Abstract
The present study aims at nanoparticles characterization and stability as well as the thermal conductivity of the hybrid nano-oil of ZnO–MWCNT/oil at the temperature range from 25 to 65 °C and the concentrations range from 0.50 to 3.2% for the solid particles. First, the nanoparticles of MWCNT and ZnO were characterized using XRD-FESEM and FTIR tests, and according to the results, the analysis of atomic, surface and chemical structure of nanoparticles was made. The nanolubricant was prepared by a two-step method. For this purpose, firstly, the stability was analyzed by the DLS test and the results show that the nanoparticles are in nanoscale after the construction of nano-oil. The thermal conductivity was measured based on the variables of temperature and volume fraction. An increasing trend was observed for the thermal conductivity for higher temperature and volume fraction of the nanoparticles. The biggest improvement of the thermal conductivity compared to the base fluid is at 65 °C, the volume fraction is 3.2%, and its value is 35.1%. Moreover, a very accurate experimental relationship was developed to determine the ratio of the thermal conductivity of nano-oil in the empirical range.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Today, oils are used extensively in the industries as lubricant and coolant for lubrication as well as thermal conductivity. In addition, oils are widely used as a lubricant and coolant in engines. The main application of engine oil is for engine parts lubricating at different temperatures to reduce the abrasion in various moving parts and cool the engine by conducting heat from movable and immovable parts. The thermal conductivity of oil plays a crucial role in this regard, and higher thermal conductivity results in a better heat transfer [1,2,3,4,5,6]. One of the methods of improving the performance of systems is the use of proper working fluid to increase the potential of heat transfer. In this case, nanoparticles can be used with a high heat transfer potential known as intelligent heat transfer fluids [7,8,9,10,11,12,13,14,15,16,17].
Nanofluids are nanoparticle-containing fluids like water, engine oil and ethylene glycol which have been introduced by Choi and Eastman [18]. They pointed out in their research that the addition of particles with a higher thermal conductivity than the base fluid would improve the base fluids properties. This characteristic has been attracted the attention of several researchers recently to use the valuable features of this novel group of fluids in various types of applications [19,20,21,22]. For the oils in engines, researchers are trying to improve these properties by the use of oxide, metal, carbon and other nanoparticles in the engine oils [23, 24].
Thermal conductivity is one of the important factors in thermal oils. Although the thermal conductivity of various nanoparticles has been extensively studied, little comprehensive research has been conducted to characterize the nanoparticles and stability of nano-oils in addition to the thermal conductivity. The studies have been made on the volume fractions less than 1%.
In the following, a summary of the literature is provided:
Maxwell was the first scientist who analyzed the thermal conductivity of a solid–liquid suspension considering a very dilute suspension of spherical particles without taking into account the interactions between particle and base fluid. He provided Eq. 1 to describe the results of his studies [25]:
Eastman et al. [26] studied the thermal conductivity of nanoparticles AL2O3, CuO and Cu, experimentally, with the diameter of nanoparticles less than 10 nm. Their results indicated that the thermal conductivity has increased by up to 40% maximum compared to the base fluid. Aberumand and Jafari Moghaddam [27] investigated the thermal behavior of nanoparticles containing Cu–engine oil at 0.5–1% volume fraction (vol. %) at a temperature range of 313 to 380°K. The maximum increase in the concentration is at 1 w/w% and the temperature of 380°K. In another research, Aberumand et al. [28] assessed the nano-oil and nanosilver-containing nanofluid in an in vitro environment. The results showed that the thermal conductivity increases for higher temperature and volume fraction. In addition, they proposed a new equation to predict the thermal conductivity in terms of temperature and volume fraction. Lee et al. [29] investigated on a nano-oil under the influence of fullerene nanoparticles in an in vitro study. The base oil was selected from the mineral type, and the test apparatus was a disk-on-a-disk system. The experiment was carried out under different volumetric concentrations and vertical loads. In this test, the temperature of the friction surface and the friction coefficient of the lubricant were calculated. They presented that the friction coefficient and abrasion are reduced for a higher amount of nanoparticles which reflects the enhancement of lubrication properties of the mineral oil by using fullerene nanoparticles (C60). Ilyas et al. [30] made an in vitro study on the alumina nanoparticle-containing nano-oil in the thermal oil as the base fluid. The volume fraction interval studied to modify the oil properties. Stability of nanofluid was investigated under different conditions. They showed an improvement in the thermal properties of the alumina nanoparticle-containing oil.
The favorable properties of nanoparticles led to the nanofluid overcome the problems of conversion and transfer of energy and also enhance the development of a lubrication process, diminish heat exchangers size, higher system efficiency, lower fuel consumption and make cost savings as an alternative fluid [31,32,33].
According to the above results, it can be seen that the thermal properties of the engine oil play a crucial role in oil performance. In the present study, a comprehensive characterization of nanoparticles, stability analysis, measurement of thermal conductivity and a new experimental equation will be provided. In this research, zinc oxide (ZnO) nanoparticles and carbon nanotubes (CNTs) are used to form compound nanoparticles. In addition to high thermal properties, it is anticipated that the heat transfer function improves. In the following, a new equation is proposed by using the laboratory results, to determine the thermal conductivity.
Materials and method
Empirical studies have shown that in preparing the nanoparticle-containing fluids, parameters such as particle materials, size, nanomaterial concentration, type of base fluid, surfactants, type and time of ultrasound, and time of accumulation and adhesion of particles are effective in the decrease or increase in the thermal conductivity [34, 35].
Compared to some other oxidized nanoparticles, ZnO nanoparticles have better thermal properties and a spherical structure. MWCNT nanoparticles are known to have high thermal conductivity, low specific gravity, high aspect ratio and high heat transfer potential. So, using a combination of ZnO and MWCNT nanoparticles, a nanofluid with long-term stability and proprietary thermal characteristics can be made.
The nanofluids creation and stability are among the main challenges, since obtaining uniform and significant properties need maintenance of stability in them. A two-stage method is employed for the creation and stability of nanofluid. The base fluid is the 20W50 oil from Total S.A., used extensively for cars. The exact specifications of the nanoparticles are presented in Table 1. Characterization of nanoparticles is carried out to investigate the surface, atomic and chemical structure of these nanoparticles employing the FESEM-XRD and FTIR tests.
To test the thermal conductivity, a compound is prepared from each sample (60 mL) according to the standard of the KD2-Pro device. Various samples are prepared using 0.05–0.1–0.2–0.4–0.8–1.6–3.2 vol.% without the use of surfactants. The nanoparticles and base fluid masses are calculated according to Eq. (2), and its values are given in Table 2. The experiment is performed at temperatures ranging from 25 to 65 °C.
In order to produce a homogenous nanofluid with long-term stability and minimum caking, nanoparticles are mixed with the base oil for a period of 60 min using a magnetic stirrer. Then, in order to homogenize the suspension and break down the particle lumps, a probed ultrasonic device is used for 60 min.
Shown in Fig. 1 are the nanoparticles and base oil used and nano-oil created.
Note that the stability is tested using the DLS test (dynamic light scattering) on the nano-oil sample.
Measuring the thermal conductivity
For the thermal conductivity measurement, KD2 Pro thermal property analyzer (Decagon Devices Inc., USA) was employed. Transient hot-wire technique was the basis of the operation of the device. To verify the device’s calibration, the standard glycerin fluid proposed by the manufacturer was used to investigate the device’s calibration before the test. In this test, the KS-1 sensor is used, and each test will be repeated three times to ensure accuracy of the results. For temperature stability, samples are placed in a bath with a 0.1 °C precision.
Validation of test results
Figure 2 displays the results of the verification of the system for measuring the thermal conductivity for pure water. In this research, the ASHRAE handbook [36] has been used to obtain the results of the pure water. Considering the varied types of oils, their comprehensive information is not available. So, product diversity was made based on emerging developments. As shown, the experimental results are consistent with the reference results, and their differences are negligible.
Results and discussion
Characterization of nanoparticles
FESEM
The FESEM results of nanoparticles are presented in Fig. 3. High-resolution images were taken for nanoparticles to evaluate their morphology and structure. The figures are presented and are magnified to allow observing and evaluating the surface structure of nanoparticles. In these results, the tubular structure and nanodimensions of the particles are clearly observed.
The morphology and structure of ZnO nanomaterials were assessed by the FESEM test, and the results are also shown in Fig. 3. FESEM images of zinc oxide nanoparticles show that these nanoparticles have a nearly spherical structure with an almost uniform diameter averaged at 30 nm. Application of spherical nanoparticles in lubricant fluids is very important for the reduction in friction and temperature and resistance increase to the abrasion resulting from friction between surfaces.
XRD
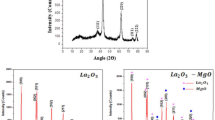
Structural characteristics of MWCNT nanoparticles analyzed by the X-ray diffraction (XRD) to investigate their atomic structure under the radiation of CuKα (λ = 0.15418 nm) and 40 kV voltage and 30 mA current at the range of 10° to 100° are shown in Fig. 4a. The diffraction peaks at the angles of 2θ, 25.15°, 42.32°, 44.40°, 53.96° and 77.82° correspond to the crystal plates of (002), (100), (101), (004) and (110) of the powders of CNT (CNT, JCPDS card No: 01-075-1621), which show the highly graphitic structure of this powder.
The crystalline structure of ZnO nanoparticles was determined by X-ray diffraction (XRD) with radiation of CuKα (λ = 0.15418 nm) under a 40 kV voltage and a 30 mA current. Figure 4b illustrates the X-ray diffraction pattern of the ZnO nanoparticles used in this study. The peaks specified at the angles of 2θ, 31.86°, 34.50°, 36.32°, 47.53°, 56.63°, 62.93°, 66.47º, 67.97°, 69.12°, 72.69°, 77.06°, 81.45°, 89.70°, 92.87°, 95.34° and 98.66° corresponding to the crystal plates of (100), (002), (101), (102), (110), (103), (200), (112), (201), (004), (202), (104), (203), (210), (211) and (114) which show the structure of polycrystalline nanoparticles (zincite, JCPDS card No: 00-036-1451). No peaks of impurities were detected to be an indication of the high purity of ZnO nanoparticles.
FTIR
The results of FTIR test on MWCNT are presented in Fig. 5a. The groups in the structure of carbon nanotubes are: a large peak at the wavelength of 3400 cm−1 for the O–H bond of the hydroxyl group; the C=C bond in 1637 cm−1 of the carbonyl group; the C–H bond in 2920 cm−1; and small peak at about 1128 cm−1 of the C–O bond. The wide peak of the hydroxyl group at the wavelength of 3400 cm−1 of the O–H bond is a result of the moisture in the sample which was not completely dried before the analysis. A large peak of C=C with a dual carbon bond confirms the hexagonal structure of carbon nanotubes. In Table 3, the links in the FTIR analysis of carbon nanotubes and their corresponding wave numbers are presented.
The results related to the ZnO sample in the range of 350–4000 cm−1 are shown in Fig. 5b. A wide peak in the range of 3100–3700 cm−1 is observed due to the O–H bond of the hydroxyl group. Also, the peak of H2O molecules is at the wavelength of about 1630 cm−1. The peak in the range of 450–550 cm−1 is related to the Zn–O bond. The bonds in the FTIR analysis of zinc oxide nanoparticles and their corresponding wave numbers are presented in Table 4.
Nano-oil stability
Making the nanofluid stable is especially important. One of the methods for creating stability conditions is the use of the DLS test. This method is able to measure the nanoparticles size inside the solution in a quick and simple way without the need for sample preparation. In this experiment, the DLS-VASCOTMy series device was used with a wavelength of 657 nm to estimate the dimensions of the nanoparticles.
The intensity of scattered light fluctuations is analyzed by radiating the laser into and passing it through the nano-oil. Then, the size of the nanoparticles is determined. The pattern of particle size measurement is based on the three measured variables of number, intensity and volume, and the results are presented in Fig. 6.
The results of the particle size measurement as a function of intensity are shown in Fig. 6a. The results of this figure are obtained by passing the laser beam through the nano-oil after the incidence, and finally, the device indicates the particle size according to the passage intensity of the beam. In this pattern, larger particles have a more intense dispersion, and this causes the effects of particles to be disappeared from the sensors and report no intensity. However, the largest peak is 35.49 nm. The general area of the particles is shown in the figure.
Figure 6b shows the results of the particles mean size according to the number variable. The average size corresponds to the diameter of the particle in one dimension, with the largest average diameter being about 35.49 nm, and the particle diameter range is less than 100 nm, which in fact confirms the nanoscale of the particles.
The diameter of the nanoparticles is presented in Fig. 6c in terms of nanomaterials concentration. The results indicate the volume occupied by nanoparticles in various dimensions. The results obtained by this methodology determine the particle diameter more generally. However, its main difference with the results of the analysis of the number of particles is that a large particle takes up more space than a small particle, and naturally, the results show a slight increase in the diameter. This method is more applicable in the study of nanofluid stability for industrial applications.
Effect of temperature on nano-oil thermal conductivity
Changes in the nano-oil thermal conductivity when using the combined particles of carbon nanotube–zinc oxide at different temperatures and concentrations are shown in Fig. 7a. It is concluded that with enhancing the temperature, the thermal conductivity of the base oil decreases. So, adding nanomaterials increases the changing trends of thermal conductivity. Moreover, for a higher concentration, the slope of the increase in thermal conductivity will be steeper. One of the reasons for this increase in the thermal conductivity coefficient is higher kinetic energy of the nanofluid molecules and higher energy levels.
As shown in Fig. 7a, with the higher volume fractions and also higher temperatures, the thermal conductivity increases very rapidly compared to the lower volume fractions. One main reason can be the increase in the heat transfer acceleration of the nanoparticles clustering rate in higher concentrations which makes a chain between nanoparticles and results in a higher heat transfer and a higher thermal conductivity.
In order to better understand the behavior of the thermal conductivity with the increase in temperature, the ratio of the thermal conductivity is calculated as follows:
The results of thermal conductivity ratio are shown in Fig. 7b. At the highest vol. % (3.2%), a higher thermal conductivity occurs with the increase in temperature from 18.3 to 35.1% compared with that for the base fluid. In the low volume fractions, due to the small amount of nanoparticles in the base fluid, variations of the thermal conductivity are lower than in high volume fractions.
In lower volume fractions, the relative thermal conductivity increases with a very low gradient when the temperature increases. However, for a higher volume concentration, the variation gradient of the relative thermal conductivity increases significantly, indicating a positive effect of the caking phenomenon in nanoparticles and higher nanoparticles Brownian diffusion with increasing temperature.
Variations in the thermal conductivity ratio of nano-oil to the base oil at different volumetric concentrations with increasing temperature are shown in Table 5. The increase in thermal conductivity is observed for higher temperature and volume concentration. The effect of the volume fraction is higher between the two variables of volume concentration and the temperature.
Effect of volume concentration of nano-oil on thermal conductivity
The results of the volume concentration effect on the thermal conductivity of the nano-oil are displayed in Fig. 8a. A higher volume concentration from 0.05 to 3.2% at constant temperature results in a higher thermal conductivity. It is quite evident that the effect of the volume fraction is much higher in comparison with the temperature, which is mainly due to the higher nanomaterials thermal conductivity compared with the base fluid. The highest volume fraction has been created at the highest volume fraction which shows the advantage of nanoparticle clustering due to the higher number of particles.
For a higher volume concentration, a higher number of particles are arranged in a chain to form larger clusters. For higher temperature, the energy level of the particles and particle interactions enhances, which, finally, accelerates the heat transfer by the clusters and increases the thermal conductivity.
As observed in Fig. 8b, for a higher volume concentration, the thermal conductivity ratio enhances. This is observed at all temperatures. Considering cooling as the main parameters deal with the oils in various engine parts in addition to their lubrication, the use of this nano-oil at higher volume fractions is recommended for cooling equipment. With all that in mind, excessive enhancement in the nanoparticles volume concentration can cause instability of the nano-oil and make it unsuspend. Also, in case of the possibility of using the nanofluid in heat transfer systems, problems could arise like increased viscosity of the nanofluid and the need for a higher-power pump [37, 38]. It should be mentioned that the highest concentration used in this oil has been quite stable.
Proposed model
The behavior of nanofluids could change under the influence of a number of parameters. Since many factors affect the thermal properties of nanofluids, e.g., manufacturing method, influences of temperature, particle distribution and size, type of nanoparticles and Brownian diffusion effect [39,40,41], researchers are not able to provide a comprehensive model for predicting the thermal conductivity with high precision. The inability of classic theoretical models has been widely reported in previous studies [42,43,44]. In this study, a precise relationship was developed to determine the thermal conductivity ratio of the combined nano-oil MWCNT–ZnO/oil in terms of temperature and volume concentration.
where \( k \) is the thermal conductivity, T0 is 25 °C, \( T \) is the nano-oil temperature in degrees centigrade and \( \varphi \) is the volume concentration. Also, the indices \( {\text{nf}} \) and \( {\text{bf}} \) are the nanofluid and the base fluid, respectively.
The precision of the equation has been investigated using Eq. (5) showing the maximum deviation of 1.08% between the experimental data and the equation. The accuracy is presented in various diagrams as shown in Fig. 9.
when the subscribe Exp and Pre show the experimental and determined values.
Conclusions
Purposeful and comprehensive characterization of the hybrid nanoparticles of carbon nanotubes–zinc oxide, production of nano-oil, stability and thermal conductivity of oil ZnO–MWCNT/oil under the influence of temperature range from 25 to 65 °C, and the volume fraction percentages were made in an in vitro environment. Results of nanoparticles characterizations showed acceptable surface and atomic structures. In the following, a summary of the conclusions of the study is presented.
- 1.
In order to create the nano-oils, the two-stage method is recommended considering their high viscosity. Mechanical and ultrasonic mixing provides suitable and acceptable results for nanolubricant production.
- 2.
Characterization of nanoparticles shows the surface structure of nanoparticles very clearly.
- 3.
Investigation of the atomic and chemical structure indicated the nature of the materials used, by which the desired details are observed in atomic and chemical aspects.
- 4.
Thermal conductivity increases significantly under the influence of volume concentrations and increases to a maximum of 35.1%.
- 5.
Thermal conductivity increases with increasing temperature. This advantageous property of the nano-oil can perform better at higher temperatures because increasing the thermal conductivity actually means increased thermal conduction. In applications, the thermal efficiency increases at higher temperatures.
- 6.
Effects of nano-oil volume fraction are much higher than temperature, which is mainly due to the much higher thermal conductivity of nanomaterials than the base fluid.
- 7.
The experimental equation of the thermal conductivity ratio has been obtained by the curve fitting method and could be used in numerical calculations and computer simulations of this nanolubricant given its high accuracy. In the worst case, the maximum error is 1.08%.
References
Ahmadi Nadooshan A, Hemmat Esfe M, Afrand M. Prediction of rheological behavior of SiO2-MWCNTs/10W40 hybrid nanolubricant by designing neural network. J Therm Anal Calorim. 2018;131(3):2741–8. https://doi.org/10.1007/s10973-017-6688-3.
Goodarzi M, Toghraie D, Reiszadeh M, Afrand M. Experimental evaluation of dynamic viscosity of ZnO–MWCNTs/engine oil hybrid nanolubricant based on changes in temperature and concentration. J Therm Anal Calorim. 2019;136(2):513–25. https://doi.org/10.1007/s10973-018-7707-8.
Sepyani K, Afrand M, Esfe MH. An experimental evaluation of the effect of ZnO nanoparticles on the rheological behavior of engine oil. J Mol Liq. 2017;236:198–204.
Asadi A, Asadi M, Rezaniakolaei A, Rosendahl LA, Afrand M, Wongwises S. Heat transfer efficiency of Al2O3-MWCNT/thermal oil hybrid nanofluid as a cooling fluid in thermal and energy management applications: an experimental and theoretical investigation. Int J Heat Mass Transf. 2018;117:474–86.
Esfe MH, Bahiraei M, Hajmohammad MH, Afrand M. Rheological characteristics of MgO/oil nanolubricants: experimental study and neural network modeling. Int Commun Heat Mass. 2017;86:245–52.
Nadooshan AA, Esfe MH, Afrand M. Evaluation of rheological behavior of 10W40 lubricant containing hybrid nano-material by measuring dynamic viscosity. Phys E. 2017;92:47–54.
Eshgarf H, Sina N, Esfe MH, Izadi F, Afrand M. Prediction of rheological behavior of MWCNTs–SiO2/EG–water non-Newtonian hybrid nanofluid by designing new correlations and optimal artificial neural networks. J Therm Anal Calorim. 2018;132(2):1029–38. https://doi.org/10.1007/s10973-017-6895-y.
Shahsavani E, Afrand M, Kalbasi R. Experimental study on rheological behavior of water–ethylene glycol mixture in the presence of functionalized multi-walled carbon nanotubes. J Therm Anal Calorim. 2018;131(2):1177–85. https://doi.org/10.1007/s10973-017-6711-8.
Alsarraf J, Rahmani R, Shahsavar A, Afrand M, Wongwises S, Tran MD. Effect of magnetic field on laminar forced convective heat transfer of MWCNT–Fe3O4/water hybrid nanofluid in a heated tube. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08078-y.
Hajatzadeh Pordanjani A, Aghakhani S, Karimipour A, Afrand M, Goodarzi M. Investigation of free convection heat transfer and entropy generation of nanofluid flow inside a cavity affected by magnetic field and thermal radiation. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-018-7982-4.
Vahedi SM, Pordanjani AH, Wongwises S, Afrand M. On the role of enclosure side walls thickness and heater geometry in heat transfer enhancement of water–Al2O3 nanofluid in presence of a magnetic field. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08224-6.
Afrand M, Esfe MH, Abedini E, Teimouri H. Predicting the effects of magnesium oxide nanoparticles and temperature on the thermal conductivity of water using artificial neural network and experimental data. Phys E. 2017;87:242–7.
Dehkordi RA, Esfe MH, Afrand M. Effects of functionalized single walled carbon nanotubes on thermal performance of antifreeze: an experimental study on thermal conductivity. Appl Therm Eng. 2017;120:358–66.
Esfahani NN, Toghraie D, Afrand M. A new correlation for predicting the thermal conductivity of ZnO–Ag (50%–50%)/water hybrid nanofluid: an experimental study. Powder Technol. 2018;323:367–73.
Esfe MH, Motahari K, Sanatizadeh E, Afrand M, Rostamian H, Ahangar MRH. Estimation of thermal conductivity of CNTs-water in low temperature by artificial neural network and correlation. Int Commun Heat Mass. 2016;76:376–81.
Nadooshan AA, Eshgarf H, Afrand M. Measuring the viscosity of Fe3O4-MWCNTs/EG hybrid nanofluid for evaluation of thermal efficiency: Newtonian and non-Newtonian behavior. J Mol Liq. 2018;253:169–77.
Shahsavani E, Afrand M, Kalbasi R. Using experimental data to estimate the heat transfer and pressure drop of non-Newtonian nanofluid flow through a circular tube: applicable for use in heat exchangers. Appl Therm Eng. 2018;129:1573–81.
Choi SU, Eastman JA. Enhancing thermal conductivity of fluids with nanoparticles: Argonne National Lab., IL (United States) 1995.
Cheraghian G, Wu Q, Mostofi M, Li M-C, Afrand M, Sangwai JS. Effect of a novel clay/silica nanocomposite on water-based drilling fluids: improvements in rheological and filtration properties. Colloids Surf, A. 2018;555:339–50.
Karimi A, Al-Rashed AA, Afrand M, Mahian O, Wongwises S, Shahsavar A. The effects of tape insert material on the flow and heat transfer in a nanofluid-based double tube heat exchanger: two-phase mixture model. Int J Mech Sci. 2019;156:397–409.
Afrand M. Experimental study on thermal conductivity of ethylene glycol containing hybrid nano-additives and development of a new correlation. Appl Therm Eng. 2017;110:1111–9.
Afrand M, Karimipour A, Nadooshan AA, Akbari M. The variations of heat transfer and slip velocity of FMWNT-water nano-fluid along the micro-channel in the lack and presence of a magnetic field. Physica E. 2016;84:474–81.
Afrand M, Najafabadi KN, Akbari M. Effects of temperature and solid volume fraction on viscosity of SiO2-MWCNTs/SAE40 hybrid nanofluid as a coolant and lubricant in heat engines. Appl Therm Eng. 2016;102:45–54.
Dardan E, Afrand M, Isfahani AM. Effect of suspending hybrid nano-additives on rheological behavior of engine oil and pumping power. Appl Therm Eng. 2016;109:524–34.
Maxwell JC. A treatise on electricity and magnetism. Clarendon. Oxford. 1881;314:1873.
Eastman JA, Choi S, Li S, Yu W, Thompson L. Anomalously increased effective thermal conductivities of ethylene glycol-based nanofluids containing copper nanoparticles. Appl Phys Lett. 2001;78(6):718–20.
Aberoumand S, Jafarimoghaddam A. Experimental study on synthesis, stability, thermal conductivity and viscosity of Cu–engine oil nanofluid. J Taiwan Inst Chem Eng. 2017;71:315–22.
Aberoumand S, Jafarimoghaddam A, Moravej M, Aberoumand H, Javaherdeh K. Experimental study on the rheological behavior of silver-heat transfer oil nanofluid and suggesting two empirical based correlations for thermal conductivity and viscosity of oil based nanofluids. Appl Therm Eng. 2016;101:362–72.
Ku B-C, Han Y-C, Lee J-E, Lee J-K, Park S-H, Hwang Y-J. Tribological effects of fullerene (C 60) nanoparticles added in mineral lubricants according to its viscosity. Int J Precis Eng Manuf. 2010;11(4):607–11.
Ilyas SU, Pendyala R, Narahari M, Susin L. Stability, rheology and thermal analysis of functionalized alumina-thermal oil-based nanofluids for advanced cooling systems. Energy Convers Manag. 2017;142:215–29.
Devendiran DK, Amirtham VA. A review on preparation, characterization, properties and applications of nanofluids. Renew Sustain Energy Rev. 2016;60:21–40.
Ranjbarzadeh R, Isfahani AM, Afrand M, Karimipour A, Hojaji M. An experimental study on heat transfer and pressure drop of water/graphene oxide nanofluid in a copper tube under air cross-flow: applicable as a heat exchanger. Appl Therm Eng. 2017;125:69–79.
Sharif M, Azmi W, Redhwan A, Mamat R. Investigation of thermal conductivity and viscosity of Al2O3/PAG nanolubricant for application in automotive air conditioning system. Int J Refrig. 2016;70:93–102.
Koca HD, Doganay S, Turgut A, Tavman IH, Saidur R, Mahbubul IM. Effect of particle size on the viscosity of nanofluids: a review. Renew Sustain Energy Rev. 2018;82:1664–74.
Hamzah MH, Sidik NA, Ken TL, Mamat R, Najafi G. Factors affecting the performance of hybrid nanofluids: a comprehensive review. Int J Heat Mass Transf. 2017;115:630.
Handbook-Fundamentals AS. American society of Heating. Refrigerating and Air-Conditioning Engineers. 2009.
Izadi F, Ranjbarzadeh R, Kalbasi R, Afrand M. A new experimental correlation for non-Newtonian behavior of COOH-DWCNTs/antifreeze nanofluid. Phys E. 2018;1(98):83–9.
Ranjbarzadeh R, Karimipour A, Afrand M, Isfahani AH, Shirneshan A. Empirical analysis of heat transfer and friction factor of water/graphene oxide nanofluid flow in turbulent regime through an isothermal pipe. Appl Therm Eng. 2017;5(126):538–47.
Ilyas SU, Pendyala R, Marneni N. Stability of nanofluids. In engineering applications of nanotechnology. Cham: Springer; 2017. p. 1–33.
Hwang YJ, Lee JK, Lee CH, Jung YM, Cheong SI, Lee CG, Ku BC, Jang SP. Stability and thermal conductivity characteristics of nanofluids. Thermochim Acta. 2007;455(1–2):70–4.
Nasiri A, Shariaty-Niasar M, Rashidi AM, Khodafarin R. Effect of CNT structures on thermal conductivity and stability of nanofluid. Int J Heat Mass Transf. 2012;55(5–6):1529–35.
Koca HD, Doganay S, Turgut A. Thermal characteristics and performance of Ag-water nanofluid: application to natural circulation loops. Energy Convers Manag. 2017;1(135):9–20.
Ranjbarzadeh R, Akhgar A, Musivand S, Afrand M. Effects of graphene oxide-silicon oxide hybrid nanomaterials on rheological behavior of water at various time durations and temperatures: synthesis, preparation and stability. Powder Technol. 2018;15(335):375–87.
Wang RT, Wang JC. Intelligent dimensional and thermal performance analysis of Al2O3 nanofluid. Energy Convers Manag. 2017;15(138):686–97.
Acknowledgements
This research is partially supported by Youth Education Research Program of Fujian (JAT170932).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Geng, Y., Al-Rashed, A.A.A.A., Mahmoudi, B. et al. Characterization of the nanoparticles, the stability analysis and the evaluation of a new hybrid nano-oil thermal conductivity. J Therm Anal Calorim 139, 1553–1564 (2020). https://doi.org/10.1007/s10973-019-08434-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08434-y