Abstract
A practical use of phase change material (PCM)-based thermal energy storage (TES) system is effectively employed for mitigating the imbalance between energy demand and energy supply. Technological development of TES is essential to overcome the drawback of the poor thermo-physical property of many PCM’s. Otherwise, the PCM-based TES system would exhibit poor thermal management. In this work, D-Mannitol (DM) sugar alcohol is considered as PCM and experimental attempts were made to accelerate the phase change behaviour of the chosen PCM by adding 1 and 2% mass fraction of micron-sized copper and aluminium metal powders. The phase change temperature and enthalpy of fusion of the plain DM and composite DM were determined by differential scanning calorimetry (DSC) measurements, and the thermal stability of composite PCM was analysed using thermogravimetric analysis (TGA). Further, charging and discharging processes were conducted for plain DM and composite DM using Therminol 55 heat transfer fluid (HTF) to study their thermal energy storage performance. The DSC results indicated superior enthalpy of fusion and phase transition temperature and TGA results depicted extended decomposition temperature with an increase of about 2 °C to 32 °C for composite DM, respectively, over plain DM. Further, from charge–discharge studies, it was identified that the total time taken for a complete phase change process for the case of DM-Cu 2% during charging process was 22% and 11% lesser compared to plain DM and DM-Al 2%, respectively. Similarly during discharging process, the total time taken for a complete phase change process of DM-Cu 2% was 16% and 10% lesser compared to plain DM and DM-Al 2%, respectively, indicating superior phase change behaviour of composite DM compared to the plain DM. Thus, when compared to plain DM and Al microparticle in DM, Cu microparticle added DM was found to be more suitable to store thermal energy at a medium temperature range.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the worldwide focus on climate change and exploring renewable energy sources to meet the power requirements, many of the countries developed their own national solar mission and unveiled solar energy-based power plants. Also, various research activities directed to prove the technological and economic viability of various solar applications. In order to reap the maximum benefit of free source solar energy, the development of efficient energy storage systems is imperative. The three main types of thermal energy storage (TES) systems are sensible, latent and thermochemical. In which sensible heat storage system is relatively economical and much less complicated. Albeit, latent heat-based TES is a good choice over sensible heat TES for effective thermal energy management because of high energy density [1].
Phase change materials (PCMs) are being used successfully in latent heat-based TES because it absorbs/releases a large amount of heat during phase change process at narrow temperature range, and small volume is enough to dump a large amount of thermal energy. The PCMs are categorized as organic (paraffins and non-paraffins), inorganic (salt hydrates and metallics) and eutectics (organic eutectics and inorganic eutectics) [2]. Due to the technological advancement in recent years, different materials with tailored phase change temperature were developed and thus large number of PCMs have successfully been employed for heating [3, 4] and cooling applications [5, 6]. Though the organic materials are congruent in melting and chemically stable on the adverse side many organic PCMs have low thermal conductivity and a high volume change between the solid and the liquid stages.
Choosing PCM for a given application is mainly based on its phase change temperature. The delivery temperature of heat transfer fluid used in solar concentrating collectors is usually in the range from medium (~ 150 °C) to high (~ 400 °C) temperature. In addition, industrial waste heat also prevailed at medium temperature range and it must be recovered for energy saving reasons. Several low-temperature PCMs were tested and proved as potential candidates to store thermal energy. In recent years, some studies have focused on developing new kinds of solid–liquid PCMs for medium/high-temperature range of applications [7, 8]. These studies revealed that sugar alcohol like erythritol [9], D-Mannitol [10, 11] and xylitol [12] can be employed as an efficient phase change material for medium temperature TES applications (100–200 °C) due to its superior thermal reliability and thermo-physical properties, respectively. In particular, D-Mannitol (DM) had high latent heat and melting temperature of about 165 °C-167 °C [8, 13] and its use as PCM in latent heat thermal storage systems (LHTSS) was successfully tested by various researchers [14,15,16]. The axis symmetric chemical structure of D-Mannitol is shown in Fig. 1.
The major limitation of the PCM’s available in the market is their low thermal conductivity which severely affects its charging and discharging behaviour. From the literature, it is observed that several techniques have been suggested to improve the phase change behaviour of PCM. These techniques include:
- 1.
Addition of high thermal conductivity additives [17, 18] in PCM.
- 2.
Addition of PCM in nanosized pores [19] and microholes of microparticle [20].
- 3.
Addition of fins in PCM containers [21].
- 4.
Reducing the size of encapsulated PCM containers. Based on the size of the PCM container, it is classified into microencapsulation (up to 1 × 106 μm or 11 × 109 nm) and nanoencapsulation (up to 11 × 103 nm) [22].
Considering DM, S Salyan et al. [17] carried out a study to analyse the thermo-physical property and cyclic stability of DM incorporated with CuO nanoparticles. High-speed ball milling technique was used to mix the CuO nanoparticles of different mass fractions (0.1, 0.2 and 0.5 mass%). It was found that the thermo-physical and heat storage characteristics of DM-CuO composite were better than DM. However, it was also reported heat of fusion for the composite DM decreased with increase in heating/cooling cycles. Similarly, in another endeavour, multi-walled carbon nanotubes (CNT) were dispersed in DM and its thermal and cyclic stability were analysed by S Salyan et al. [18]. The effect of CNT addition on energy storage performance was tested for 100 thermal cycles. Experimental analysis indicated that DM-CNT composite exhibited good thermal/chemical stability with improved heat transfer rate.
The detailed thermal performance improvement study of DM-SiO2 composite DM that involved addition of DM into nanosized pores of SiO2 was carried out by Sagara et al. [19]. Experimental analysis indicated that the thermal degradation period of the DM-SiO2 composite PCM was three times higher than that of pure DM, and the authors concluded that the composite DM can be employed as an efficient shape stable PCM. In another endeavour, Xu et al. [20] carried out an investigation on DM/expanded graphite (EG) PCM where DM was uniformly distributed in EG micro holes. It was found that the thermal conductivity of the DM/EG PCM improved substantially while the energy storage period was influenced by the mass fraction of the EG and density of the DM/EG PCM, respectively. Effect of addition of fins in the DM LHTSS was analysed by R P Singh et al. [21]. A comparative analysis with addition of fins, addition of graphene nano plates (GNP) and combination of both indicated that the total melting time reduced by 68% with usage of finned LHTSS system containing DM with 5% GNP compared to a conventional system without fins.
Among the above techniques, the addition of nanoparticles in PCMs has gained momentum in recent years [17, 18, 23]. Though many researchers claimed the benefits of nanoparticles added in PCM, it has technical issues involved in it. Narasimhan et al. [24] summarized the drawbacks of nanoparticle added PCMs. The authors also concluded that, instead of the addition of nanoparticles in PCM, the other scale like macro/microparticles in PCM appeared to be a promising option for improved performance of LHTSS.
The main aim of this study is to accelerate the phase change process of DM PCM encapsulated in the spherical container by the introduction of microparticles. The phase change temperature, decomposition temperature and enthalpy of fusion of microparticles (copper and aluminium) added DM PCM are studied by using differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). The charging and discharging study on plain DM and composite DM (DM-Al and DM-Cu) is also performed using Therminol-55 as heat transfer fluid (HTF).
Experimental setup and procedures
The experimental setup is constructed to investigate the charging and discharging process of plain PCM and composite PCM which is encapsulated in a spherical container made up of stainless steel material. The schematic diagram and photograph of the experimental setup are shown in Fig. 2.
The developed experimental setup consisted of a vertically oriented cylindrical tank containing one spherical capsule having 8.8 cm internal diameter which was filled with PCM. The cylindrical tank was further loaded with 8 litres of Therminol-55 oil, and immersion-type electric heater of 2 kW capacity was used to heat the Therminol-55 oil. The oil temperature inside the cylindrical tank was regulated using a temperature controller attached to the electric heater. To ensure negligible heat loss from the test cylindrical tank, it was well insulated using glass wool insulation of thickness ~ 4 cm. To measure the temperature of the oil and PCM in the cylindrical tank, K-type (chromel–alumel) thermocouples (± 2.2 °C accuracy) were placed along the oil path and at the centre of spherical capsule, respectively.
Composite PCM preparation
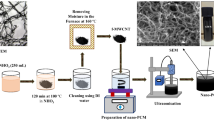
Microparticles of Aluminium (Al) and Copper (Cu) were uniformly mixed at 1 and 2 mass% to DM PCM. The composition of DM with microparticles is shown in Fig. 3. The composition of PCM and metal powder is heated to 230 °C, and the melted composition is evenly mixed by the magnetic stirrer as shown in Fig. 4. The properties of DM and microparticles (Al and Cu) are given in Tables 1 and 2, respectively.
A sample of pure DM, DM-Al, DM-Cu weighing 12 mg was taken for DSC and TGA. The calorimetry test was carried out at N2 atmosphere, and the temperature range 40–220 °C with a heating rate of 20 °C min−1 was considered during the DSC analysis.
The morphologies of PCM + Al 1%, PCM + Cu 1%, PCM + Al 1% and PCM + Cu 2% were studied using scanning electron microscope (SEM) observations with energy-dispersive X-ray analyser (EDX). The SEM images and its corresponding EDX graphs are shown in Fig. 5a–h, respectively. The SEM images depict the structure of DM with irregular shape and size along with sharp edges, similar to that of Salyan et al. [18]. The corresponding peaks in the EDX graphs confirm the presence of suspended Al and Cu microparticles in DM.
Experimental procedure
The phase change temperature and enthalpy of fusion for plain and composite DM were determined by differential scanning calorimeter (NETZSCH DSC 214 manufactured by Netzsch, Germany). Further, the thermal stability of the plain and composite DM was determined using thermogravimetric analyser (TGA Q50 V20.5 Build 30). The accuracy of both the instruments remained ± 0.05 °C.
After careful examination of the DSC & TGA analysis of composite DM and plain DM, the charging and discharging experiment of all the samples were carried out. For the experimental study, totally five stainless steel spherical capsules (internal diameter 88 mm, wall thickness 1 mm) were fabricated and for each capsule, the opening for filling of PCM is provided at the top. All five capsules filled with 0.45 kg mass of plain DM/Composite DM. At molten state, it is ensured carefully that 85% of the volume of the spherical capsule occupied by the samples. The charging/discharging process of all the five capsules was conducted separately using Therminol 55 heat transfer fluid (HTF). Before charging process, the cylindrical tank is filled with HTF at room temperature. The temperature controlled electrical heater is kept in a cylindrical tank. The charging process is initiated by maintaining HTF temperature of 190 °C with the help of a temperature controller. This set temperature is well above the phase change temperature of the composite DM and plain DM. Before the start of the discharging process, the hot oil is completely drained out from the tank and the tank wall was allowed to cool until it reaches the room temperature. Then, the fresh HTF at ambient temperature was filled in the tank and the heater is switched off during the entire discharging process. The change in temperature of plain DM/composite DM is observed during the study for a regular time interval.
Results and discussion
DSC results
The DSC results of pure DM and composite DM is shown in Fig. 6a–e. The rising curve in the figure indicates the endothermic peak corresponding to the melting process. The area under the peak curve represents the enthalpy of fusion. The phase transition temperature is taken as the onset obtained by line fitting of the rising part of the peak curve. The phase transition range of PCM is determined between onset temperature and curve peak temperature. Figure 6a shows that the melting process as an onset point of 163.3 °C and the curve then peaks at a temperature of 166.9 °C. Thus, the phase transition range of plain DM is 163.3–166.9 °C and also the enthalpy of fusion of DM is measured as 261.7 J g−1. The phase transition temperature and enthalpy of fusion of pure DM were observed for the heating rate of 20 °C min−1. Similarly, the phase transition temperature and enthalpy of fusion of composite DM are given in Fig. 6b–e. The values of onset, peak, phase transition temperature and enthalpy of fusion of composite DM shown in the figure are given in Table 3. The variations of phase transition range of composite PCM are due to the presence of microparticles. It is known that the addition of nanoparticles in PCM tends to pull down its latent heat of fusion [24], but the presence of microparticles does not encounter such an issue. Table 4 clearly shows the improvement of the latent heat of fusion values for composite DM over plain DM.
TGA results
The thermal stability of plain DM and composite DM were evaluated in TGA analysis. The two major parameters inferred from TGA analysis include decomposition temperature and mass loss temperature. Decomposition temperature is the temperature at which the plain DM and composite DM begin to chemically decompose, and mass loss temperature is the temperature at which maximum percentage of plain DM and composite DM masses are lost, respectively, during endothermic reaction. The results obtained from the TGA analysis are collectively shown in Fig. 7a–e. It is inferred from the Fig. 7a that the early decomposition temperature (277.57 °C) and mass loss temperature (365.52 °C) are seen for plain DM, whereas the addition of microparticles of aluminium and copper protects pure DM from early decomposition. The composite PCM of DM-Al 2% pertains maximum withstanding temperature (309.42 °C) against decomposition. The values of decomposition temperature and maximum mass loss temperature of composite PCM shown in the Fig. 7b–e is given in Table 4. The results of DSC and TGA analysis show that the composite PCM has improved phase transition temperature and good thermal stability at high temperature.
Charging process
The temperature variation of plain DM/composite DM encapsulated in the spherical capsule during the charging process is shown in Fig. 8a. The temperature variation of plain DM and 1% added both Al and Cu in DM during the charging and discharging process shows almost similar trend, and the time taken for complete melting and solidification also shows the same response. Thus, the reading of 1% added both Al and Cu with DM during the charging and discharging process is not reported in Fig. 8a–b. The initial temperature of plain DM and composite DM during the charging process is maintained the same as 36 °C. The HTF temperature is maintained at a constant temperature of 190 °C which is higher than the phase change temperature of PCM’s considered in the present analysis. It can be inferred from the Fig. 8a that at any instance, the rise in the temperature of composite DM is higher compared to the plain DM due to the presence of microparticles. Further, an abrupt rising trend for all the PCM temperatures can be identified for the initial 15 min followed by a steady temperature rise up to the end of the charging process. Comparatively, during the phase change process, faster response exists for DM-Cu 2% PCM followed by DM-Al 2% PCM and finally plain DM with slower response effect. This can be attributed to higher conductivity of suspended Cu microparticles compared to Al microparticles (Table 2) which plays a predominant role in temperature rise during the charging phase change process.
Discharging process
The temperature variation of plain PCM/composite PCM with respect to time during the discharging process is represented in Fig. 8b. The discharging process is carried out from the initial temperature of 170 °C for all PCM considered in the analysis. The discharging process is allowed to continue until the PCM’s reach 90 °C. During this experiment, the HTF temperature increases and at the end of the experiment the temperature of HTF in the tank is noted as 81 °C, 85 °C and 87 °C for the case of plain DM, DM-Al 2% and DM- Cu 2%, respectively. From Fig. 8b, it can be inferred that DM-Cu 2% PCM incurred lesser time duration than DM-Al 2% PCM and plain PCM, respectively, to reach a minimum discharging temperature of about 90 °C. The plain DM PCM shows a slower charge in temperature response than composite PCM. Again the presence of composite powder in PCM enables faster phase change process due to improved heat transfer rate which is reflected in higher increasing temperature of the HTF.
From Fig. 8a and b, it can be identified that the total time taken for complete charging process of DM-Cu 2% was 22% and 11% lesser compared to plain DM and DM-Al 2%, respectively. Similarly, the total time taken for the complete discharging process of DM-Cu 2% reduced up to 16% and 10% when compared to DM and DM-Al 2%, respectively.
Conclusions
In this work, an attempt has been made to accelerate the phase change process of D-Mannitol (DM) PCM by adding 1 and 2 mass% of micro-sized Al and Cu metal powders. The PCM’s phase change and enthalpy of fusion behaviour were evaluated by using DSC analysis, and decomposition performance of PCM was observed by using TGA analysis, respectively. The DSC results showed improved enthalpy of fusion and phase transition temperature for composite DM over plain DM. The extended decomposition temperature (an increase of 2–32 °C) of composite DM PCM’s was observed from TGA results. It implies that composite PCM’s have good thermal stability at a higher temperature. Thus, undoubtedly composite DM PCM is suitable to store solar thermal energy for systems which operate at a medium temperature range. The total time taken for a complete phase change process (solid–liquid) of DM-Cu 2% was 22% and 11% lesser when compared to plain DM and DM-Al 2%, respectively. This accelerated phase change behaviour was also observed in discharging study (liquid–solid). In this process, the total time taken for DM-Cu 2% was reduced to 16% and 10% when compared to plain DM and DM-Al 2%, respectively. Thus, when compared to Al powder in DM, Cu powder was found to be more suitable to store energy at a medium temperature range.
References
Alva G, Lin Y, Fang G. An overview of thermal energy storage systems. Energy. 2017;144:341–78.
Hariharan K, Kumar GS, Kumaresan G, Velraj R. Investigation on phase change behavior of paraffin phase change material in a spherical capsule for solar thermal storage units. Heat Transf Eng. 2018;39:775–83.
Devaux P, Farid MM. Benefits of PCM underfloor heating with PCM wallboards for space heating in winter. Appl Energy. 2017;191:593–602.
Li Y, Huang G, Wu H, Xu T. Feasibility study of a PCM storage tank integrated heating system for outdoor swimming pools during the winter season. Appl Therm Eng. 2018;134:490–500.
Sudhakar P, Kumaresan G, Velraj R. Experimental analysis of solar photovoltaic unit integrated with free cool thermal energy storage system. Sol Energy. 2017;158:837–44.
Panchabikesan K, Vincent AA, Ding Y, Ramalingam V. Enhancement in free cooling potential through PCM based storage system integrated with direct evaporative cooling (DEC) unit. Energy. 2018;144:443–55.
del Barrio EP, Godin A, Duquesne M, Daranlot J, Jolly J, Alshaer W, Kouadio T, Sommier A. Characterization of different sugar alcohols as phase change materials for thermal energy storage applications. Sol Energy Mater Sol Cells. 2017;159:560–9.
Kumaresan G, Velraj R, Iniyan S. Thermal analysis of d-mannitol for use as phase change material for latent heat storage. J Appl Sci. 2011;11:3044–8.
Leng G, Qiao G, Xu G, Vidal T, Ding Y. Erythritol-Vermiculite form-stable phase change materials for thermal energy storage. Energy Procedia. 2017;142:3363–8.
Mojiri A, Grbac N, Bourke B, Rosengarten G. D-mannitol for medium temperature thermal energy storage. Sol Energy Mater Sol Cells. 2018;176:150–6.
Beemkumar N, Yuvarajan D, Karthikeyan A, Ganesan S. Comparative experimental study on parabolic trough collector integrated with thermal energy storage system by using different reflective materials. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-018-07989-6.
Biçer A, Sarı A. Synthesis and thermal energy storage properties of xylitol pentastearate and xylitol pentapalmitate as novel solid–liquid PCMs. Sol Energy Mater Sol Cells. 2012;102:125–30.
Gombás Á, Szabó-Révész P, Regdon G, Erős I. Study of thermal behaviour of sugar alcohols. J Therm Anal Calorim. 2003;73:615–21.
Kumaresan G, Vigneswaran VS, Esakkimuthu S, Velraj R. Performance assessment of a solar domestic cooking unit integrated with thermal energy storage system. J Energy Storage. 2016;6:70–9.
Peiró G, Gasia J, Miró L, Cabeza LF. Experimental evaluation at pilot plant scale of multiple PCMs (cascaded) vs. single PCM configuration for thermal energy storage. Renew Energy. 2015;83:729–36.
Kumaresan G, Santosh R, Raju G, Velraj R. Experimental and numerical investigation of solar flat plate cooking unit for domestic applications. Energy. 2018;157:436–47.
Salyan S, Suresh S. Study of thermo-physical properties and cycling stability of D-Mannitol-copper oxide nanocomposites as phase change materials. J Energy Storage. 2018;15:245–55.
Salyan S, Suresh S. Multi-walled carbon nanotube laden with D-Mannitol as phase change material: characterization and experimental investigation. Adv Powder Technol. 2018;29:3183–91.
Sagara A, Nomura T, Tsubota M, Okinaka N, Akiyama T. Improvement in thermal endurance of D-Mannitol as phase-change material by impregnation into nanosized pores. Mater Chem Phys. 2014;146:253–60.
Xu T, Chen Q, Huang G, Zhang Z, Gao X, Lu S. Preparation and thermal energy storage properties of D-Mannitol/expanded graphite composite phase change material. Sol Energy Mater Sol Cells. 2016;155:141–6.
Singh RP, Kaushik SC, Rakshit D. Melting phenomenon in a finned thermal storage system with graphene nano-plates for medium temperature applications. Energy Convers Manag. 2018;163:86–99.
Cabeza LF, Castellon C, Nogues M, Medrano M, Leppers R, Zubillaga O. Use of microencapsulated PCM in concrete walls for energy savings. Energy Build. 2007;39:113–9.
Andreani T, Kiill CP, de Souza AL, Fangueiro JF, Doktorovová S, Garcia ML, Gramião MP, Silva AM, Souto EB. Effect of cryoprotectants on the reconstitution of silica nanoparticles produced by sol–gel technology. J Therm Anal Calorim. 2015;120:1001–7.
Narasimhan NL, Veeraraghavan V, Ramanathan G, Bharadwaj BS, Thamilmani M. Studies on the inward spherical solidification of a phase change material dispersed with macro particles. J Energy Storage. 2018;15:158–71.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pramothraj, M., Santosh, R., Swaminathan, M.R. et al. Study of effect of Al and Cu microparticles dispersed in D-Mannitol PCM for effective solar thermal energy storage. J Therm Anal Calorim 139, 895–904 (2020). https://doi.org/10.1007/s10973-019-08469-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08469-1