that engine stability is not affected. Can et al. [152] found COVimep in the range of 2.15–3.06 in the tests conducted with canola oil biodiesel–diesel fuel blends. Uyumaz [153] showed that the critical threshold for COVimep was 10% and that biodiesel blended fuels undergo a more stable combustion process.
The cost analysis of safflower oil biodiesel
The cost analysis is a powerful and necessary method in order to decide the competitiveness of safflower oil biodiesel as opposed to the petroleum-based diesel fuel. It can also conclude the profitability and feasibility of biodiesel fuel. The conversion process composes of two main subsections as follows: The first is the oil extraction process from safflower seeds by appropriate technique, and the second is the production of biodiesel from this oil via transesterification method.
In this work, safflower oil was procured from a local supplier as mentioned above and the cost of oil is $1.19 L−1. One of the most significant contributors to the biodiesel fuel’s cost is the raw material. It has been calculated that the cost of feedstocks constitutes about 70–80% of the total cost of biodiesel production [154, 155]. The total cost of biodiesel is found to be at approximately $1.60 L−1 when all costs such as methanol and electricity are added. Today, the diesel price reaches $1.09 L−1 in our country. Still, the price of biodiesel fuel is higher than that of conventional diesel fuel; therefore, biodiesel fuel is tax-favored for the promotional intention in several countries [156].
The countries have mostly preferred cottonseed oil, karanja oil, rice bran oil, soybean oil, and sunflower oil for the production of biodiesel [157]. The biodiesel fuel production from safflower oil in a commercial scale has not been reported before to the best of the authors’ knowledge. The safflower oil price is the major component of the biodiesel cost. This price can be taken down thanks to the higher production rate of safflower plant by farmers or supplying larger quantities. However, the safflower oil biodiesel is a promising alternative fuel for diesel applications and competitive with neat diesel fuel if the safflower oil price can be less than $0.65 L−1. Furthermore, it should be not too hard in Turkey. Although 7.2% of the world safflower production, which was the fifth rank in the world in 2017, was satisfied with our country, there is a limited amount of consumption as a portion of food by people.
Contribution of the present study to the literature
The aim of the present study is to investigate the influence of pentanol addition into the safflower oil biodiesel–diesel fuel as an additive at various percentages of 5–20% by volume on the engine performance, exhaust emissions, and combustion characteristics under four different engine loads. All of the test fuels were performed in a single-cylinder, four-stroke, air-cooled, naturally aspirated, direct-injection diesel engine. The experimental outputs of this work were compared with other investigations related to the pentanol with the biodiesel, diesel fuel, or biodiesel–diesel fuel blends conducted in recent dates. Table 10 exhibits the comparative analysis of the results found in this study with the previous researches. When the technical literature was reviewed, pentanol has been utilized not more than 50% in the fuel blends. In addition, the earlier studies showed that the pentanol was preferred as a secondary fuel in the biodiesel or diesel fuel. Nowadays, the ternary fuel blends of pentanol–biodiesel–diesel fuel have been investigated by most of the authors. Moreover, the combustion characteristics of ternary blends have not been carried out comprehensively. As shown in Table 10, the present study and all the previous studies pointed out that the higher alcohol of pentanol-added fuels has led to decrease the NOX, CO, HC, and smoke emissions in general while increasing BSFC in the diesel engine behaviors. Moreover, the outcomes have verified that the diesel engine could be operated with pentanol-blended fuel without any major modifications. In addition, the present study is able to supply additional information for using ternary blends of biodiesel–pentanol–diesel fuel blends in the unmodified CI engines for short-term applications thanks to the experimental results. It is noteworthy that the experimental results of the present study will ensure a perspective about the appropriate ternary blend concentration to the new authors in order to obtain better engine performance and combustion characteristics, and lower exhaust emission profiles. On the other hand, the stability of biodiesel–pentanol–diesel fuel blends should be performed with the aid of convenient testing methods prior to the utilization for long-term applications. Meanwhile, there is no study considering long-term durability and material compatibility to the best of the authors’ knowledge; therefore, these studies should be included in the literature.
Conclusions
As known, the higher alcohols are encouraging alternative, renewable, and sustainable resources because of their favorable influence on the environmental and economic outputs for the purpose of utilization in the CI engines as an additives. Among the higher alcohols, pentanol having five carbons in the chemical structure attracts attention all over the world in recent years due to the higher oxygen content and higher energy content as compared to the lower alcohols. Considering this incentive in the sense, the present study was performed. In this experimental work, the engine performance, exhaust emissions, combustion characteristics of a single-cylinder, four-stroke, direct-injection diesel engine operating with conventional diesel fuel, a binary blend of safflower oil biodiesel–diesel fuel, and four different ternary blends of biodiesel–pentanol–diesel fuel were examined and the results were discussed. Particularly, increasing concentrations up to 20% (by volume), higher alcohol blends were preferred. The following findings could be summarized according to the test outcomes:
The cold flow properties of ternary blends were advanced with the addition of pentanol, while density, kinematic viscosity, heating value, and cetane number were deteriorated. Moreover, the oxygen content of test fuels was increased when the pentanol was added.
In light of the observations and test results, all test fuels were safely operated in a diesel power generator without any engine alteration.
The pentanol blends led to becoming higher BSFC and mass fuel consumption in contrast to the baseline diesel fuel; however, there was no significant change in their BTE values as the more oxygen could improve the combustion efficiency.
Although B20 fuel blend showed higher EGT than that of diesel fuel at each engine loads, an increase in pentanol concentration in the blends had a positive influence on the reduction in the EGT.
The HC and CO emissions were decreased for all test fuels where the mean largest reductions were found to be at 30.47% and 31.61%, respectively, for 20% of pentanol proportion due to the improvement of the combustion process occurring in the combustion chamber because of the high oxygen content.
Nevertheless, the CO2 emissions for both alternative fuels exhibited dissimilar inclination as compared to the diesel fuel owing to the oxygen concentration. The highest CO2 emission was observed as 13.15 vol% for B20P20 fuel blend under the maximum engine load of 1250 W, which was 38.72% and 21.87% higher than those of diesel and biodiesel fuels, respectively.
One of the most substantial results of this work is that NOX emissions were diminished for all ternary blends compared to the B20 fuel blend. B20 fuel demonstrated an increase of 15.38% on average for NOX emissions, whereas NOX emissions of B20P5, B20P10, B20P15, and B20P20 decreased on average 3.16%, 11.85%, 21.58%, and 31.44%, respectively, as compared to B20 fuel blend. This is probably due to the cooling impact of the pentanol having higher latent heat of vaporization. The cooling effect could be verified from the EGT results.
Obviously, the smoke opacities of the pentanol-blended fuels also showed reductions from 1.20 to 8.15% in comparison with the neat diesel fuel.
The peak gas pressure inside the cylinder of the tested fuels was found to be lower at the low load, but higher at the higher engine operating conditions. At the highest engine load, the peak pressures of tested fuels were observed between 74.85 and 77.65 bar.
The combustion analysis results showed that D100 (27.87 J deg−1) gave higher HRR than B20 fuel blend (26.06 J deg−1) because of the higher calorific value of diesel under the maximum engine load condition. The average maximum HRR values of B20P5, B20P10, B20P15, and B20P20 were found to be at 19.82 J deg−1, 18.94 J deg−1, 19.54 J deg−1, and 16.10 J deg−1, respectively.
The ID period of the biodiesel was observed lesser than that of diesel fuel owing to the higher cetane number of biodiesel. But, addition of pentanol into the biodiesel–diesel blend has led to increase the ID gradually.
According to the overall conclusions of this work, both safflower oil biodiesel–diesel fuel binary blend and safflower oil biodiesel–pentanol–diesel fuel ternary blends could be used in the diesel engine applications devoid of any engine modification. In addition, experimental results clearly indicated that pentanol could be a viable oxygenated additive because of possessing many significant advantages. In particular, ternary blends can be evaluated in order to reduce the exhaust emissions. On the other hand, these test fuels should be experimented further at various engine operating conditions and long-term trials in order to point out their effects on the engine performances, combustion behaviors, and environmental pollutants. In addition, a diesel engine fueled with the prepared test fuels can be evaluated in the aspects of the thermodynamic, environmental, and economic.
Abbreviations
- DF:
-
100% diesel fuel
- BD:
-
100% biodiesel
- P:
-
Pentanol
- BTE:
-
Brake thermal efficiency
- BSFC:
-
Brake-specific fuel consumption
- HC:
-
Hydrocarbon
- CO2 :
-
Carbon dioxide
- CO:
-
Carbon monoxide
- NOX :
-
Nitrogen oxides
- PM:
-
Particulate matter
- BMEP:
-
Brake mean effective pressure
- IMEP:
-
Indicated mean effective pressure
- CA (θ):
-
Crank angle
- SOC:
-
Start of combustion
- ID:
-
Ignition delay
- Pr:
-
Propanol
- B:
-
Butanol
- PAH:
-
Polycyclic aromatic hydrocarbon
- EGR:
-
Exhaust gas recirculation
- bTDC:
-
Before top dead center
- IV:
-
Iodine value
- CN:
-
Cetane number
- OS:
-
Oxidation stability
- HHV:
-
Higher heating value
- COV:
-
Coefficient of variations
- EGT:
-
Exhaust gas temperature
- HRR:
-
Heat release rate
- DU:
-
Degree of unsaturation
- LCSF:
-
Long-chain saturated factor
- FFA:
-
Free fatty acid
- SN:
-
Saponification number
- Q :
-
Energy amount
- P :
-
Cylinder pressure
- W net :
-
Net work
- V stroke :
-
Swept volume of the cylinder
- R :
-
The function of the independent variables
- w :
-
Uncertainty
- D :
-
Number of double bonds
- A i :
-
The proportion of each fatty acid
- MMi :
-
Molecular mass of each fatty acid
- \(\eta_{ }\) :
-
Kinematic viscosity
- N s :
-
Number of double bonds in the saturated fatty acids
- y :
-
Estimated value of the property
- c :
-
Blending ratio
- x :
-
Independent variables
- v :
-
Titration solution volume
- b :
-
Blank volume
- m :
-
Oil sample mass
- N :
-
Normality
- V :
-
Cylinder volume
- γ :
-
Specific heat ratio
- \(\sigma\) :
-
Standard deviation
- ϕ :
-
Respective property
References
Tamilvanan A, Balamurugan K, Vijayakumar M. Effects of nano-copper additive on performance, combustion and emission characteristics of Calophyllum inophyllum biodiesel in CI engine. J Therm Anal Calorim. 2019;136(1):317–30.
Ashok B, Nanthagopal K, Darla S, Chyuan OH, Ramesh A, Jacob A, Sahil G, Thiyagarajan S, Geo VE. Comparative assessment of hexanol and decanol as oxygenated additives with calophyllum inophyllum biodiesel. Energy. 2019;173:494–510.
Demirbas A. Biodiesel production from vegetable oils via catalytic and non-catalytic supercritical methanol transesterification methods. Prog Energy Combust Sci. 2005;31(5–6):466–87.
Atabani AE, Silitonga AS, Badruddin IA, Mahlia TMI, Masjuki HH, Mekhilef S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew Sustain Energy Rev. 2012;16:2070–93.
Xue J, Grift TE, Hansen AC. Effect of biodiesel on engine performances and emissions. Renew Sustain Energy Rev. 2011;15(2):1098–116.
Soares S, Rocha FR. Fast Spectrophotometric determination of iodine value in biodiesel and vegetable oils. J Braz Chem Soc. 2018;29(8):1701–6.
Ozsezen AN, Canakci M. Determination of performance and combustion characteristics of a diesel engine fueled with canola and waste palm oil methyl esters. Energy Convers Manag. 2011;52:108–16.
Galavi M, Romroudi M, Tavassoli A. Effect of micronutrientes foliar application on yield and seed oil content of safflower (Carthamus tinctorius). Afr J Agric Res. 2012;7:482–6.
Ayas N, Yılmaz O. A shrinking core model and empirical kinetic approaches in supercritical CO2 extraction of safflower seed oil. J Supercrit Fluids. 2014;94:81–90.
Food and Agricultural Organization of the United Nations. http://www.fao.org/home/en/. Accessed 14 May 2019.
Çelebi Y, Aydın H. Investigation of the effects of butanol addition on safflower biodiesel usage as fuel in a generator diesel engine. Fuel. 2018;222:385–93.
Ilkılıç C, Aydın S, Behcet R, Aydin H. Biodiesel from safflower oil and its application in a diesel engine. Fuel Process Technol. 2011;92(3):356–62.
Al-Samaraae RR, Atabani AE, Uguz G, Kumar G, Arpa O, Ayanoglu A, Mohammed MN, Farouk H. Perspective of safflower (Carthamus tinctorius) as a potential biodiesel feedstock in Turkey: characterization, engine performance and emissions analyses of butanol–biodiesel–diesel blends. Biofuels. 2017. https://doi.org/10.1080/17597269.2017.1398956.
Ors I, Bakircioglu V. An experimental and ANNs study of the effects of safflower oil biodiesel on engine performance and exhaust emissions in a CI engine. Int J Automot Eng Technol. 2016;5(3):125–35.
Kul BS, Kahraman A. Energy and exergy analyses of a diesel engine fuelled with biodiesel–diesel blends containing 5% bioethanol. Entropy. 2016;18(11):387.
Venu H, Raju VD, Subramani L. Combined effect of influence of nano additives, combustion chamber geometry and injection timing in a DI diesel engine fuelled with ternary (diesel–biodiesel–ethanol) blends. Energy. 2019;174:386–406.
Keskin A, Resitoglu IA, Ozcanli M. Effects of butanol-ethanol and gasoline blends on specific fuel consumption and emissions in a spark ignition engine. Çukurova Univ Eng Architect Dep J. 2009;24:147–56.
Kumar BR, Saravanan S. Use of higher alcohol biofuels in diesel engines: a review. Renew Sustain Energy Rev. 2016;60:84–115.
Li L, Wang J, Wang Z, Xiao J. Combustion and emission characteristics of diesel engine fueled with diesel/biodiesel/pentanol fuel blends. Fuel. 2015;156:211–8.
Campos-Fernández J, Amal JM, Gomez J, Lacalle N, Dorado MP. Performance tests of a diesel engine fueled with pentanol/diesel fuel blends. Fuel. 2013;107:866–72.
Yesilyurt MK. Investigating the effects of different alcohol additives in biodiesel–diesel fuel blends on performance, combustion and emission characteristics of the diesel engines. PhD Thesis, Bozok University 2017.
Zhu L, Xiao Y, Cheung CS, Guan C, Huang Z. Combustion, gaseous and particulate emission of a diesel engine fueled with n-pentanol (C5 alcohol) blended with waste cooking oil biodiesel. Appl Therm Eng. 2016;102:73–9.
Imdadul HK, Masjuki HH, Kalam MA, Zulkifli NWM, Alabdulkarem A, Rashed MM, Teoh YH, How HG. Higher alcohol-biodiesel–diesel blends: an approach for improving the performance, emission, and combustion of a light-duty diesel engine. Energy Convers Manag. 2016;111:174–85.
Imdadul HK, Masjuki HH, Kalam MA, Zulkifli NWM, Alabdulkarem A, Kamruzzaman M, Rashed MM. A comparative study of C4 and C5 alcohol treated diesel-biodiesel blends in terms of diesel engine performance and exhaust emission. Fuel. 2016;179:281–8.
Atmanli A. Comparative analyses of diesel-waste oil biodiesel and propanol, n-butanol or 1-pentanol blends in a diesel engine. Fuel. 2016;176:209–15.
Wei LJ, Cheung CS, Huang ZH. Effect of n-pentanol addition on the combustion performance and emission characteristics of a direct-injection diesel engine. Energy. 2014;70:172–80.
Nanthagopal K, Ashok B, Saravanan B, Patel D, Sudarshan B, Ramasamy RA. An assessment on the effects of 1-pentanol and 1-butanol as additives with Calophyllum Inophyllum biodiesel. Energy Convers Manag. 2018;158:70–80.
Devarajan Y, Nagappan BK, Munuswamy DB. Performance and emissions analysis on diesel engine fuelled with cashew nut shell biodiesel and pentanol blends. Korean J Chem Eng. 2017;34(4):1021–6.
Babu D, Anand R. Effect of biodiesel–diesel-n-pentanol and biodiesel–diesel-n-hexanol blends on diesel engine emission and combustion characteristics. Energy. 2017;133:761–76.
Dhanasekaran R, Krishnamoorthy V, Rana D, Saravanan S, Nagendran A, Kumar BR. A sustainable and eco-friendly fueling approach for direct-injection diesel engines using restaurant yellow grease and n-pentanol in blends with diesel fuel. Fuel. 2017;193:419–31.
Yilmaz N, Atmanli A. Experimental assessment of a diesel engine fueled with diesel-biodiesel-1-pentanol blends. Fuel. 2017;191:190–7.
Yilmaz N, Ileri E, Atmanli A. Performance of biodiesel/higher alcohols blends in a diesel engine. Int J Energy Res. 2016;40:1143–243.
Zhang ZH, Balasubramanian R. Investigation of particulate emission characteristics of a diesel engine fueled with higher alcohols/biodiesel blends. Appl Energy. 2016;163:71–80.
Imdadul HK, Masjuki HH, Kalam MA, Zulkifli NWM, Alabdulkarem A, Rashed MM, Ashraful AM. Influences of ignition improver additive on ternary (diesel-biodiesel-higher alcohol) blends thermal stability and diesel engine performance. Energy Convers Manag. 2016;123:252–64.
Chuah LF, Yusup S, Aziz ARA, Klemeš JJ, Bokhari A, Abdullah MZ. Influence of fatty acids content in non-edible oil for biodiesel properties. Clean Technol Environ Policy. 2016;18(2):473–82.
Bello EI, Agge M. Biodiesel production from ground nut oil. J Emerg Trends Eng Appl Sci. 2012;3(2):276–80.
Anand K, Sharma RP, Mehta PS. A comprehensive approach for estimating thermo-physical properties of biodiesel fuels. Appl Therm Eng. 2011;31:235–42.
Rathore V, Tyagi S, Newalkar B, Badoni RP. Jatropha and karanja oil derived DMC–biodiesel synthesis: a kinetics study. Fuel. 2015;140:597–608.
Correia LM, de Sousa Campelo N, Novaes DS, Cavalcante CL Jr, Cecilia JA, Rodríguez-Castellón E, Vieira RS. Characterization and application of dolomite as catalytic precursor for canola and sunflower oils for biodiesel production. Chem Eng J. 2015;269:35–43.
Ramos MJ, Fernández CM, Casas A, Rodríguez L, Pérez Á. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol. 2009;100(1):261–8.
Ma F, Hanna MA. Biodiesel production: a review. Bioresour Technol. 1999;70:1–15.
Zullaikah S, Lai C, Vali SR, Ju Y. A two-step acid-catalyzed process for the production of biodiesel from rice bran oil. Bioresour Technol. 2005;96:1889–96.
Helwani Z, Othman MR, Aziz N, Fernando WJN, Kim J. Technologies for production of biodiesel focusing on green catalytic techniques: a review. Fuel Process Technol. 2009;90:1502–14.
Atadashi IM, Aroua MK, Aziz AA, Sulaiman NMN. The effects of water on biodiesel production and refining technologies: a review. Renew Sustain Energy Rev. 2012;16(5):3456–70.
Meng X, Chen G, Wang Y. Biodiesel production from waste cooking oil via alkali catalyst and its engine test. Fuel Process Technol. 2008;89:851–7.
Betiku E, Okunsolawo SS, Ajala SO, Odedele OS. Performance evaluation of artificial neural network coupled with generic algorithm and response surface methodology in modeling and optimization of biodiesel production process parameters from shea tree (Vitellaria paradoxa) nut butter. Renew Energy. 2015;76:408–17.
Jeyalakshmi P, Subramanian R. The application of response surface methodology for the optimization of pretreatment process parameters of paradise seed (Simarouba Glauca) oil. Energy Sources Part A. 2013;35:2087–95.
Djibril D, Lamine DM, Mamadou F. Biodiesel production from Neem seeds (Azadirachta indica A. Juss) oil by its base-catalyzed transesterification and its blending with diesel. Res J Chem Sci. 2015;5:13–9.
Saydut A, Duz MZ, Kaya C, Kafadar AB, Hamamci C. Transesterified sesame (Sesamum indicum L.) seed oil as a biodiesel fuel. Bioresour Technol. 2008;99(14):6656–60.
Manigandan S, Gunasekar P, Devipriya J, Nithya S. Emission and injection characteristics of corn biodiesel blends in diesel engine. Fuel. 2019;235:723–35.
Onukwuli DO, Emembolu LN, Ude CN, Aliozo SO, Menkiti MC. Optimization of biodiesel production from refined cotton seed oil and its characterization. Egypt J Pet. 2017;26(1):103–10.
Verma P, Sharma MP, Dwivedi G. Evaluation and enhancement of cold flow properties of palm oil and its biodiesel. Energy Rep. 2016;2:8–13.
Jham GN, Moser BR, Shah SN, Holser RA, Dhingra OD, Vaughn SF, Berhow MA, Winkler-Moser JK, Isbell TA, Holloway RK, Walter EL, Natalino R, Anderson JC, Stelly DM. Wild Brazilian mustard (Brassica juncea L.) seed oil methyl esters as biodiesel fuel. J Am Oil Chem Soc. 2009;86(9):917–26.
Rashid U, Anwar F, Moser BR, Ashraf S. Production of sunflower oil methyl esters by optimized alkali-catalyzed methanolysis. Biomass Bioenergy. 2008;32:1202–5.
Atapour M, Kariminia HR, Moslehabadi PM. Optimization of biodiesel production by alkali-catalyzed transesterification of used frying oil. Process Saf Environ. 2014;92:179–85.
Aksoy L. Biodiesel as a alternative energy source and its production process. Electr J Veh Technol. 2010;2(3):45–52 (in Turkish).
Sánchez M, Bergamin F, Peña E, Martínez M, Aracil J. A comparative study of the production of esters from Jatropha oil using different short-chain alcohols: optimization and characterization. Fuel. 2015;143:183–8.
Meneghetti SMP, Meneghetti MR, Wolf CR, Silva EC, Lima GES, Silva LL, Serra TM, Cauduro F, Oliveira LG. Biodiesel from castor oil: a comparison of ethanolysis versus methanolysis. Energy Fuels. 2006;20:2262–5.
Rashid U, Ibrahim M, Ali S, Adil M, Hina S, Bukhari IH, Yunus R. Comparative study of the methanolysis and ethanolysis of maize oils using alkaline catalysts. Grasas Aceites. 2012;63(1):35–43.
Jeyalakshmi P. Characterization of Simarouba glauca seed oil biodiesel. J Therm Anal Calorim. 2019;136(1):267–80.
Ramírez-Verduzco LF, Rodríguez-Rodríguez JE, Jaramillo-Jacob AR. Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel. 2012;91(1):102–11.
Alagumalai A. Combustion characteristics of lemongrass (Cymbopogon flexuosus) oil in a partial premixed charge compression ignition engine. Alex Eng J. 2015;54(3):405–13.
Holman P. Experimental methods for engineers. 8th ed. New York: McGraw-Hill; 2012.
Heywood JB. Internal combustion engine fundamentals. USA: McGraw-Hill; 1988.
Ibrahim A. Performance and combustion characteristics of a diesel engine fuelled by butanol–biodiesel–diesel blends. Appl Therm Eng. 2016;103:651–9.
Kumar S, Cho JH, Park J, Moon I. Advances in diesel–alcohol blends and their effects on the performance and emissions of diesel engines. Renew Sustain Energy Rev. 2013;22:46–72.
Yilmaz N, Vigil FM. Potential use of a blend of diesel, biodiesel, alcohols and vegetable oil in compression ignition engines. Fuel. 2014;124:168–72.
Makarevičienė V, Lebedevas S, Rapalis P, Gumbyte M, Skorupskaite V, Žaglinskis J. Performance and emission characteristics of diesel fuel containing microalgae oil methyl esters. Fuel. 2014;120:233–9.
Candan F, Ciniviz M, Ors I. Effect of cetane improver addition into diesel fuel-methanol mixtures on performance and emissions at different injection pressures. Therm Sci. 2017;21(1B):555–66.
Ong HC, Masjuki HH, Mahlia TMI, Silitonga AS, Chong WT, Leong KY. Optimization of biodiesel production and engine performance from high free fatty acid Calophyllum inophyllum oil in CI diesel engine. Energy Convers Manag. 2014;81:30–40.
Puhan S, Vedaraman N, Sankaranarayanan G, Ram BVB. Performance and emission study of Mahua oil (madhuca indica oil) ethyl ester in a 4-stroke natural aspirated direct injection diesel engine. Renew Energy. 2005;30(8):1269–78.
Devan PK, Mahalakshmi NV. Study of the performance, emission and combustion characteristics of a diesel engine using poon oil-based fuels. Fuel Process Technol. 2009;90(4):513–9.
Panwar NL, Shrirame HY, Rathore NS, Jindal S, Kurchania AK. Performance evaluation of a diesel engine fueled with methyl ester of castor seed oil. Appl Therm Eng. 2010;30(2–3):245–9.
Rakopoulos CD, Antonopoulos KA, Rakopoulos DC. Experimental heat release analysis and emissions of a HSDI diesel engine fueled with ethanol-diesel fuel blends. Energy. 2007;32:1791–808.
Anand K, Sharma RP, Mehta PS. Experimental investigations on combustion, performance and emissions characteristics of neat karanji biodiesel and its methanol blend in a diesel engine. Biomass Bioenergy. 2011;35(1):533–41.
Cheung CS, Zhu L, Huang Z. Regulated and unregulated emissions from a diesel engine fueled with biodiesel and biodiesel blended with methanol. Atmos Environ. 2009;43(32):4865–72.
Yasin MH, Mamat R, Yusop AF, Aziz A, Najafi G. Comparative study on biodiesel–methanol–diesel low proportion blends operating with a diesel engine. Energy Proc. 2015;75:10–6.
Palash SM, Kalam MA, Masjuki HH, Masum BM, Fattah IR, Mofijur M. Impacts of biodiesel combustion on NOx emissions and their reduction approaches. Renew Sustain Energy Rev. 2013;23:473–90.
Cheikh K, Sary A, Khaled L, Abdelkrim L, Mohand T. Experimental assessment of performance and emissions maps for biodiesel fueled compression ignition engine. Appl Energy. 2016;161:320–9.
Anderson A, Devarajan Y, Nagappan B. Effect of injection parameters on the reduction of NOx emission in neat bio-diesel fuelled diesel engine. Energy Sources Part A. 2017;40(2):186–92.
Özçelik AE, Aydoğan H, Acaroğlu M. Determining the performance, emission and combustion properties of camelina biodiesel blends. Energy Convers Manag. 2015;96:47–57.
Mofijur M, Masjuki HH, Kalam MA, Atabani AE, Arbab MI, Cheng SF, Gouk SW. Properties and use of Moringa oleifera biodiesel and diesel fuel blends in a multi-cylinder diesel engine. Energy Convers Manag. 2014;82:169–76.
How HG, Masjuki HH, Kalam MA, Teoh YH, Chuah HG. Effect of Calophyllum Inophyllum biodiesel–diesel blends on combustion, performance, exhaust particulate matter and gaseous emissions in a multi-cylinder diesel engine. Fuel. 2018;227:154–64.
Rashed MM, Kalam MA, Masjuki HH, Mofijur M, Rasul MG, Zulkifli NWM. Performance and emission characteristics of a diesel engine fueled with palm, jatropha, and moringa oil methyl ester. Ind Crops Prod. 2016;79:70–6.
Karavalakis G, Stournas S, Bakeas E. Light vehicle regulated and unregulated emissions from different biodiesels. Sci Total Environ. 2009;407:3338–46.
Kalligeros S, Zannikos F, Stournas S, Lois E, Anastopoulos G, Teas C, Sakellaropoulos F. An investigation of using biodiesel/marine diesel blends on the performance of a stationary diesel engine. Biomass Bioenergy. 2003;24(2):141–9.
Xue Y, Zhao W, Ma P, Zhao Z, Xu G, Yang C, Chen H, Lin H, Han S. Ternary blends of biodiesel with petro-diesel and diesel from direct coal liquefaction for improving the cold flow properties of waste cooking oil biodiesel. Fuel. 2016;177:46–52.
Puhan S, Vedaraman N, Ram BVB, Sankarnarayanan G, Jeychandran K. Mahua oil (Madhuca Indica seed oil) methyl ester as biodiesel-preparation and emission characterstics. Biomass Bioenergy. 2005;28:87–93.
Sahoo PK, Das LM, Babu MKG, Naik SN. Biodiesel development from high acid value polanga seed oil and performance evaluation in a CI engine. Fuel. 2007;86:448–54.
Lapuerta M, Armas O, Ballesteros R, Fernández J. Diesel emissions from biofuels derived from Spanish potential vegetable oils. Fuel. 2005;84:773–80.
Çelik M, Ors I, Bayindirli C, Demiralp M. Experimental investigation of impact of addition of bioethanol in different biodiesels, on performance, combustion and emission characteristics. J Mech Sci Technol. 2017;31(11):5581–92.
Mahalingam A, Munuswamy DB, Devarajan Y, Radhakrishnan S. Emission and performance analysis on the effect of exhaust gas recirculation in alcohol-biodiesel aspirated research diesel engine. Environ Sci Pollut Res. 2018;25:12641–7.
Rizwanul Fattah IM, Masjuki HH, Kalam MA, Mofijur M, Abedin MJ. Effect of antioxidant on the performance and emission characteristics of a diesel engine fueled with palm biodiesel blends. Energy Convers Manag. 2014;79:265–72.
Mahalingam A, Devarajan Y, Radhakrishnan S, Vellaiyan S, Nagappan B. Emissions analysis on mahua oil biodiesel and higher alcohol blends in diesel engine. Alex Eng J. 2018;57(4):2627–31.
Sidharth, Kumar N. Performance and emission studies of ternary fuel blends of diesel, biodiesel and octanol. Energy Sources Part A. 2019. https://doi.org/10.1080/15567036.2019.1607940.
Sharon H, Shiva Ram PJ, Fernando KJ, Murali S, Muthusamy R. Fueling a stationary direct injection diesel engine with diesel-used palm oil–butanol blends—an experimental study. Energy Convers Manag. 2013;73:95–105.
Ashok B, Nanthagopal K, Saravanan B, Azad K, Patel D, Sudarshan B, Ramasamy RA. Study on isobutanol and Calophyllum inophyllum biodiesel as a partial replacement in CI engine applications. Fuel. 2019;235:984–94.
EL-Seesy AI, Hassan H, Kosaka H. Improving the performance of a diesel engine operated with jojoba biodiesel–diesel–n-butanol ternary blends. Energy Proc. 2019;156:33–7.
Devarajan Y, Munuswamy DB, Mahalingam A. Performance, combustion and emission analysis on the effect of ferrofluid on neat biodiesel. Process Saf Environ Prot. 2017;111:283–91.
Behcet R, Aydın H, Ilkilic C, Iscan B, Aydin S. Diesel engine applications for evaluation of performance and emission behavior of biodiesel from different oil stocks. Environ Prog Sustain Energy. 2015;34(3):890–6.
Özsezen AN, Çanakçi M. An investigation of the effect of methyl ester produced from waste frying oil on the performance and emissions of an IDI diesel engine. J Facul Eng Architect Gazi Univ. 2008;23(2):395–404.
Sayın C. Diesel engine emissions improvements by the use of sun flower methyl ester/diesel blends. J Therm Sci Technol. 2013;33(2):83–8.
Hulwan DB, Joshi SV. Performance, emission and combustion characteristic of a multicylinder DI diesel engine running on diesel-ethanol-biodiesel blends of high ethanol content. Appl Energy. 2011;88(12):5042–55.
Sureshkumar K, Velraj R, Ganesan R. Performance and exhaust emission characteristics of a CI engine fueled with Pongamia pinnata methyl ester (PPME) and its blends with diesel. Renew Energy. 2008;33(10):2294–302.
Randazzo ML, Sodre JR. Exhaust emissions from a diesel powered vehicle fuelled by soybean biodiesel blends (B3-B20) with ethanol as an additive (B20E2-B20E5). Fuel. 2011;90(1):98–103.
Alptekin E, Canakci M, Ozsezen AN, Turkcan A, Sanli H. Using waste animal fat based biodiesels-bioethanol-diesel fuel blends in a DI diesel engine. Fuel. 2015;157:245–54.
Akar MA. Performance and emission characteristics of compression ignition engine operating with false flax biodiesel and butanol blends. Adv Mech Eng. 2016;8(2):1–7.
De Oliveira A, De Morais AMAM, Valente OS, Sodre JR. Combustion characteristics, performance and emissions from a diesel power generator fuelled by B7-ethanol blends. Fuel Process Technol. 2015;139:67–72.
Ramesh A, Ashok B, Nanthagopal K, Pathy MR, Tambare A, Mali P, Phuke P, Patil S, Subbarao R. Influence of hexanol as additive with Calophyllum Inophyllum biodiesel for CI engine applications. Fuel. 2019;249:472–85.
Gumus M, Sayin C, Canakci M. The impact of fuel injection pressure on the exhaust emissions of a direct injection diesel engine fueled with biodiesel–diesel fuel blends. Fuel. 2012;95:486–94.
Ajav EA, Singh B, Bhattacharya TK. Experimental study of some performance parameters of a constant speed stationary diesel engine using ethanol-diesel blends as fuel. Biomass Bioenergy. 1999;17(4):357–65.
Choi CY, Reitz RD. An experimental study on the effects of oxygenated fuel blends and multiple injection strategies on DI diesel engine emissions. Fuel. 1999;78(11):1303–17.
Tuccar G, Ozgur T, Aydın K. Effect of diesel–microalgae biodiesel–butanol blends on performance and emissions of diesel engine. Fuel. 2014;132:47–52.
Dogan O. The influence of n-butanol/diesel fuel blends utilization on a small diesel engine performance and emissions. Fuel. 2011;90(7):2467–72.
Gonca G, Dobrucali E. Theoretical and experimental study on the performance of a diesel engine fueled with diesel–biodiesel blends. Renew Energy. 2016;93:658–66.
Rajesh Kumar B, Muthukkumar T, Krishnamoorthy V, Saravanan S. A comparative evaluation and optimization of performance and emission characteristics of a DI diesel engine fueled with n-propanol/diesel, n-butanol/diesel and n-pentanol/diesel blends using response surface methodology. RSC Adv. 2016;6(66):61869–90.
Atmanli A, Ileri E, Yuksel B. Experimental investigation of engine performance and exhaust emissions of a diesel engine fueled with diesel–n-butanol–vegetable oil blends. Energy Convers Manag. 2014;81:312–21.
Gonca G, Dobrucali E. The effects of engine design and operating parameters on the performance of a diesel engine fueled with diesel-biodiesel blends. J Renew Sustain Energy. 2016;8(2):025702.
Rajesh Kumar B, Saravanan S. Effect of exhaust gas recirculation (EGR) on performance and emissions of a constant speed DI diesel engine fueled with pentanol/diesel blends. Fuel. 2015;160:217–26.
Devarajan Y, Jayabal RK, Ragupathy D, Venu H. Emissions analysis on second generation biodiesel. Front Environ Sci Eng. 2016;11:1.
Devarajan Y, Munuswamy DB, Nagappan B. Emissions analysis on diesel engine fuelled with cashew nut shell biodiesel and pentanol blends. Environ Sci Pollut Res. 2017;24(14):13136–41.
Li DG, Zhen H, Xingcai L, Wu-gao Z, Guang YJ. Physico-chemical properties of ethanol-diesel blends fuel and its effect on performance and emission of diesel engines. Renew Energy. 2005;30:967–76.
Yao M, Wang H, Zheng Z, Yue Y. Experimental study of n-butanol additive and multi-injection on HD diesel engine performance and emissions. Fuel. 2010;89:2191–201.
Ong HC, Masjuki HH, Mahlia TMI, Silitonga AS, Chong WT, Yusaf T. Engine performance and emissions using Jatropha curcas, Ceiba pentandra and Calophyllum inophyllum biodiesel in a CI diesel engine. Energy. 2014;69:427–45.
Ren Y, Huang Z, Miao H, Di Y, Jiang D, Zeng K, Wang X. Combustion and emissions of a DI diesel engine fuelled with diesel-oxygenate blends. Fuel. 2008;87(12):2691–7.
Selvan T, Nagarajan G. Combustion and emission characteristics of a diesel engine fuelled with biodiesel having varying saturated fatty acid composition. Int J Green Energy. 2013;10:952–65.
Ozener O, Yüksek L, Ergenç AT, Özkan M. Effects of soybean biodiesel on a DI diesel engine performance, emission and combustion characteristics. Fuel. 2014;115:875–83.
Silitonga AS, Masjuki HH, Mahlia TMI, Ong HC, Chong WT. Experimental study on performance and exhaust emissions of a diesel engine fuelled with Ceiba pentandra biodiesel blends. Energy Convers Manag. 2013;76:828–36.
Chauhan BS, Kumar N, Cho HM. A study on the performance and emission of a diesel engine fueled with Jatropha biodiesel oil and its blends. Energy. 2012;37:616–22.
Aliyu B, Shitanda D, Walker S, Agnew B, Masheiti S, Atan R. Performance and exhaust emissions of a diesel engine fuelled with Croton megalocarpus (musine) methyl ester. Appl Therm Eng. 2011;31(1):36–41.
Jeyakumar N, Narayanasamy B. Effect of Basil antioxidant additive on the performance, combustion and emission characteristics of used cooking oil biodiesel in CI engine. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-019-08699-3.
Hebbal OD, Reddy KV, Rajagopal K. Performance characteristics of a diesel engine with deccan hemp oil. Fuel. 2006;85(14–15):2187–94.
Rakopoulos DC. Combustion and emissions of cottonseed oil and its bio-diesel in blends with either n-butanol or diethyl ether in HSDI diesel engine. Fuel. 2013;105:603–13.
Emiroglu AO, Sen M. Combustion, performance and exhaust emission characterizations of a diesel engine operating with a ternary blend (alcohol–biodiesel–diesel fuel). Appl Therm Eng. 2018;133:371–80.
Rakopoulos DC, Rakopoulos CD, Kyritsis DC. Butanol or DEE blends with either straight vegetable oil or biodiesel excluding fossil fuel: comparative effects on diesel engine combustion attributes, cyclic variability and regulated emissions trade-off. Energy. 2016;115:314–25.
Yang K, Wei L, Cheung CS, Tang C, Huang Z. The effect of pentanol addition on the particulate emission characteristics of a biodiesel operated diesel engine. Fuel. 2017;209:132–40.
Siwale L, Kristóf L, Adam T, Bereczky A, Mbarawa M, Penninger A, Kolesnikov A. Combustion and emission characteristics of n-butanol/diesel fuel blend in a turbo-charged compression ignition engine. Fuel. 2013;107:409–18.
Tsolakis A, Megaritis A, Wyszynski M, Theinnoi K. Engine performance and emissions of a diesel engine operating on diesel-RME (rapeseed methyl ester) blends with EGR (exhaust gas recirculation). Energy. 2007;32(11):2072–80.
Yuvarajan D, Venkata Ramanan M. Experimental analysis on neat mustard oil methyl ester subjected to ultrasonication and microwave irradiation in four stroke single cylinder Diesel engine. J Mech Sci Technol. 2016;30(1):437–46.
Choi B, Jiang X. Individual hydrocarbons and particulate matter emission from a turbocharged CRDI diesel engine fueled with n-butanol/diesel blends. Fuel. 2015;154:188–95.
Ashok B, Jeevanantham AK, Nanthagopal K, Saravanan B, Kumar MS, Johny A, Mohan A, Kaisan MU, Abubakar S. An experimental analysis on the effect of n-pentanol-Calophyllum Inophyllum Biodiesel binary blends in CI engine characteristcis. Energy. 2019;173:290–305.
Tse H, Leung CW, Cheung CS. Investigation on the combustion characteristics and particulate emissions from a diesel engine fueled with diesel-biodiesel-ethanol blends. Energy. 2015;83:343–50.
Qi DH, Chen H, Geng LM, Bian YZ, Ren XC. Performance and combustion characteristics of biodiesel–diesel–methanol blend fuelled engine. Appl Energy. 2010;87(5):1679–86.
Anbarasu A, Saravanan M, Loganathan M. The effect of ethanol addition in a biodiesel operated DI diesel engine on combustion, performance, and emission characteristics. Int J Green Energy. 2013;10(1):90–102.
Balasubramanian K, Purushothaman K. Effect of acetylene addition on performance, emission and combustion characteristics of neem biodiesel and corn biodiesel-fueled CI engine. J Therm Anal Calorim. 2019;138(2):1405–14.
Lujaji F, Kristóf L, Bereczky A, Mbarawa M. Experimental investigation of fuel properties, engine performance, combustion and emissions of blends containing croton oil, butanol, and diesel on a CI engine. Fuel. 2011;90(2):505–10.
Madhankumar S, Stanley MJ, Thiyagarajan S, Geo VE, Karthickeyan V, Chen Z. Effect of oxygen enrichment on CI engine behavior fueled with vegetable oil: an experimental study. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-019-09179-4.
Murugapoopathi S, Vasudevan D. Performance, combustion and emission characteristics on VCR multi-fuel engine running on methyl esters of rubber seed oil. J Therm Anal Calorim. 2019;138(2):1329–43.
Gharehghani A, Mirsalim M, Hosseini R. Effects of waste fish oil biodiesel on diesel engine combustion characteristics and emission. Renew Energy. 2017;101:930–6.
Ibrahim A, Bari S. An experimental investigation on the use of EGR in a supercharged natural gas SI engine. Fuel. 2010;89(7):1721–30.
Gharehghani A, Hosseini R, Mirsalim M, Yusaf TF. A computational study of operating range extension in a natural gas SI engine with the use of hydrogen. Int J Hydrogen Energy. 2015;40(17):5966–75.
Can Ö, Öztürk E, Yücesu HS. Combustion and exhaust emissions of canola biodiesel blends in a single cylinder DI diesel engine. Renew Energy. 2017;109:73–82.
Uyumaz A. Combustion, performance and emission characteristics of a DI diesel engine fueled with mustard oil biodiesel fuel blends at different engine loads. Fuel. 2018;212:256–67.
Praveena V, Martin MLJ, Geo VE. Experimental characterization of CI engine performance, combustion and emission parameters using various metal oxide nanoemulsion of grapeseed oil methyl ester. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-019-08722-7.
Mahmudul HM, Hagos FY, Mamat R, Adam AA, Ishak WFW, Alenezi R. Production, characterization and performance of biodiesel as an alternative fuel in diesel engines—a review. Renew Sustain Energy Rev. 2017;72:497–509.
Fahd MEA, Lee PS, Chou SK, Wenming Y, Yap C. Experimental study and empirical correlation development of fuel properties of waste cooking palm biodiesel and its diesel blends at elevated temperatures. Renew Energy. 2014;68:282–8.
Siluvaimuthu S, Thiyagarajan S, Martin LJ, Nagalingam B. Comparative analysis of premixed combustion and blending of alcohols with neem and wintergreen oil biofuel blends in CI engine. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-019-08956-5.
Yilmaz N, Atmanli A. Experimental evaluation of a diesel engine running on the blends of diesel and pentanol as a next generation higher alcohol. Fuel. 2017;210:75–82.
Ramakrishnan P, Kasimani R, Peer MS, Rajamohan S. Assessment of n-pentanol/Calophyllum inophyllum/diesel blends on the performance, emission, and combustion characteristics of a constant-speed variable compression ratio direct injection diesel engine. Environ Sci Pollut Res. 2018;25(14):13731–44.
Acknowledgements
The author would like to pay special thankfulness, warmth, and appreciation to Assoc. Professor M. ARSLAN, Head of Thermodynamic Division, Mechanical Engineering, Yozgat Bozok University, Turkey, for support, technical suggestions, and continuous motivation during the course of this work.
Funding
This study was supported by Scientific Research Projects Unit of Yozgat Bozok University, Yozgat, Turkey, for financial support under the contact numbers of projects: 6602b-MÜH/19-274.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yesilyurt, M.K., Yilbasi, Z. & Aydin, M. The performance, emissions, and combustion characteristics of an unmodified diesel engine running on the ternary blends of pentanol/safflower oil biodiesel/diesel fuel. J Therm Anal Calorim 140, 2903–2942 (2020). https://doi.org/10.1007/s10973-020-09376-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09376-6