Abstract
Many studies have been done on the stability, thermo-mechanical degradation and pyrolysis of polymers with hydrocarbon skeleton. According to the main structure, side groups, with and without double bond in the structure, polymer has different responses in reference to the thermo-mechanical and thermal degradations. The polymers in extruder are faced with thermo-mechanical degradation while with appropriate stability against thermo-mechanical degradation, the shelf time of the final products increases, and the polymeric wastes are reduced. On the other hand, the structural and process parameters can significantly affect the resulting pyrolysis products as suitable process to reduce the non-recyclable polymers. Also the literature review in this field containing reactor and TG studies shows that the chemical bonds and the related degradation mechanisms can affect the quality and quantity of the pyrolytic products obviously. For this purpose, the effects of different molecular specifications, additives and related effective parameters on the thermal stability and the thermo-mechanical degradation of plastics are considered. Meanwhile, the mechanisms of degradation, the share of each mechanism, the related products under different structural and process parameters and the needed activation energy for all of the studied polymers are investigated to reduce the polymeric wastes sent to landfills.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Decades have passed since the commodity polymers identification as one of the most usable resources for the different industries, and before that, with a very limited knowledge of their structure and properties, they had restricted applications. Since 1956, Ziegler–Natta catalysts have been used in the commercial manufacture of various polyolefins and synthetic rubbers and the rate of polymer production has accelerated obviously [1,2,3]. With identifying varied properties, the polymers with different structures and grades were introduced to the commercial markets in the short time [4]. Each of these polymers has been widely used in the different industries according to the structure, molecular mass and molecular mass distribution, the used mineral and organic additives and fillers and in many cases alloys with the other polymers [5, 6]. With easy molding and low cost, polymers are replacing stones, glass, metal, wood, natural textiles, etc. [7]. Perhaps, if the commercial polymers were not introduced to the world, the electric power industry and the other industries without polymeric insulators, did not find the home appliances.
On the other hand, the commodity polymers and highly consumed rubbers containing polyethylene, polypropylene, polystyrene, polyvinyl chloride, natural rubber, styrene butadiene rubber, polybutadiene rubber, etc., have a hydrocarbon skeleton. The hydrocarbon skeleton with no side groups (polyethylene), methyl group (polypropylene), phenyl group (polystyrene), chlorine group (PVC), double bond (polybutadiene rubber), methyl group and double bond (natural rubber) and the random copolymer of styrene and butadiene as styrene butadiene rubber are more than 90% of the world polymer market that may be due to their production dependence on oil and natural gas [8].
With understanding of polymer structure, related degradation mechanisms, effective process parameters and suitable stability against the degradation during extrusion and molding, we can increase the shelf time of final products [9,10,11]. The polymeric products with low shelf time are shortly discharged into landfills and constitute a serious threat to the environment. On the other hand, the widespread and increasing applications of polymers in various industries have led to the generation of polymeric wastes as significant part of the landfills. While the organic wastes decompose and disintegrate in the landfills and environment in short time, polymeric wastes have remained in the environment for many decades as one of the sources of greenhouse gases will intensify their effects in the coming decades and threaten the environment heavily [12].
In this regard, the most important solution is the effective methods for recycling and reducing the disposal of polymeric wastes in the landfills. Pyrolysis is one of the best methods for disposing the polymeric wastes and rubbers and producing the valuable products. Pyrolysis is generally defined as the breakdown of polymer chains, converting to low molecular mass hydrocarbons under thermal degradation in the absence of oxygen [13,14,15]. The catalytic pyrolysis process can lead to the controlled scission of polymer chains, narrowing the molecular mass distribution of products and also affecting the share and type of products [16,17,18].
The pyrolysis of polymeric wastes has the acceptable potential to achieve economic efficiency, and in order to increase the efficiency of production and get the valuable products, it is necessary to better understand the polymers structure and their responses to the different process parameters [19].
The studies show that many process parameters such as type of reactor [20], temperature [21], mixing type [19], heating rate [22], type and amount of catalyst [23], carrier gas [24], etc., are effective in the pyrolysis, while the influence level of the said parameters depends directly on the polymer structure. The polymers with the double bonds in the main structure such as rubbers [25, 26] or the produced double bonds during the thermal degradation such as PVC [27] tend to follow the cross-linking degradation mechanism, and the cyclic products increase significantly. On the other hand, the polymers with saturated structure have a high tendency to follow the linear scission and/or un-zipping mechanisms [28, 29]. Without phenyl group in the saturated structure, the pyrolysis tends to produce the non-cyclic products though the process parameters can affect the pyrolysis products significantly [28]. In general, the process parameters in the related suitable trend can accelerate the secondary reactions such as Diels–Alder reaction as cyclization process and increase the cyclic products [28].
The catalysts have a significant effect on the type and size of products, depending on their acidity and porosity [26]. The higher pore sizes produce the pyrolytic products with high molecular mass hydrocarbons and low coke [26], while more acidity [30] tends to produce the aromatic compounds though the polymers with different structures give different responses to the catalysts.
As novelty, in this paper by reviewing the related studies on the polymers with hydrocarbon structure, the effects of process and structural parameters are considered on the extruder degradation and pyrolysis. For this purpose, the effective degradation mechanisms and pyrolytic products under different process and structural parameters have been studied. By evaluating the effective process parameters and degradation mechanisms, it is possible to minimize the producing cost of the valuable products from these polymers in a mixture and/or separately and found a suitable design to do pyrolysis more economically.
The thermal study of different polymers
Figure 1 shows the schematic structure of commodity polymers and highly consumed rubbers with hydrocarbon structure. In these structures, tacticity and isomerization play an important role that has not been studied. In the following, the degradation of these polymers is investigated.
Polyethylene
Polyethylene with a simple structure is known as the most widely used polymer with many applications [31]. Generally in polymers, the first degradation occurs under thermal and shear stress in the extruder and the polymer degradation is increased with the number of extrusion [32]. The degradation mechanism of polyethylene in the extruder is somewhat different from the other polyolefins [33]. Almost most polyolefins follow chain scission as the main mechanism of thermo-mechanical degradation [33, 34], while the degradation of polyethylene is slightly different [33]. Table 1 shows the effect of the amount of multiple extrusions on the molecular mass and the polydispersity index (PDI). Under multiple extrusions, the increase in molecular mass (150,000–148,000 g mol−1) and PDI (2.16–20.14) of polyethylene indicate the formation of high molecular mass hydrocarbons and the activation of the cross-linking mechanism, while under multiple extrusions of polypropylene, the molecular mass (418,400–140,650 g mol−1) and PDI (5.34–3.06) decrease as function of chain scission mechanism [35, 36].
In many cases, after polyethylene processing in the extruder, the molecular mass (103,000–121,000 g mol−1) and molecular weight distribution (5.9–13.2) increase under cross-linking mechanism [35, 36]. In total, the cross-linking over chain scission in polyethylene thermo-mechanical degradation depends on the molecular mass, molecular mass distribution, antioxidant content, etc. [36]. Higher molecular mass and temperature, narrower molecular mass distribution, lower antioxidant content and inappropriate design of the extruder increase the cross-linking over chain scission ratio in the degradation [36]. Gel points as direct result of cross-linking mechanism act as stress concentration points, affect the physical–mechanical properties of the final products and reduce the shelf time especially in film products [35]. The use of additives such as antioxidants (Irganox 1010 and Irgafos 168) as well as increasing the process-ability of polyethylene is effective in reducing the creation of gel points in the final product [35]. In general, the parameters that reduce the process-ability of polyethylene melt in the extruder are very effective in creating hot spots and increasing the cross-linking rate [36]. Increasing the shelf time of polymer products is the first way to reduce the polymeric wastes sent to the environment and landfills.
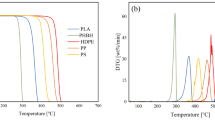
The TG study of different commercial polyethylenes shows that the structure of polyethylene and tertiary carbons content can affect the degradation significantly (Fig. 2). Low density polyethylene (LDPE) with higher content of tertiary carbons has lower thermal stability and degrades at lower temperatures, while linear low density polyethylene (LLDPE) with lower tertiary carbons and high density polyethylene (HDPE) with the lowest have the better thermal stabilities and degrade at high temperatures [37, 38]. On the other hand, LLDPE with 1-hexene co-monomer exhibits a higher thermal resistance than that with 1-butene co-monomer because the samples with 1-hexene co-monomer have about half co-monomer and tertiary carbons compared to LLDPE with 1-butene content [39, 40]. The consideration of HDPE degradation using TG instrument shows that flow rate ratio as function of molecular mass distribution and density as function of co-monomer content and/or tertiary carbons are effective in the degradation. The HDPE grades with higher density and/or lower flow rate ratio are degraded at higher temperatures and require more activation energy for degradation (276–252 kJ mol−1) [38].
The TG curves for the polyethylene samples in N2 atmosphere at heating rate of 30 K min−1 [37]
Green [41] studied the effect of temperature on polyethylene pyrolysis products from 400 to 1000 °C (Fig. 3). The thermal pyrolysis of polyethylene tends to produce wax at the degradation temperature less than 600 °C due to the specific and almost identical structure compared to other polyolefins. With temperature increasing, the high molecular mass hydrocarbons are reduced and the aromatics, low molecular mass hydrocarbons and gas contents are increased in the products. Also as the temperature rises, Diels–Alders reactions become more active and poly-aromatics and coke content increase. At temperature of around 800 °C, ethylene is about 40% and aromatics about 20% of the products. Certainly, the heating rate can affect the products and change the composition of products at different degradation temperatures [41].
Generated individual and the total yields of polyethylene pyrolysis versus temperature [41]
At low degradation temperatures, the polyethylene pyrolysis usually follows the chain scission mechanism and produces high molecular mass hydrocarbons such as wax, while with increasing temperature, the secondary reactions such as Diels–Alder become more active in the production of cyclic compounds. Also un-zipping shows a good potential in polyethylene degradation with ethylene production at high temperatures (> 800 °C). The products of polyethylene pyrolysis at about 800 °C containing more than 75% C1–C4 can provide a very suitable feed for the olefin plants of polyethylene and polypropylene petrochemical plants [41, 42].
In the degradation of polyethylenes, the produced liquid usually show an optimum with the increasing temperature. In the case of suitable heat and mass transfers and proper mixing, 450 °C is the optimum temperature, but generally, at temperatures between 400 and 500 °C, liquid production is higher than other conditions (with the yield up to 95%). At temperatures above 500 °C, gas and coke increase with increasing temperature [19, 24, 43].
Catalysts can play an important role in the polymer pyrolysis. In addition to reducing activation energy, the catalysts reduce the needed degradation temperature. Meanwhile, they influence the type and size of the products [44, 45]. Zeolite-based catalysts have been widely used in the pyrolysis of polyolefins because of their effective role in degradation [46]. On the other hand, two parameters of porosity [47] and acidity [48] of zeolite catalysts have significant effects on the final product. The catalysts with smaller pore sizes produce the lower mass hydrocarbons [49], and on the other hand, the catalysts with higher acidities and/or the lower ratios of Si/Al tend to follow the Diels–Alder reactions and the aromatic production increases obviously [48]. On the other hand, the type of catalyst and the size [49] and structure [46] of the pore channels are also effective in the production of aromatic acids. The use of metal-impregnated zeolites is also very effective in the production of aromatic compounds, and the zeolite catalysts promoted with different metals such as gallium and iron as aromatization catalysts are highly effective in the production of aromatics (Fig. 4) [50]. If liquid fuel is the main goal of pyrolysis of polyethylene, the produced wax will become the lower molecular mass hydrocarbons using various catalysts, especially HZSM-5 at high temperatures and pressures [48].
Hydrocarbon composition of oil produced from pyrolysis of HDPE, over Y-zeolite catalyst and with 1% metal-Y-zeolite catalysts [50]
Table 2 shows that carrier gas is another important parameter in the polyethylene pyrolysis. The use of hydrogen as a carrier gas decreases the cyclic compounds and olefins and increases paraffinic product as saturated products significantly. On the other hand, the use of other hydrocarbon gases such as ethylene and propylene as carrier gases also reduces the produced gases. This is because the reactive gases can shift the equilibrium to generate a greater liquid yield [19, 24, 43, 51].
The suitable heat and mass transfer and narrower temperature profile in the reactor produce the condensed products with narrower molecular mass distribution and lower coke and gas. Meanwhile, the type of reactor with attention to heat and mass transfer, geometry and temperature profile is also effective in the size and type of productions [52].
In general, the obvious differences between the pyrolysis results obtained from different papers on a polymer such as polyethylene depend on the polyethylene grade [38], polymer shape and size [53], ratio of the polymer mass over reactor volume [26], reactor type [52], mixing and heating rate [43], degradation temperature, carrier gas, condensers and their cooling power, the accuracy of gas chromatography–mass spectrometry (GC/MS) instrument, etc. [43,44,45]. Figure 5 shows the effect of structural and process parameters on the stability, thermo-mechanical and pyrolysis of polyethylene.
Polypropylene
Polypropylene is the most widely used polymer after polyethylene and has many applications in various industries [31]. Unlike polyethylene, which is commercially categorized according to density, polypropylene is classified into two types as homo-polymer and copolymer with a few percent of ethylene to improve the polymer impact compared to the homo type [54]. In general, the degradation of polypropylene during the extruding follows the chain scission mechanism and the molecular mass of the final product decreases (Table 1) [55]. In general, antioxidants and some additives such as metal stearates are used to reduce the degradation of polypropylene during the thermo-mechanical degradation [56]. Antioxidants significantly inhibit the loss of molecular mass in the extruder and generally improve the thermal resistance of polypropylene, and as the amount of antioxidant increases from 0 to 0.6%, the processing degradation index decreases from about 160 to 10 [55, 56]. On the other hand, the TG study indicates that antioxidants protect the polymer against thermal degradation and in the presence of antioxidants, the polymer is degraded at higher temperatures [57]. The degradation of polypropylene in extruder reduces the impact resistance and shelf time of the final products. In polypropylenes, the impact resistance depends directly on the molecular mass [55, 56, 58].
Having an additional methyl group in the structure of propylene monomer relative to ethylene is the main difference of polypropylene with polyethylene. The TG study shows that due to the presence of tertiary carbons in the polypropylene structure, which is much more than that in polyethylene, this polymer has a lower thermal resistance than polyethylenes and is degraded at lower temperatures [38, 59]. Tertiary carbons in polyolefins have a very significant effect on degradation at high temperatures [38]. Depending on the type of polypropylene grade, homo- or copolymer, additives, molecular mass and other parameters, the activation energy is about 178–195 kJ mol−1 [38].
Ethylene propylene diene monomer (EPDM) as olefinic rubber has lower thermal stability in comparison with polypropylene and polyethylene at ambient temperature, but the TG results indicate that propylene content controls the EPDM degradation and the samples with higher propylene contents degrade at lower temperatures (Fig. 6). As remarkable result, all of the EPDM samples degrade between polypropylene and polyethylene degradation curves [60].
Curves of the degradation of different EPDM samples, heating rate 2 °C min−1 [60] (the number index is ethylene content in different EPDM samples)
The results of polypropylene pyrolysis show that the mechanism of chain scission is the dominant mechanism in the degradation of this polymer, while as the temperature rises, the secondary reactions such as Diels–Alder reactions became more active and aromatics increase in the products [28, 61].
Based on the TG studies, the polypropylene wastes with high yellowness index as function of double bond and degradation have lower thermal stability and degrade at lower temperatures in comparison with virgin polypropylene [62]. While in the case of high density polyethylene, the waste sample with high yellowness index begins to degrade at lower temperatures compared to virgin polyethylenes but the final reminded of waste polyethylene is degraded at higher temperatures and shows the broader DTG curve in comparison with the virgin polyethylene [63].
Heavy hydrocarbons as the main product of polypropylene pyrolysis at low degradation temperatures (less than 500 °C) have a relatively high viscosity, although it is obviously less than the wax viscosity produced from the polyethylene degradation [28]. Meanwhile, in most cases, polypropylene thermal degradation produces less aromatics compared to polyethylenes and usually LDPE produces significantly more aromatics in comparable conditions than HDPE and polypropylene [24, 28, 43, 64].
Temperature as key process parameter has a great influence on the polypropylene pyrolysis [65]. Typically, the produced liquid like polyethylenes shows a peak with increasing temperature, usually between 400 and 500 °C [28]. On the other hand, at the higher temperatures resulting in the greater number of scissions, the thermal degradation of the polypropylene leads to the production of light hydrocarbons, aromatics and gases [28, 66].
The catalysts especially zeolites take part in the pyrolysis of polypropylene and decrease the needed temperature and activation energy [67] to obtain the valuable products obviously [68]. The zeolite catalysts with larger pores [68] and more acidity [17] play effective role in the pyrolysis of polyolefins.
In general, the produced liquid using zeolite catalysts has lighter color and less viscosity in comparison with thermal degradation product [28]. Based on the consumed catalyst and its porosity and acidity, the catalytic degradation of polypropylene produces the products with different sizes and types but in the specific and narrow range [68, 69]. The use of effective catalysts such as zeolites can produce significant amounts of valuable products in the range of gasoline or other commercial fuels [28, 68]. FCC catalysts are suitable for producing liquids in the gasoline range of polypropylene pyrolysis. A few heavy metals on the catalyst surface of used FCC catalysts—with suitable regeneration—can contribute significantly to the polyolefin pyrolysis [24]. The fresh and used forms of FCC catalysts play a very effective role in pyrolysis of polyolefins and, under suitable conditions, can produce up to 90% of liquid in the gasoline range [28].
Rotary kiln reactors with suitable stability against needed high temperatures, suitable heat and mass transfer have acceptable potential to be used as commercial reactor especially in polyolefin pyrolysis [70].
In total, many process parameters affect the products and trend of polypropylene degradation such as temperature, heating rate, reactor and carrier gas.[28, 43]. Figure 7 shows the effect of structural and process parameters on the stability, thermo-mechanical and pyrolysis of polypropylene.
Polystyrene
Polystyrene as one of the commodity polymers is used in the fields of building and construction, foam, packaging, etc. The commercial types of polystyrene are general purpose polystyrene (GPPS), expanded polystyrene (EPS) and high impact polystyrene (HIPS). EPS is used as foam, and pentane is the blowing agent, while HIPS is the copolymer of polystyrene and butadiene rubber with improved toughness in comparison with polystyrene [71].
Polystyrene is polymerized from styrene monomer, and due to the presence of tertiary carbon connected to a phenyl group in the structure, it has the lower stability compared to polyethylene and polypropylene [72]. Polystyrene is sensitive to shear stress in the extruder, and with increasing extruder speed, the thermo-mechanical degradation will be significantly increased and the molecular mass decreases in the final product [73]. During extrusion, the thermo-mechanical degradation of polystyrene produces a small amount of very low molecular mass hydrocarbons such as monomer and dimer [74]. The degradation of polystyrene resulting in the production of monomer and dimer, especially in food applications, can be very dangerous for human health [75]. Generally, the thermo-mechanical degradation of polystyrene in the extruder follows the chain scission and un-zipping mechanisms [76]. Using the extruder with suitable geometry and low shear stress, the appropriate amount of antioxidants and metal stearates will significantly reduce the degradation of polystyrene [77].
In general, the thermal resistance of a polymer depends on the chemical bond and the polymer with weaker bonds has lower thermal resistance. In the saturated hydrocarbon polymers, tertiary carbon is a weakness and the bulky groups such as phenyl connected to the tertiary carbon will significantly reduce the thermal resistance and requires less energy to degrade compared to polyethylene and polypropylene. As measured by TG, polystyrene is degraded at lower temperatures, in a narrower temperature range, and has a lower activation energy compared to polyethylene and polypropylene [72]. Also, as measured by TG, the activation energy of polystyrene is calculated from 50 to 80 kJ mol−1 according to different calculation methods which is significantly lower than that of polyethylene and polypropylene [78].
Due to the specific structure of polystyrene and the presence of phenyl group in the structure, the aromatic products exceed 90% and are sometimes up to 99% of the pyrolysis products [79, 80]. Thermal degradation of polystyrene has a tendency to produce styrene and follows the zipping mechanism [80]. With decreasing the degradation temperature, the styrene production of polystyrene pyrolysis is significantly increased and in some cases, up to 85% of styrene can be produced [80].
The catalytic pyrolysis of polystyrene shows that by using zeolite catalysts such as FCC [81] and HZSM-5 [82], the role of the un-zipping mechanism in the degradation is diminished and the produced styrene is decreased obviously, while with some catalysts such as BaO, styrene is increased obviously [81]. On the other hand, high percentage of styrene monomer in the product of thermal and catalytic pyrolysis of polystyrene has a good potential for styrene re-polymerization and polystyrene production [81]. Figure 8 shows the effect of structural and process parameters on the stability, thermo-mechanical and pyrolysis of polystyrene.
PVC
Polyvinyl chloride as one of the most widely used polymers is used in different applications such as pipes, films, electrical insulation cables, profiles and sheets. In the synthetic polymers, polyvinyl chloride is the most produced plastic after polyethylene and polypropylene [31]. PVC is available in rigid and flexible form with DOP plasticizer [82]. PVC has mainly an atactic stereochemistry, which means that the relative stereochemistry of the chloride centers is random. Some degree of syndiotacticity of the chain gives a few percent crystallinity that is influential on the properties of the material [83]. Chlorine accounts for about 57% of PVC mass, and almost all of PVC’s special properties are due to chlorine [84]. PVC like polyolefins has a hydrocarbon skeleton, but the presence of chlorine in the structure makes this polymer quite different from the polyolefins in terms of physical and mechanical properties [84, 85]. This polymer is highly sensitive to thermo-mechanical degradation, and therefore, the extruders designed for PVC have the least shear stresses, and the twin-screw PVC extruders in contrast to polyolefins are counter-rotating [86]. The degradation of PVC during extrusion is very important for this polymer compared to polyolefins, and additives such as heat stabilizers, metal stearate, antioxidants have a great impact on the properties of the final product. Hydrochloric acid is the hazardous byproduct of thermo-mechanical degradation of PVC in the extruder, and HCl release can have a lot of harm to humans and the environment, so special equipment to prevent HCl emission is considered in PVC extruders [87].
In the thermal degradation of PVC in the reactor, many process parameters such as temperature [88, 89], heating rate [88, 89] and catalyst [90, 91] are effective and HCl as toxic and dangerous substance is the main pyrolysis product [88,89,90,91]. PVC loses more of the mass as HCl in the temperatures less than 300 °C [89], and depending on the atmosphere such as oxygen, air, nitrogen and steam, the degree of degradation is different [92]. The release of HCl results in the formation of radicals and double bonds in the polymer structure, and the degradation mechanism after the formation of new bonds is significantly different [88]. Figure 9 shows the degradation trend of chlorinated polymers such as PVC and PVDC compared to polyolefins. The results show that the degradation mechanism of chlorinated polymers such as PVC is completely different from polyolefins. The degradation of polyvinyl chloride has about three steps with different mechanisms. In the first step, the thermal stability is very low and released HCl changes the polymer structure to a poly-aromatic polymer [88, 93, 94]. At the same time with the release of HCl, the formation of radicals and double bonds creates a convenient space for creating 2D cross-linking networks and the residual polymer, which has lost almost all of the chlorines, becomes a poly-aromatic network. At the next step, cross-linked networks have high thermal resistance and strongly resist degradation and do not lose mass at the related temperature interval—about 50–100 °C depending on the heating rate [88]. The created two-dimensional networks are so strong that some of them resist the rise in temperature and proceed to the production of non-degradable coke, and the weak part of these two-dimensional networks is degraded at this stage and produces the aromatics especially benzene [88]. In most cases, degradation of polyvinyl chloride has produced about 10–20% of coke, which is a high percentage for the studied polymers [88, 93, 94]. Also, for three degradation stages of PVC, the activation energy is equal to 163.0, 107.5 and 113.5 kJ mol−1, respectively [95]. The aromatics due to cross-linking and chain stripping degradation mechanisms beside HCl are the products of PVC pyrolysis [88]. Figure 10 shows the effect of structural and process parameters on the stability, thermo-mechanical and pyrolysis of polyvinyl chloride.
TG analysis of PVDC, PVC, PP, PE and PS samples [92]
Natural rubber
Natural rubber is used as the most usable rubber in various industries, including tire production and all kinds of industries that need flexibility. This rubber is produced from the latex sap of Hevea tree and is supplied after several stages of purification and various mechanical and mastication processes under special commercial grades [96]. Vulcanization of natural rubber creates cross-link bonds between chains, which limits the degrees of freedom and results in chains that tighten more quickly for a given strain, thereby increasing the elastic force constant and making the rubber harder and less extensible [97].
The non-recyclable used tires are the unsolved problem in most countries and as a huge hydrocarbon source can be a serious threat to the environment. Very few used tires are being recycled under different processes such as ultrasonic de-vulcanization, the use of tire powder in bitumen, etc. Pyrolysis can be an economical way to convert used tires into hydrocarbon compounds that have been studied by researchers in recent years. In this way, the wire and carbon black are separated and the rubber is converted to oil liquids [98, 99].
The effective degradation mechanisms of natural rubber pyrolysis significantly depend on the process parameters such as temperature, heating rate, catalyst and initial cross-linking. [99, 100].
Temperature as the most important pyrolysis parameter plays an important role in the degradation of natural rubber. By increasing the temperature, cross-linking mechanism and Diels–Alder reactions affect highly the degradation due to the presence of double bond in the polymer structure and aromatics increase with temperature, while the share of chain scission and un-zipping mechanisms in the degradation decreases with temperature. On the other hand, in natural rubber pyrolysis, the heating rate has a significant effect on the degradation mechanisms and final products compared to polyolefins. With increasing heating rate, the ratio of chain scission over cross-linking and un-zipping increases and more products tend to be linear [100,101,102].
On the other hand, the zeolite-based catalysts are very effective in the pyrolysis of natural rubber and depending on the pore size of catalyst, the products have narrow molecular mass distribution in a specific range. FCC catalysts produce liquids in the gasoline range, while HZSM-5 leads to further gas production. With the gallium FCC catalyst, the most aromatic content is produced in comparison with FCC and HZSM-5 catalysts. The initial artificial cross-linking has important role in the degradation and can affect the products obviously. The initial cross-linking can be a suitable area for cross-linking mechanism, and the aromatics and coke content increases with initial cross-linking increasing. The produced liquid decreases with initial cross-linking, while the residence time of the products in the reactor increases obviously [100]. Figure 11 shows the effect of structural and process parameters on the pyrolysis of natural rubber.
Polybutadiene rubber
Polybutadiene is a widely used synthetic rubber for about a quarter of total global consumption of synthetic rubbers, which has almost the same structure as polyethylene and differs in the presence of a double bond in all four carbons. Polybutadiene rubber like the other unsaturated rubbers vulcanizes and, due to the cross-linked bonds, cannot be reused [103]. Polybutadiene rubber in terms of the presence of double bonds and the absence of a side group in the structure is highly susceptible to degradation [26, 104, 105]. For the polymers sensitive to thermal degradation like PBR, the heating rate has important role in the degradation and cross-linking mechanism role decreases with heating rate obviously, while chain scission can be the effective mechanism using high heating rates. In the usual polymers, the polymer sample degrades at higher temperatures using higher heating rates because the heating rate has no significant effect on the degradation mechanisms, while in the degradation of PBR, heating rate can change the degradation mechanisms basically and the polymer sample can partly be degraded at lower temperatures under higher heating rates. At high heating rates, the energy input is very high and prevents cross-link preparation. While at low heating rates, due to the moderate and low energy input, the presence of double bonds and the absence of side groups, two-dimensional nets—like PVC—are created. The created nets with high thermal resistance are degraded at higher temperatures. Generally, on the rubbers with double bonds in the structure, the amount and direction of energy input and the side group type greatly affect the final product and degradation mechanisms [26, 105].
Temperature like heating rate can affect the degradation mechanisms of polybutadiene rubber significantly, and with temperature increasing, polybutadiene rubber pyrolysis tends to produce more aromatics and coke.
Zeolite-based catalysts also have a significant effect on the PBR pyrolysis. In the presence of FCC catalyst, more of the pyrolytic products are the hydrocarbon liquid in the gasoline range, while the thermal degradation produces the viscous and darker liquid with broad molecular mass distribution. On the other hand, HZSM-5 catalyst has a tendency to produce aromatics and, with attention to the small pore size, produces higher gas content compared to FCC catalyst. By increasing the catalyst content of FCC, aromatics increase significantly and the linear product reduces [26]. Figure 12 shows the effect of structural and process parameters on the pyrolysis of polybutadiene rubber.
SBR
Due to the dynamic and mechanical properties of styrene butadiene rubber (SBR), it is widely used in different industries, including the production of tire tread [106]. This polymer is produced by in situ polymerization of butadiene and styrene [107], the cross-linked final products have low recycle-ability, and similar to other types of rubbers, pyrolysis can be a suitable method to recycle SBR [25, 108]. Due to the presence of styrene and butadiene in the structure, the degradation behavior is partly between the degradation behavior of plastics and polybutadiene rubber. At low heating rates, the degradation depends on competition between the cross-linking mechanism and the chain scission through the styrene affect the degradation. With increasing heating rate, the cross-linking mechanism loses its importance and the degradation follows chain scission mechanism [25].
Due to the presence of styrene in the structure of SBR, aromatics are the significant portion of pyrolysis product under any conditions. With increasing temperature and intensification of Diels–Alder reactions, the aromatics and coke increase and the high degradation temperatures increase the gaseous products obviously. Zeolite catalysts are also very effective in the degradation of this polymer, and the pyrolysis products using FCC catalyst are often the condensed liquid in the gasoline range. HZSM-5 as catalyst with small pore size decreases the liquid product and increases aromatics and gasoline range content obviously. Generally, the specific zeolite catalyst maybe have different Si/Al and acidity, and as a result, the specific catalyst can have different effects on the aromatic production. On the other hand, the metal-impregnated zeolite catalysts such as Ga/FCC catalyst with high acidity produce significantly more aromatics [25].
By increasing the FCC catalyst content, although aromatics increase, the condensed liquid decreases obviously. To improve the performance of the catalyst during pyrolysis, by increasing the catalyst, the pyrolysis temperature should be decreased to not have a negative effect on the final products [25]. On the other hand, the studies show that the vacuum pyrolysis of rubbers and used tires can increase the production of liquid products in comparison with other methods in addition to increasing pyrolysis efficiency [109, 110]. Figure 13 shows the effect of structural and process parameters on the pyrolysis of polybutadiene rubber.
Conclusions
In this paper, the effect of different process and structural parameters such as temperature, heating rate, catalyst, carrier gas, extruder and additives on the thermal and thermo-mechanical degradation of polymers with hydrocarbon structure and plastic stabilization was evaluated. The results showed that in most cases, except PVC and the release of HCl gas, in almost the other cases, the effect of structural and process parameters on polymer degradation is the same, and a basic road map for their degradation and pyrolysis can be considered.
In the first step, it is necessary to use a variety of metal stearates, appropriate antioxidant levels for each extrusion time of the plastic materials, suitable process-ability and appropriate extruder design, and in general, to stabilize the polymers, increase the shelf time and reduce the polymeric wastes disposal.
In the second step of pyrolysis of polymers either individually or simultaneously, it must first be determined which products are needed to control the process parameters in its optimum amount. It should be noted that in order to achieve a suitable pyrolysis product and to use optimum process parameters, a reactor with suitable geometry and proper heating and mixing is required, which can be used to maximize the process parameters effect. The results show that in most cases, the condensed liquid at the temperature range of 400–500 °C can be produced up to about 80 percent and at least 800 °C should be supplied to reach the suitable gaseous products.
On the other hand, catalysts can play an important role in the production of hydrocarbons in the desired range. Depending on the porosity and acidity of the catalyst, the valuable products can be significantly increased. As one of the most important results, the metal-impregnated zeolite catalysts can be the potential catalysts to produce the enriched aromatic liquid that can be used as fuel.
Carrier gases and vacuum pyrolysis can affect the mass transfer significantly, the active carrier gases such as hydrogen are also very effective on the products, and hydrogen tends to produce paraffins as standard fuel.
In general, the type of feeding into the reactor and size of the polymers, due to the poor heat transfer coefficient of polymers, can also affect the final products. It seems that the use of extruder for feeding the plastics into the pyrolysis reactor, the use of hydrogen as carrier gas, Ga/FCC catalyst and temperatures between 400 and 500 °C are very suitable for producing petroleum fluids in the gasoline range using a semi-batch rotary kiln reactor. On the other hand, the temperatures higher than 800 °C without catalyst are suitable for the production of olefin gas such as ethylene and propylene. For pyrolysis of rubbers and tires, rubber and tire fragmentation and vacuum pyrolysis are suitable at temperatures between 350 and 450 °C using a semi-batch rotary kiln reactor.
References
Sauter DW, Taoufik M, Boisson Ch. Polyolefins a success story. Polymers. 2017;9:185.
Hutley TJ, Ouederni M. Polyolefins—the history and economic impact. In: Al-Ali AlMa'adeed M, Krupa I, editors. Polyolefins compounds and materials. Springer series on polymer and composite materials. Cham: Springer; 2016. p. 13–50.
Stalzer MM, Delferro M, Marks TJ. Supported single-site organometallic catalysts for the synthesis of high-performance polyolefins. Catal Lett. 2014;145:3–14.
Al-Ali AlMa'adeed M, Krupa I. Introduction. In: Al-Ali AlMa'adeed M, Krupa I, editors. Polyolefin compounds and materials. Springer Series on Polymer and Composite Materials. Cham: Springer; 2016. p. 1–11.
Shubhra QTH, Alam AKMM, Quaiyyum MA. Mechanical properties of polypropylene composites: a review. J Thermoplast Compos Mater. 2013;26:362–91.
Hassan A, Akbari A, Hing NK, Ratnam CT. Mechanical and thermal properties of ABS/PVC composites: effect of particles size and surface treatment of ground calcium carbonate. Polym Plas Technol Eng. 2012;51:473–9.
Hopewell J, Dvorak R, Kosior E. Plastics recycling: challenges and opportunities. Philos Trans R Soc Lond B Biol Sci. 2009;364:2115–266.
Akovali G. Plastics, rubber and health. Shawbury: iSmithers Rapra Publishing; 2007.
Ghani MHA, Salleh MN, Chen RS, Ahmad S, Hamid MRY, Hanafi I, Royan NRR. The effects of antioxidants content on mechanical properties and water absorption behaviour of biocomposites prepared by single screw extrusion process. J Polym. 2014. https://doi.org/10.1155/2014/243078.
Dopico-García MS, López-Vilariñó JM, González-Rodríguez MV. Antioxidant content of and migration from commercial polyethylene, polypropylene, and polyvinyl chloride packages. J Agric Food Chem. 2007;55:3225–311.
Ojeda T, Freitas A, Birck K, Dalmolin E, Jacques R, Bento F, Camargo F. Degradability of linear polyolefins under natural weathering. Polym Degrad Stab. 2011;96:703–7.
Lee U, Han J, Wang M. Evaluation of landfill gas emissions from municipal solid waste landfills for the life-cycle analysis of waste-to-energy pathways. J Cleaner Prod. 2017;166:335–42.
Jr NJ, Silva AA, Marques MRC. Enhanced diesel fuel fraction from waste high-density polyethylene and heavy gas oil pyrolysis using factorial design methodology. Waste Manag. 2015;36:166–76.
Kordoghli S, Khiari B, Paraschiv M, Zagrouba F, Tazerout M. Impact of different catalysis supported by oyster shells on the pyrolysis of tyre wastes in a single and a double fixed bed reactor. Waste Manag. 2017;67:288–97.
Kumar PS, Bharathikumar M, Prabhakaran C, Vijayan S, Ramakrishnan K. Conversion of waste plastics into low-emissive hydrocarbon fuels through catalytic depolymerization in a new laboratory scale batch reactor. Int J Energy Environ Eng. 2017;8:167–73.
Gaurh P, Pramanik H. Production of benzene/toluene/ethyl benzene/xylene (BTEX) via multiphase catalytic pyrolysis of hazardous waste polyethylene using low cost fly ash synthesized natural catalyst. Waste Manag. 2018;77:114–30.
Coelho A, Costa L, Marques MM, Fonseca IM, Lemos MANDA, Lemos F. The effect of ZSM-5 zeolite acidity on the catalytic degradation of high-density polyethylene using simultaneous DSC/TG analysis. Appl Catal A. 2012;413–414:183–91.
Gaurh P, Pramanik H. A novel approach of solid waste management via aromatization using multiphase catalytic pyrolysis of waste polyethylene. Waste Manag. 2018;71:86–96.
Abbas-Abadi MS, Haghighi MN, Yeganeh H. The effect of temperature, catalyst, different carrier gases and stirrer on the produced transportation hydrocarbons of LLDPE degradation in a stirred reactor. J Anal Appl Pyrolysis. 2012;95:198–204.
Funke A, Henrich E, Dahmen N, Sauer J. Dimensional analysis of auger-type fast pyrolysis reactors. Energy Technol. 2017;5:119–29.
Kalargaris I, Tian G, Gu S. The utilisation of oils produced from plastic waste at different pyrolysis temperatures in a DI diesel engine. Energy. 2017;131:179–85.
Pei T, Xiao-bo M, De-zhen Ch, Hai W. Pyrolysis of waste plastics: effect of heating rate on product yields and oil properties. Adv Mater Res. 2013;666:1–10.
Chandrasekaran SR, Kunwar B, Moser BR, Rajagopalan N, Sharma BK. Catalytic thermal cracking of postconsumer waste plastics to fuels. 1. Kinetics and optimization. Energy Fuels. 2015;29:6068–77.
Abbas-Abadi MS, McDonald AG, Haghighi MN, Yeganeh H. Estimation of pyrolysis product of LDPE degradation using different process parameters in a stirred reactor. Polyolefins J. 2015;2:39–47.
Abbas-Abadi MS, Haghighi MN. The consideration of different effective zeolite based catalysts and heating rate on the pyrolysis of Styrene Butadiene Rubber (SBR) in a stirred reactor. Energy Fuels. 2017;31:12358–63.
Salmasi SS, Abbas-Abadi MS, Haghighi MN, Abedini H. The effect of different zeolite based catalysts on the pyrolysis of polybutadiene rubber. Fuel. 2015;160:544–8.
Yu J, Sun L, Ma Ch, Qiao Y, Yao H. Thermal degradation of PVC: a review. Waste Manag. 2016;48:300–14.
Abbas-Abadi MS, Haghighi MN, Yeganeh H, McDonald AG. Evaluation of pyrolysis process parameters on polypropylene degradation products. J Anal Appl Pyrolysis. 2014;109:272–7.
Sarker M, Rashid MM, Molla M, Rahman MS. Thermal conversion of waste plastics (HDPE, PP and PS) to produce mixture of hydrocarbons. Amer J Env Eng sci. 2012;2:128–36.
Wangi J-L, Wang L-L. Catalytic pyrolysis of municipal plastic waste to fuel with nickel-loaded silica–alumina catalysts. Energy Sources Part A. 2011;33:1940–8.
Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci Adv. 2017. https://doi.org/10.1126/sciadv.1700782.
Dostál J, Kašpárková V, Zatloukal M, Muras J, Šimek L. Influence of the repeated extrusion on the degradation of polyethylene. Structural changes in low density polyethylene. Eur Polym J. 2008;44:2652–8.
Hinsken H, Moss S, Pauquet J-R, Zweifel H. Degradation of polyolefins during melt processing. Polym Degrad Stab. 1991;34:279–93.
Delva L, Ragaert K, Degrieck J, Cardon L. The effect of multiple extrusions on the properties of montmorillonite filled polypropylene. Polymers. 2014;6:2912–27.
Andersson T, Stålbom B, Wesslén B. Degradation of polyethylene during extrusion II. Degradation of low-density polyethylene, linear low-density polyethylene, and high-density polyethylene in film extrusion. J Appl Polym Sci. 2004;3:1525–37.
Massa S, Zweifel H. Degradation and stabilization of high density polyethylene during multiple extrusions. Polym Degrad Stab. 1989;25:217–45.
Park JW, Oh SCh, Lee HP, Kim HT, Yoo KO. Kinetic analysis of thermal decomposition of polymer using a dynamic model. Korean J Chem Eng. 2000;17:489–96.
Abbas-Abadi MS, Haghighi MN, Yeganeh H. Effect of the melt flow index and melt flow rate on the thermal degradation kinetics of commercial polyolefins. J Appl Polym Sci. 2012;126:1739–45.
Kebritchi A, Nekoomanesh M, Mohammadi F, Khonakdar HA. The interrelationships between microstructure and melting, crystallization and thermal degradation behaviors of fractionated ethylene/1-butene copolymer. Iran Polym J. 2015;24:267–77.
Kebritchi A, Nekoomanesh M, Mohammadi F, Khonakdar HA. The role of 1-hexene comonomer content in thermal behavior of medium density polyethylene (MDPE) synthesized using Phillips catalyst. Polyolefins J. 2014;1:117–29.
Green AES, Sadrameli SM. Analytical representations of experimental polyethylene pyrolysis yields. J Anal Appl Pyrolysis. 2004;72:329–35.
Mastral FJ, Esperanza E, Garcıa P, Juste M. Pyrolysis of high-density polyethylene in a fluidised bed reactor. Influence of the temperature and residence time. J Anal Appl Pyrolysis. 2002;63:1–15.
Abbas-Abadi MS, Haghighi MN, Yeganeh H. Evaluation of pyrolysis product of virgin High Density Polyethylene degradation using different process parameters in a stirred reactor. Fuel Process Technol. 2012;109:90–5.
Bagri R, Williams PT. Catalytic pyrolysis of polyethylene. J Anal Appl Pyrolysis. 2002;63:29–41.
Marcilla A, Beltrán MI, Navarro R. Thermal and catalytic pyrolysis of polyethylene over HZSM5 and HUSY zeolites in a batch reactor under dynamic conditions. Appl Catal B. 2009;86:78–86.
Agullo J, Kumar N, Berenquer D, Kubicka D, Marcilla A, Gómez A, Salmi T, Murzin DY. Catalytic pyrolysis of low density polyethylene over H-β, H-Y, H-Mordenite, and H-Ferrierite zeolite catalysts: Influence of acidity and structures. Kinet Catal. 2007;48:535–40.
Elordi G, Olazar M, Lopez G, Castaño P, Bilbao J. Role of pore structure in the deactivation of zeolites (HZSM-5, Hβ and HY) by coke in the pyrolysis of polyethylene in a conical spouted bed reactor. Appl Catal B. 2011;102:224–31.
Artetxe M, Lopez G, Amutio M, Elordi G, Bilbao J, Olazar M. Cracking of high density polyethylene pyrolysis waxes on HZSM-5 catalysts of different acidity. Ind Eng Chem Res. 2013;52:10637–45.
Manos G, Garforth A, Dwyer J. Catalytic degradation of high-density polyethylene over different zeolitic structures. Ind Eng Chem Res. 2000;39:1198–202.
Akubo K, Nahil MA, Williams PT. Aromatic fuel oils produced from the pyrolysis-catalysis of polyethylene plastic with metal-impregnated zeolite catalysts. J Energy Ins. 2017. https://doi.org/10.1016/j.joei.2017.10.009.
Williams PT, Slaney E. Analysis of products from the pyrolysis and liquefaction of single plastics and waste plastic mixtures. Resour Conserv Recycl. 2007;51:754–69.
Chen D, Yin L, Wang H, He P. Reprint of: Pyrolysis technologies for municipal solid waste: a review. Waste Manag. 2015;37:116–36.
Abbas-Abadi MS, Haghighi MN, Yeganeh H, Bozorgi B. The effect of melt flow index, melt flow rate, and particle size on the thermal degradation of commercial high density polyethylene powder. J Therm Anal Calorim. 2013;114:1333–9.
Chen HB, Karger-Kocsis J, Wu JS, Varga J. Fracture toughness of α- and β-phase polypropylene homopolymers and random- and block-copolymers. Polymer. 2002;43:6505–14.
Canevarolo SV. Chain scission distribution function for polypropylene degradation during multiple extrusions. Polym Degrad Stab. 2000;70:71–6.
Tochacek J, Jancar J. Processing degradation index (PDI) – A quantitative measure of processing stability of polypropylene. Polym Test. 2012;31:1115–20.
Oliveira RJB, Forrester AMS, Marques MFV. In-reactor stabilization of poly(propylene) with natural antioxidants. Macromol Symp. 2011;299(300):215–9.
Tochacek J, Jancar J, Kalfus J, Zborilova P, Buran Z. Degradation of polypropylene impact-copolymer during processing. Polym Degrad Stab. 2008;93:770–5.
Bhaskar T, Tanabe M, Muto A, Sakata Y, Liu C-F, Chen M-D, Chao CC. Analysis of chlorine distribution in the pyrolysis products of poly(vinylidene chloride) mixed with polyethylene, polypropylene or polystyrene. Polym Degrad Stab. 2005;89:38–42.
Gamlin C, Dutta N, Roy-Choudhury N, Kehoe D, Matisons J. Influence of ethylene–propylene ratio on the thermal degradation behaviour of EPDM elastomers. Thermochim Acta. 2001;367–368:185–93.
Jung S-H, Cho M-H, Kang B-S, Kim J-S. Pyrolysis of a fraction of waste polypropylene and polyethylene for the recovery of BTX aromatics using a fluidized bed reactor. Fuel Process Technol. 2010;91:277–84.
Ciliz NK, Ekinci E, Snape CE. Pyrolysis of virgin and waste polypropylene and its mixtures with waste polyethylene and polystyrene. Waste Manag. 2004;24:173–81.
Kayacan I, Dogan OM. Pyrolysis of low and high density polyethylene. Part I: non-isothermal pyrolysis kinetics. Energy Sources Part A. 2008;30:385–91.
Lee K-H, Shin D-H. A comparative study of liquid product on non-catalytic and catalytic degradation of waste plastics using spent FCC catalyst. Korean J Chem Eng. 2006;23:209–15.
Ahmad I, Khan MI, Khan H, Ishaq M, Tariq R, Gul K, Ahmad W. Pyrolysis study of polypropylene and polyethylene in to premium oil products. Int J Green Energy. 2014;12:663–71.
Westerhout RWJ, Kuipers JAM, van Swaaij WPM. Experimental determination of the yield of pyrolysis products of polyethene and polypropene. Influence of reaction conditions. Ind Eng Chem Res. 1998;37:841–7.
Durmus A, Koc SN, Pozan GS, Kasgoz A. Thermal-catalytic degradation kinetics of polypropylene over BEA, ZSM-5 and MOR zeolites. Appl Catal B. 2005;61:316–22.
Lin Y-H, Yen H-Y. Fluidised bed pyrolysis of polypropylene over cracking catalysts for producing hydrocarbons. Polym Degrad Stab. 2005;89:101–8.
Wang Y, Huang Q, Zhou Zh, Yang J, Qi F, Pan Y. Online study on the pyrolysis of polypropylene over the HZSM-5 zeolite with photoionization time-of-flight mass spectrometry. Energy Fuels. 2015;29:1090–8.
Czajczyńska D, Anguilano L, Ghazal H, Krzyżyńska R, Reynolds AJ, Spencer N, Jouhara H. Potential of pyrolysis processes in the waste management sector. Therm Sci Eng Prog. 2017;3:171–97.
Wünsch JR. Polystyrene: synthesis, production and applications. Shawbury: iSmithers Rapra Publishing; 2000.
Peterson JD, Vyazovkin S, Wigh ChA. Kinetics of the thermal and thermo-oxidative degradation of polystyrene, polyethylene and poly(propylene). Macromol Chem Phys. 2001;202:775–84.
Farahanchi A, Malloy R, Sobkowicz MJ. Effects of ultrahigh speed twin screw extrusion on the thermal and mechanical degradation of polystyrene. Polym Eng Sci. 2016;56:743–51.
Maharana T, Negi YS, Mohanty B. Review article: recycling of polystyrene. Polym Plast Technol Eng. 2007;46:729–36.
Paraskevopoulou D, Achiliasa DS, Paraskevopoulou A. Migration of styrene from plastic packaging based on polystyrene into food simulants. Polym Int. 2012;61:141–8.
Capone C, Landro LD, Inzoli F, Penco M, Sartore L. Thermal and mechanical degradation during polymer extrusion processing. Polym Eng Sci. 2007;47:1813–9.
Zweifel H. Stabilization of polymeric materials. Berlin: Springer; 1998.
Nishizaki H. Comparative study of various methods for thermogravimetric analysis of polystyrene degradation. J Appl Polym Sci. 1980;25:2869–77.
Lee K-H. Composition of aromatic products in the catalytic degradation of the mixture of waste polystyrene and high-density polyethylene using spent FCC catalyst. Polym Degrad Stab. 2008;93:1284–9.
Ojha DK, Vinu R. Resource recovery via catalytic fast pyrolysis of polystyrene using zeolites. J Anal Appl Pyrolysis. 2015;113:349–59.
Achilias DS, Kanellopoulou I, Megalokonomos P, Antonakou E, Lappas AA. Chemical recycling of polystyrene by pyrolysis: potential use of the liquid product for the reproduction of polymer. Macromol Mater Eng. 2007;292:923–34.
Navarro R, Perrino MP, Tardajos MG, Reinecke H. Phthalate plasticizers covalently bound to PVC: plasticization with suppressed migration. Macromolecules. 2010;43:2377–81.
Reinecke H, Mijangos C, Brulet A, Guenet J-M. Molecular structures in poly(vinyl chloride) thermoreversible gels: effect of tacticity and of solvent type. Macromolecules. 1997;30:959–65.
Miranda R, Yang J, Roy Ch, Vasile C. Vacuum pyrolysis of commingled plastics containing PVC. I. Kinetic study. Polym Degrad Stab. 2001;72:469–91.
Miranda R, Pakdel H, Roy C, Vasile C. Vacuum pyrolysis of commingled plastics containing PVC. II. Product analysis. Polym Degrad Stab. 2001;73:47–67.
Garcia JL, Koelling KW, Xu G, Summers JW. PVC degradation during injection molding: experimental evaluation. J Vinyl Add Technol. 2004;10:17–40.
Bhunia K, Sablani SS, Tang J, Rasco B. Migration of chemical compounds from packaging polymers during microwave, conventional heat treatment, and storage. Compr Rev Food Sci Food Saf. 2013;12:523–45.
Gui B, Qiao Y, Wan D, Liu S, Han Z, Yao H, Xu M. Nascent tar formation during polyvinylchloride (PVC) pyrolysis. Proc Combust Inst. 2013;34:2321–9.
Ma S, Lu J, Gao J. Study of the low temperature pyrolysis of PVC. Energy Fuels. 2002;16:338–42.
Wootthikanokkhan J, Jaturapiree A, Meeyoo V. Effect of metal compounds and experimental conditions on distribution of products from PVC pyrolysis. J Polym Environ. 2003;11:1–6.
Ali MF, Siddiqui MN. Thermal and catalytic decomposition behavior of PVC mixed plastic waste with petroleum residue. J Anal Appl Pyrolysis. 2005;74:282–9.
Hirschler MM. Thermal decomposition (STA and DSC) of PVC compounds under a variety of atmospheres and heating rates. Eur Polym J. 1986;22:153–60.
Bhaskar T, Negoro R, Muto A, Sakata Y. Prevention of chlorinated hydrocarbons formation during pyrolysis of PVC or PVDC mixed plastics. Green Chem. 2006;8:697–700.
Ma S, Lu J, Gao J. Study on the pyrolysis dechlorination of PVC waste. Energy Sources. 2004;26:387–96.
Dudkina LM, Ya Gerasimov G, Khaskhachikh VV. Thermogravimetric and kinetic study of pyrolysis of chlorine-containing medical waste. Earth Environ Sci. 2019;272:022117.
Ehabe EE, Bonfils F, Sainte-Beuve J, Collet A, Schué F. High-temperature mastication of raw natural rubber: changes in macrostructure and mesostructure. Polym Eng Sci. 2006;46:222–7.
Hernández M, Valentín JL, López-Manchado MA, Ezquerra TA. Influence of the vulcanization system on the dynamics and structure of natural rubber: comparative study by means of broadband dielectric spectroscopy and solid-state NMR spectroscopy. Eur Polym J. 2015;68:90–103.
Ahmad N, Abnisa F, Daud WMAW. Liquefaction of natural rubber to liquid fuels via hydrous pyrolysis. Fuel. 2018;218:227–35.
Kan T, Strezov V, Evans T. Fuel production from pyrolysis of natural and synthetic rubbers. Fuel. 2017;191:403–10.
Tamri Z, Yazdi AV, Haghighi MN, Abbas-Abadi MS, Heidarinasab A. The effect of temperature, heating rate, initial cross-linking and zeolitic catalysts as key process and structural parameters on the degradation of natural rubber (NR) to produce the valuable hydrocarbons. J Anal Appl Pyrolysis. 2018;134:35–42.
Hall WJ, Zakaria N, Williams PT. Pyrolysis of latex gloves in the presence of Y-zeolite. Waste Manag. 2009;29:797–803.
Ahmad N, Abnisa F, Daud WMAW. Potential use of natural rubber to produce liquid fuels using hydrous pyrolysis—a review. RSC Adv. 2016;6:68906–21.
Friebe L, Nuyken O, Obrecht W. Neodymium-based Ziegler/Natta catalysts and their application in diene polymerization. Adv Polym Sci. 2006;204:1–154.
Williams PT, Besler S. Pyrolysis–thermogravimetric analysis of tyres and tyre components. Fuel. 1995;14(9):1277–83.
Brazier DW, Schwartz NV. The effect of heating rate on the thermal degradation of polybutadiene. J Appl Polym Sci. 1978;22:113–24.
Grieco E, Bernardi M, Baldi G. Styrene−butadiene rubber pyrolysis: products, kinetics, modelling. J Anal Appl Pyrolysis. 2008;82:304–11.
Harandi MH, Alimoradi F, Rowshan Gh, Faghihi M, Keivani M, Abadyan M. Morphological and mechanical properties of styrene butadiene rubber/nano copper nanocomposites. Results Phys. 2017;7:338–44.
Martínez JD, Puy N, Murillo R, García T, Navarro MV, Mastral AM. Waste tyre pyrolysis—a review. Renew Sustain Energy Rev. 2013;23:179–21313.
Lopez G, Olazar M, Aguado R, Elordi G, Amutio M, Artetxe M, Bilbao J. Vacuum pyrolysis of waste tires by continuously feeding into a conical spouted bed reactor. Ind Eng Chem Res. 2010;49:8990–7.
Frigo S, Seggiani M, Puccini M, Vitolo S. Liquid fuel production from waste tyre pyrolysis and its utilisation in a Diesel engine. Fuel. 2014;116:399–408.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abbas-Abadi, M.S. The effect of process and structural parameters on the stability, thermo-mechanical and thermal degradation of polymers with hydrocarbon skeleton containing PE, PP, PS, PVC, NR, PBR and SBR. J Therm Anal Calorim 143, 2867–2882 (2021). https://doi.org/10.1007/s10973-020-09344-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09344-0