Abstract
In the present study, TEIA bioresin was blended with the diglycidyl ether bisphenol A (DGEBA) epoxy resin in different ratios (i.e. 10, 20, 30, 40 mass%), cured with methylhexahydrophthalic anhydride curing agent in the presence of 2-methylimidazole catalyst. The optimized composition of DGEBA and TEIA bioresin blends system was employed as an adhesive strength. The adhesive strength of the TEIA-modified DGEBA epoxy resin blend system was increased from 4.14 to 6.31 MPa on an aluminium substrate compared to the DGEBA epoxy resin. The curing kinetics of non-isothermal, DGEBA epoxy resin and its bio-based blend systems were investigated employing differential scanning calorimetry. An increase in the peak temperature and reduction in a heat of curing as well as activation energy in DGEBA epoxy resin were observed with the addition of TEIA bioresin content. The activation energy (Ea) of the DGEBA resin and their bio-based blend system were obtained from Kissinger and Flynn–Wall–Ozawa methods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Owing to the increasing of environmental, uncertainty of finite petrochemical feedstock as economic concern has spurred much research in developing bio-based epoxy resin [1, 2]. However, a further advantage of the bio-based building blocks compounds exhibits a new structure feature and demonstrates an unprecedented property compared to the petroleum feedstock. Until now, bio-based epoxy resin derived from natural resources such as eugenol [3,4,5], rosin acid [6,7,8], vegetable oil [9,10,11,12,13] and isosorbide [14,15,16] earlier have been reported.
Diglycidyl ether bisphenol A (DGEBA) epoxy resin is one of the most extensively employed epoxy resins because of its higher chemical resistance, good thermal properties and high adhesion strength to various substrates. These properties bestow various applications of DGEBA epoxy resin in different fields such as coatings, adhesive, paint and other engineering applications [17]. The properties of DGEBA epoxy resin are decided mostly via their chemical composition and their curing conditions. Conversely, cured DGEBA epoxy resin demonstrates a lower toughness due to the existence of rigid aromatic ring structure in the backbone. The toughness of the brittle DGEBA epoxy resin is enhanced by the incorporation of elastomers, thermosetting and thermoplastic polymers. Because of the molecular structure of DGEBA, it can be easily modified for various applications considering its versatile properties [18].
The curing kinetics, process of the thermosetting polymers by using cyclic anhydride as a curing agent have been investigated by numerous researchers [19,20,21] and bestows sufficient detail of the process development and to improving the quality of the final product. The properties of the cured DGEBA system depend on the character and functionality of the DGEBA epoxy resin and curing agent. Commonly, amine and carboxylic acid anhydride are utilized as curing agent for DGEBA epoxy resins. Therefore, amine-cured DGEBA exhibits poorly cross-linked network, while anhydride-cured bio-based epoxies are more rigid and have a higher glass transition temperature (Tg) and less curing shrinkage [22,23,24]. Further, the anhydride curing agent has a number of benefits such as low viscosity, readily miscible with DGEBA epoxy resin, very low exotherm, extremely long pot life and less hazardous compared to the amine curing agent. Extensively, the anhydride-cured product has good electrical, chemical and mechanical properties.
In the current research, several researchers studied the effect of various anhydride-based curing agents and catalysts on properties of cured epoxidized plant oils and their blends [25,26,27]. Amongst the anhydride-based curing agents, methylhexahydrophthalic anhydride (MHHPA) is a low viscous liquid at room temperature and highly preferable due to its properties such as high reactivity, short molecular chain and rigid structure.
Methylhexahydrophthalic anhydride (MHHPA)-cured epoxidized linseed oil (ELO) catalyzed by 2-methylimidazole exhibits good thermal stability reported by Pin et al. [28]. MHHPA is a high-temperature curing agent, and the addition of 2-methylimidazole (2-MI) as a catalyst balances the pot life and drastically reduced the curing temperature of the reaction, and improves the mechanical, thermal properties of the DGEBA and its blend system since it acts as a more active initiator for faster cross-linking [29]. Furthermore, 2-MI was more effective than 1-methylimidazole, tertiary amines, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), or dimethylaminopyridine in initiating the polymerization of epoxidized soybean oil and DGEBA epoxy resin, and the resulting network revealed a higher anhydride conversion.
Patel et al. [30] investigated the effect of anhydride structure on the curing kinetics, and the results exhibit higher curing reactivity of hexahydrophthalic anhydride (HHPA) compared to phthalic anhydride (PA). Nowadays, many researchers reported the curing kinetics of DGEBA epoxy resin and bioresin. Differential scanning calorimetry (DSC) is one of the extensively utilized techniques to investigate the curing kinetics of DGEBA epoxy resin in isothermal or dynamic mode using various kinetic models. The Kinetics Committee of the International Confederation for Thermal Analysis and Calorimetry (ICTAC) recommendations were followed for collection of kinetic data and performing kinetic computations [31].
Current investigation based on the development of TEIA network from the decarboxylation, and epoxidation of itaconic acid (IA) and prepared DGEBA/TEIA blend system were cured with MHHPA in the presence of a 2-MI catalyst, which is not reported until now. Curing kinetics and mechanical properties of DGEBA epoxy and its bio-based blend system were investigated. The activation energy (Ea) of the DGEBA epoxy resin and DGEBA/TEIA blend systems was determined by using Kissinger and Flynn–Wall–Ozawa methods (FWO). The adhesive properties such as lap shear strength (LSS) of the DGEBA epoxy resin and its bio-based blend system were investigated with the universal testing machine (UTM).
Experimental
Raw materials
Araldite GY 250 (epoxide equivalent weight (EEW) of 183–189 g eq−1), diglycidyl ether of bisphenol-A (DGEBA) epoxy resin were purchased from Huntsman International (India) Pvt. Ltd., Mumbai. Methylhexahydrophthalic anhydride (MHHPA > 99%, equivalent weight 168), allyl bromide and 2-methylimidazole (> 99% 2-MI) and itaconic acid (IA > 99%) (M.wt.130.10) were obtained from TCI Chemicals (India) Pvt. Ltd. All other chemicals and materials were employed as received. The structures of the used chemicals are shown in Fig. 1.
Synthesis of itaconic acid based bioresin
Step I: Dehydrobromination of itaconic acid and allyl bromide
In a 500 mL three-necked round-bottom flask equipped with a magnetic stirrer, a thermometer and reflux condenser was filled with itaconic acid (IA) (25 g), allyl bromide (93.5 g), acetone (250 g), N,N-dimethylformamide (50 g) and K2CO3 (58.5 g). The solution was stirred at room temperature for 15 min. After the mixture was heated at 60 °C and reflux for 12 h, the product was formed after removing acetone and un-reacted allyl bromide. The product was diluted with dichloromethane and washed with deionized water, and produced modified itaconic acid (MIA) as amber-like colour of 97% yield.
Step II: Epoxidation of modified itaconic acid (MIA) with m-CPBA
Further, the modified itaconic acid (MIA) (35 g), dichloromethane (153.6 g), and m-chloroperoxybenzoic acid (m-CPBA, 153.8 g) were charged into a 500 mL three-necked round-bottom flask equipped with a magnetic stirrer, a thermometer and reflux condenser and maintained the temperature at 40 °C for 4 days. After cooling, the solution was filtered and washed with 10% aqueous solution of sodium sulphite (Na2SO3) followed by distilled water. The bio-based epoxy resin from itaconic acid (TEIA) was obtained after removing dichloromethane (DCM), and the yield was 85%. The epoxy value and an epoxy equivalent weight (EEW) of bio-based epoxy resin (TEIA) were investigated by the hydrochloric acid-acetone method and are found to be 1.02 and 98 g eq−1, respectively [32, 33]. The reaction scheme is shown in Fig. 2.
Synthesis of bio-based DGEBA blend adhesive network
DGEBA/TEIA blend adhesives network were prepared by blending the two epoxy resins of various weight ratios of DGEBA/TEIA shown in (100/0, 90/10, 80/20, 70/30, 60/40). The DGEBA and TEIA were mixed using a digital mechanical stirrer followed by ultrasonication for 30 min. Then, the mixture was kept in vacuum oven at 70 °C for 20 min to remove air bubbles. Subsequently, the stoichiometric amount of MHHPA and 2-MI catalyst was added to the DGEBA/TEIA resin mixture as per the epoxy equivalent weight (EEW) of the resin mixture and continuously stirred for homogenization. Then, it was degassed in a vacuum oven at 60 °C for 15 min and poured into a preheated mould sprayed with silicon spray. The samples were cured at 160 °C for 2 h to obtain a completely cured resin. All the specimens were cured under the same temperature condition.
Characterization techniques
FT-IR and 1H NMR analysis
Fourier-transform infrared (FT-IR) spectra of IA, MIA and TEIA resin were recorded using FT-IR spectrometer (Thermo Scientific, Nicolet 6700, USA). Each spectrum was obtained by co-adding 64 consecutive scans with a resolution of 4 cm−1 within the range of 500–4000 cm−1. 1H-NMR spectra were measured on a Bruker AVANCE 400 MHz spectrometer with CDCl3 as a solvent.
Lap shear strength
The adhesive behaviour of DGEBA and DGEBA/TEIA blends was determined by lap shear strength, joint test (LSS) using aluminium substrates according to ASTM-D 1002. The adhesion test specimen size is 25.4 mm (the width of the aluminium) × 12.7 mm (length of aluminium overlap) shown in Fig. 3 and cleaned using acetone prior to use. The two aluminium specimens were attached by DGEBA and DGEBA/TEIA blend systems and cured at 160 °C for 2 h, in an oven. The adhesive thickness was controlled at about 0.29 mm by film applicator. The bonded test specimens were pulled apart at the room temperature with a cross-head speed of 1.27 mm min−1 on an Instron 3382, UK Universal Testing Machine. The total six specimen replicates were tested, and the average value has been reported.
Non-isothermal curing kinetics
The DSC measurement was taken on a (Q20, M/s TA Instrument, USA) to investigate the non-isothermal curing behaviour of DGEBA and its blends. The 5–8 mg samples were placed into the aluminium pan from 30 to 250 °C temperatures range at a various heating rate of 5, 10 and 15 °C min−1 under a constant flow of nitrogen of 50 mL min−1.
Theory of curing kinetics
The curing kinetics of DGEBA epoxy resin and their blends were measured from DSC analysis assuming that the degree of consumption of the reactive groups is proportional to the amount of heat evolved. The degree of conversion (α) of the curing reaction and the reaction rate as a function of time (t) can be measured by using Eqs. (1) and (2) [34].
where α is degree of conversion, \(\Delta H_{\text{t}}\) is the enthalpy of the reaction in certain temperature and time t. \(\Delta H_{{\text{total}}}\) is the total enthalpy of the reaction completely cured sample. The dα/dt and dH(t)/dt represent the rate of curing reaction and heat flow rate, respectively. The enthalpy of the curing reaction DGEBA epoxy resin and DGEBA/30%TEIA blend system up to the time (t) can be determined by integration of the heat flow and over the time (t) of the exothermic peak.
The kinetic analysis of non-isothermal curing process was employed by using two kinetic methods, the Kissinger and Flynn–Wall–Ozawa as expressed in Eqs. (3) and (4) [35].
Kissinger method:
where Tp represents the maximum peak temperature, β is the heating rates, Ea is the activation energy of the system. R is the gas constant (8.314 J mol−1 K−1), and A is the exponential factor. The activation energy of the DGEBA and DGEBA/30%TEIA is calculated from the plot of ln(β/T 2p ) versus 1/Tp, with the correlation of experimental data depicted in Fig. 8a and the activation energy data is shown in Table 2 [36].
Flynn–Wall–Ozawa method (FWO):
All other parameters are described earlier. Ea of each individual degree of cure was measured by the plot of ln(β) versus 1000/Tp.
Results and discussion
FT-IR analysis
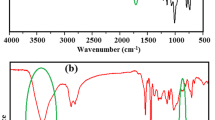
FT-IR spectra of itaconic acid (IA), modified itaconic acid (MIA) and bio-based (TEIA) are depicted in Fig. 4a–c, respectively. In Fig. 4a, the observed broadband peak at 3500 cm−1 is attributed to the O–H stretching vibration of carboxylic acid and peak at 2916 cm−1 corresponds to –CH2 [37]. The characteristic peaks that appeared at 1705 cm−1 and 1623 cm−1 are associated with the C=O stretching and C=C stretching of unsaturated carboxylic acid, respectively [38].
The appeared peak at 3083 cm−1 and 2944 cm−1 for MIA shown in Fig. 4b can be ascribed to =CH and –CH stretching. The intense peak at 1727 cm−1 is attributed to C=O stretching of ester in MIA. The O–C–C stretching vibration of ester group appeared at 1168 cm−1. After modification, the carboxylic group converts into ester group and the two peaks at 1447 cm−1 and 1375 cm−1 correspond to the –CH2, and –CH bending modes. The characteristic absorption peaks of the C=CH2 stretching from the allyl group appeared at 1647 cm−1, and bending vibration of C–H out of plane was observed at 986–920 cm−1 and 815 cm−1 assigned to the allyl group. The characteristic peak of at 906 cm−1 is attributed to the stretching vibration of the oxirane group in TEIA bioresin [39].
1H NMR analysis
1H NMR spectra of MIA Fig. 5a demonstrate the distinguished peaks of protons P1, P2, P13, P14 and the tertiary protons P3, P12 on the C=C bond of CH2=CH–CH2– backbone at 5.1–5.24 and 5.75–5.84 ppm, respectively [33, 40]. The peak of the P8, P9 of –CH2=C appeared at 5.6 and 6.3 ppm, respectively. The peak 4.5–4.6 ppm represents the characteristic peak of the protons P4, P5, P10, P11 on the CH2–O–(CO)–backbone, whereas the peaks of the protons P6, P7 on C-atom between CH2–O–(CO)– and ester appeared at 3.4 ppm.
In the case of 1H NMR spectra of TEIA bioresin depicted in Fig. 4b, all the protons above 5 ppm disappeared. The characteristic peak of proton P1, P2, P3, P12, P13, P14 on glycidyl epoxides CH2–(O)–CH–, P8, P9 on pendent epoxide CH2–(O)–C–, proton P6, P7 on the C-atom present between the ester bond –CH2–COO– and pendent epoxide were observed at 2.68–3.45 ppm. The peaks of other protons P4, P5, P10, P11 on the C-atom next to the glycidyl epoxide appeared at 3.92–.52 ppm [40].
Adhesive properties of DGEBA and its blend
DGEBA epoxy resins are extensively used as adhesives because of their excellent properties. Therefore, it was required to investigate the adhesive properties of the DGEBA and DGEBA/TEIA blend system. As an efficient demonstration, the adhesion strength of the DGEBA and its bio-based blend systems for aluminium were studied in this work. As depicted in Fig. 6, the adhesion strength of DGEBA epoxy resin to aluminium was about 4.3 MPa, much lower than that of DGEBA/TEIA blends. The adhesive strength of the DGEBA epoxy system was correlated with the brittle nature reported by Ma et al. [40] and Okamatsu et al. [41]. The increased brittleness of the DGEBA system lowers the adhesion strength. After the incorporation of TEIA, its adhesion strength to aluminium was increased up 30 mass%, reduced the brittle nature of DGEBA and absorbed the sufficient amount of energy.
However, the adhesive strength decreased with 40 mass% TEIA content in the system because of the plasticization effect of a pendant epoxide group of TEIA which enhanced the flexibility and freedom for the movements of the molecules in the DGEBA/40%TEIA blend system, mostly owing to low cross-linking density and plasticizing effect of TEIA. This effect can be attributed to the lower reactivity of epoxy group in TEIA compared to the DGEBA epoxy resin. On the other hand, the amount of TEIA reacted with DGEBA epoxy resin and remained un-reacted parts in the continuous phase, which in reduces in the adhesive strength of DGEBA/40%TEIA blend system.
Study of curing behaviour of DGEBA epoxy resin and their bio-based blend systems
Non-isothermal curing kinetics of DGEBA and DGEBA/30%TEIA blends was carried out with MHHPA as curing agent catalyzed by 2-MI at 5,10, 15 °C min−1 heating rate and are shown in Fig. 7. The total heat of reaction, ∆Hcure, onset cure temperature (Tonset) of all the systems are summarized in Table 1. As anhydride is a high-temperature curing agent, therefore imidazole-type catalysts are frequently used to reduce the curing temperature of the reaction earlier finding [42]. Figure 7 demonstrated a single exothermic peak that corresponds to the ring opening reaction of the oxirane group of DGEBA epoxy resin with MHHPA group. Table 1 that indicates the DGEBA systems demonstrated lower Tonset temperature at a lower heating rate and higher heat of reaction compared the its blend system which is mainly due to the lower cross-linking.
In case of DGEBA/30%TEIA blend system exhibit higher Tonset and heat of reaction at 10, 15 °C heating rate were observed as compared to the DGEBA epoxy resin formulation. This is mainly ascribed to the less reactive internal pendent epoxy groups of TEIA. However, the Tonset enhanced from 81 to 84 °C and demonstrate that the rigid aromatic group of the DGEBA epoxy resin replaced to the flexible aliphatic molecules of the TEIA bioresin.
Table 1 also reveals that the heat of reaction obtained for the DGEBA, at 10, 15 °C heating rates is higher as compared to the DGEBA/30%TEIA system. This is demonstrating that the mixture of the DGEBA/30%TEIA blend system required extra heat of the curing process.
The curing kinetics parameters of DGEBA epoxy resin and DGEBA/30%TEIA systems were explored by non-isothermal DSC analysis at different heating rates: 5, 10, 15 °C min−1 depicted in Table 2. All the systems exhibited only single exothermic peak throughout the entire experiment. Figure 7 and Table 2 indicated that the peak temperature (Tp) of DGEBA and its blend systems shifted to the higher-temperature region with the increase in heating rate. This is attributed to the higher heating rate which does not allow enough time for the reactive groups to react in the curing process [43, 44].
The Tp of DGEBA (125.71 °C) was slightly lower than that of DGEBA/30%TEIA blend system (130.98 °C) at a heating rate 5 °C min−1, which indicates that the reactivity of the DGEBA epoxy resin is slightly decreased with addition of 30 mass% TEIA due to the pendent epoxide group and steric hindrance induced by intermolecular interaction in the DGEBA/30%TEIA blend system [7].
Calculation of activation energy (E a)
The Ea of the 2-MI catalyzed DGEBA epoxy resin, and DGEBA/30%TEIA blend systems was obtained from the slope plotting ln(β/T 2p ) versus (1/Tp) and ln(β) versus 1000/Tp by employing Kissinger and FWO method, Eqs. (3) and (4), respectively. The Ea of all the systems are shown in Fig. 8, and the data are summarized in Table 2.
Addition of TEIA bioresin into the DGEBA matrix enhanced the activation energy (Ea) due to the presence of an internal pendent epoxide group of blend system and increased number of oxirane groups. The Ea of the DGEBA/30%TEIA system was higher than DGEBA epoxy resin owing to the existence of a pendent epoxide group of the TEIA bioresin and decreased reactivity of the curing system in the curing time.
The higher Ea indicates the lower reactivity of the systems. The reactivity of the cured DGEBA system is slightly lower compared to cured DGEBA/30%TEIA blend system, due to the higher reactivity of the epoxide group in the chain ends. FWO method demonstrates the higher activation energy of all the systems than Kissinger method. A similar result was also been reported by earlier studies [45, 46]. The results indicate that the curing behaviour of the bio-based system is in good agreement with Kissinger method. Conversely, FWO method required more energy to achieve the complete curing shown in Fig. 8.
Further, the activation energy (Ea) at various degrees of conversions (α) was obtained from the slope of the linear plots of ln(β/T 2p ) versus (1/Tp). The variation of the Ea at different relative α values of DGEBA epoxy resin and DGEBA/30%TEIA blend system by using Eqs. (1) and (3) is shown in Fig. 9. The activation energy of DGEBA epoxy resin at every temperature was much lower than DGEBA/30%TEIA blend system owing to the more reactive epoxide groups present in the DGEBA epoxy resin.
In the case of DGEBA system, the activation energy of unmodified DGEBA epoxy resin steadily increased up to α = 0.1–0.7 and then declined at the conversion range 0.8–0.9. This can be explained via the 2-methylimidazole addition to the DGEBA system as a catalyst reacting with the epoxy group to form alkoxide and additionally reacting with the anhydride group to form a carboxylate anion. This carboxylate anion participated in the curing reaction, and as a result, it reduced the activation energy of reaction [47].
The DGEBA/30%TEIA blend system demonstrates slightly higher activation energy (Ea) than DGEBA epoxy resin at every conversion. In the initial stage of curing, the Ea was lower in both TEIA and DGEBA epoxy resins. This is ascribed to the reduced viscosity in the blend system and allowed better compatibility of the resin and anhydride curing agent loading to improved diffusion of curing agent into less viscous resin blend system and increase the rate of curing reaction as earlier reported by Sbirrazzuoli, et al. [48]. The Ea at the lower temperature was less due to the faster reaction of DGEBA anhydride curing agent reaction. But at the end of the curing process of blend, the cross-linked density becomes higher and the movement of the chain segment restricted by the gelation which makes the reactive sites more difficult to react [45].
In the next step, Kissinger plots were plotted by using ln (β/T 2p ) versus 100/Tp in the conversion range 0.1–0.9 with a step of 0.1. Figure 10 demonstrates the change in activation energy of DGEBA epoxy resin and DGEBA/30%TEIA systems with a degree of curing (α) from the Kissinger plots.
The curing reaction mechanism of DGEBA and its blend with the MHHPA curing agent in the presence of a 2-MI catalyst depicted in two steps such as initiation and propagation are shown in Scheme 1. The use of 2-MI catalyst reduced the Ea and enhanced the propagation rate. In the initiation step of curing, the ring opening of the anhydride-curing agent (MHHPA) via the 2-MI catalyst involves in the SN2 reaction to form zwitterions. Additionally, the zwitterions react with an oxirane group in the DGEBA ring, formed an ester linkage and produced alkoxide anion species. In the second steps, alkoxide anion reacts with another molecule of the MHHPA curing agent resulting in a carboxylate anion [49,50,51].
This reaction continued until all the anhydrides were exhausted. Finally, the carboxylate anions react with the DGEBA epoxy resin and an epoxy monomer to generate the reaction intermediate product; and after curing, the polyesterification in the DGEBA/MHHPA, DGEBA/30%TEIA/MHHPA mixture is completed and forms a polyester-type linkage Tan et al. [52, 53] and Tao et al. [42] also reported similar results.
The activation energy ranged from α = 0.7–0.8 almost constantly. Figure 9 shows that activation energy at higher conversions (0.9) slightly increased due to higher cross-linked density at the end of the curing reaction, and gelation restricts the mobility of chains which makes the reactive species more difficult to react. The DGEBA/30%TEIA blend required more energy for the reaction.
Conversion and reaction kinetics
The degree of conversion (α) versus temperature curves of DGEBA epoxy resin and DGEBA/30%TEIA systems at various heating rates is depicted in Fig. 11a, b. The α values were obtained by the integration of the exothermic peak corresponding to Eq. (2). The degree of reaction was normalized within (0 and 1) using the total reaction heat of DGEBA and DGEBA/30%TEIA blend systems at same heating rates. The result shows that the curing behaviour, the degree of conversion of DGEBA epoxy and DGEBA/30%TEIA blends occur at lower temperatures [53].
The maximum conversion (αp) of the DGEBA epoxy and DGEBA/30%TEIA blends curing at DSC peak is shown in Fig. 12. The αM values are independent of the heating rate of this reaction. The \(\alpha_{P}^{\infty } \ne 0.632\) characteristic values are used for determining the kinetic model.
Conclusions
In this study, trifunctional bio-based TEIA resin was successfully synthesized from the itaconic acid and a blend with DGEBA epoxy resin. The adhesive properties of the DGEBA/TEIA blends system were enhanced with the addition of TEIA bioresin up to 30 mass%. Non-isothermal DSC measurement was employed to determine the curing behaviour of the DGEBA epoxy resin and their bio-based blend systems. By the addition of the 30 mass% TEIA bioresin, the heat of reaction was slightly decreased due to the pendent epoxide group of the TEIA bioresin. The activation energy (Ea) determined by using Kissinger equation was found to be lower than the Ea determined from FWO equation. This result indicated that the Kissinger equation is well fitted for the DGEBA/TEIA blend systems.
References
Kumar S, Samal SK, Mohanty S, Nayak SK. Epoxidized soybean oil-based epoxy blend cured with anhydride-based cross-linker: thermal and mechanical characterization. Ind Eng Chem Res. 2017;56:687–98.
Kumar S, Krishnan S, Samal SK, Mohanty S, Nayak SK. Itaconic acid used as a versatile building block for the synthesis of renewable resource based resins and polyesters for future prospective: a review. Polym Int. 2017;66:1349–63.
Qin J, Liu H, Zhang P, Wolcott M, Zhang J. Use of eugenol and rosin as feedstocks for biobased epoxy resins and study of curing and performance properties. Polym Int. 2004;63:760–5.
Wan J, Gan B, Li C, Molina-Aldareguia J, Kalali EN, Wang X, Wang DY. A sustainable, eugenol-derived epoxy resin with high biobased content, modulus, hardness and low flammability: synthesis, curing kinetics and structure–property relationship. Chem Eng J. 2016;284:1080–93.
Wan J, Zhao J, Gan B, Li C, Molina-Aldareguia J, Zhao Y, Wang DY. Ultrastiff biobased epoxy resin with high T g and low permittivity: from synthesis to Properties. ACS Sustain Chem Eng. 2016;4:2869–80.
Liu X, Zhang J. High-performance biobased epoxy derived from rosin. Polym Int. 2010;59:607–9.
Wang H, Liu B, Liu X, Zhang J, Xian M. Synthesis of biobased epoxy and curing agents using rosin and the study of cure reactions. Green Chem. 2008;10:1190–6.
Atta AM, Mansour R, Abdou MI, El-Sayed AM. Synthesis and characterization of tetra-functional epoxy resins from rosin. J Polym Res. 2005;12:127–38.
Jin FL, Park SJ. Thermomechanical behavior of epoxy resins modified with epoxidized vegetable oils. Polym Int. 2008;57:577–83.
Ratna D. Mechanical properties and morphology of epoxidized soyabean-oil-modified epoxy resin. Polym Int. 2001;50:179–84.
Sudha GS, Kalita H, Mohanty S, Nayak SK. Biobased epoxy blends from epoxidized castor oil: effect on mechanical, thermal, and morphological properties. Macromol Res. 2017;25:420–30.
Xia Y, Larock RC. Vegetable oil-based polymeric materials: synthesis, properties, and applications. Green Chem. 2010;12:1893–909.
Sahoo SK, Mohanty S, Nayak SK. Synthesis and characterization of bio-based epoxy blends from renewable resource based epoxidized soybean oil as reactive diluents. Chin J Polym Sci. 2015;33:137–52.
Lukaszczyk J, Janicki B, Kaczmarek M. Synthesis and properties of isosorbide based epoxy resin. Eur Polym J. 2011;47:1601–6.
Chrysanthos M, Galy J, Pascault JP. Preparation and properties of bio-based epoxy networks derived from isosorbide diglycidyl ether. Polymer. 2011;52:3611–20.
Hong J, Radojcic D, Ionescu M, Petrovic ZS, Eastwood E. Advanced materials from corn: isosorbide-based epoxy resins. Polym Chem. 2014;5:5360–8.
Kumar S, Samal SK, Mohanty S, Nayak SK. Recent development of bio-based epoxy resins: a review. Polym Plast Technol Eng. 2018;57:133–55.
Mohan P. A critical review: the modification, properties, and applications of epoxy resins. Polym Plast Technol Eng. 2013;52:107–25.
Chen Y, Xi Z, Zha L. Curing kinetics of bio-based epoxy resin based on epoxidized soybean oil and green curing agent. AIChE J. 2017;63:147–53.
Hwang SH, Jung JC. Curing kinetics and thermal properties of diglycidylether of bisphenol A with various diamines. J Appl Polym Sci. 2001;81:279–84.
Ampudia J, Larrauri E, Gil EM, Rodriguez M, Leon LM. Thermal scanning rheometric analysis of curing kinetic of an epoxy resin. I. An anhydride as curing agent. J Appl Polym Sci. 1999;71:1239–45.
Miyagawa H, Mohanty AK, Misra M, Drzal LT. Thermo-physical and impact properties of epoxy containing epoxidized linseed oil, 1. Macromol Mater Eng. 2004;289:629–35.
Miyagawa H, Mohanty AK, Misra M, Drzal LT. Thermo-physical and impact properties of epoxy containing epoxidized linseed oil, 2. Macromol Mater Eng. 2004;289:636–41.
Espinoza-Perez JD, Nerenz BA, Haagenson DM, Chen Z, Ulven CA, Wiesenborn DP. Comparison of curing agents for epoxidized vegetable oils applied to composites. Polym Compos. 2011;32:1806–16.
Altuna FI, Esposito LH, Ruseckaite RA, Stefani PM. Thermal and mechanical properties of anhydride-cured epoxy resins with different contents of biobased epoxidized soybean oil. J Appl Polym Sci. 2011;120:789–98.
Chen Y, Yang L, Wu J, Ma L, Finlow DE, Lin S, Song K. Thermal and mechanical properties of epoxy resin toughened with epoxidized soybean oil. J Therm Anal Calorim. 2013;113:939–45.
Boquillon N, Fringant C. Polymer networks derived from curing of epoxidised linseed oil: influence of different catalysts and anhydride hardeners. Polymer. 2000;41:8603–13.
Pin JM, Sbirrazzuoli N, Mija A. From epoxidized linseed oil to bioresin: an overall approach of epoxy/anhydride cross-linking. Chemsuschem. 2015;8:1232–43.
Supanchaiyamat N, Shuttleworth PS, Hunt AJ, Clark JH, Matharu AS. Thermosetting resin based on epoxidised linseed oil and bio-derived crosslinker. Green Chem. 2012;14:1759–65.
Patel SR, Patel RG. Effect of the anhydride structure on the curing kinetics and thermal stability of tetrafunctional epoxy resin. Thermochim Acta. 1992;202:97–104.
Vyazovkin S, Burnham AK, Criado JM, Perez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
He Z, Wang Y, Zhao T, Ye Z, Huang H. Ultrasonication-assisted rapid determination of epoxide values in polymer mixtures containing epoxy resin. Anal Methods. 2014;6:4257–61.
Kumar S, Samal SK, Mohanty S, Nayak SK. Synthesis and characterization of itaconic-based epoxy resins. Polym Adv Technol. 2018;29:160–70.
Kumar S, Samal SK, Mohanty S, Nayak SK. Study of curing kinetics of anhydride cured petroleum-based (DGEBA) epoxy resin and renewable resource based epoxidized soybean oil (ESO) systems catalyzed by 2-methylimidazole. Thermochim Acta. 2017;654:112–20.
Vyazovkin S, Sbirrazzuoli N. Isoconversional kinetic analysis of thermally stimulated processes in polymers. Macromol Rapid Comm. 2006;27:1515–32.
Natarajan M, Murugavel SC. Cure kinetics of bio-based epoxy resin developed from epoxidized cardanol–formaldehyde and diglycidyl ether of bisphenol–A networks. J Therm Anal Calorim. 2016;125:387–96.
Dobic SN, Filipovic JM, Tomic SL. Synthesis and characterization of poly (2-hydroxyethyl methacrylate/itaconic acid/poly (ethylene glycol) dimethacrylate) hydrogels. Chem Eng J. 2012;179:372–80.
Dai J, Ma S, Zhu L, Wang S, Yang L, Song Z, Zhu J. UV-thermal dual cured anti-bacterial thiol-ene networks with superior performance from renewable resources. Polymer. 2017;108:215–22.
Ma S, Liu X, Fan L, Jiang Y, Cao L, Tang Z, Zhu J. Synthesis and properties of a bio-based epoxy resin with high epoxy value and low viscosity. Chemsuschem. 2014;7:555–62.
Ma S, Liu X, Jiang Y, Tang Z, Zhang C, Zhu J. Bio-based epoxy resin from itaconic acid and its thermosets cured with anhydride and comonomers. Green Chem. 2013;15:245–54.
Okamatsu T, Ochi M. Effect on the toughness and adhesion properties of epoxy resin modified with silyl-crosslinked urethane microsphere. Polymer. 2002;43:721–30.
Tao Q, Su L, Frost RL, He H, Theng BK. Effect of functionalized kaolinite on the curing kinetics of cycloaliphatic epoxy/anhydride system. Appl Clay Sci. 2014;95:317–22.
Zhang Y, Ferdosian F, Yuan Z, Xu CC. Sustainable glucose-based phenolic resin and its curing with a DGEBA epoxy resin. Taiwan Inst Chem Eng. 2017;71:381–7.
Ferdosian F, Zhang Y, Yuan Z, Anderson M, Xu CC. Curing kinetics and mechanical properties of bio-based epoxy composites comprising lignin-based epoxy resins. Eur Polym J. 2016;82:153–65.
El-Thaher N, Mekonnen T, Mussone P, Bressler D, Choi P. Nonisothermal DSC study of epoxy resins cured with hydrolyzed specified risk material. Ind Eng Chem Res. 2013;52:8189–99.
Huang K, Zhang P, Zhang J, Li S, Li M, Xia J, Zhou Y. Preparation of biobased epoxies using tung oil fatty acid-derived C21 diacid and C22 triacid and study of epoxy properties. Green Chem. 2013;15:2466–75.
Yang T, Zhang C, Zhang J, Cheng J. The influence of tertiary amine accelerators on the curing behaviors of epoxy/anhydride systems. Thermochim Acta. 2014;577:11–6.
Sbirrazzuoli N, Mititelu-Mija A, Vincent L, Alzina C. Isoconversional kinetic analysis of stoichiometric and off-stoichiometric epoxy-amine cures. Thermochim Acta. 2006;447:167–77.
Matejka L, Lovy J, Pokorny S, Bouchal K, Dusek K. Curing epoxy resins with anhydrides: model reactions and reaction mechanism. J Polym Sci. A. 1983;21:2873–85.
Leukel J, Burchard W, Kruger RP, Much H, Schulz G. Mechanism of the anionic copolymerization of anhydride-cured epoxies–analyzed by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS). Macromol Rapid Commun. 1996;17:359–66.
Ham YR, Kim SH, Shin YJ, Lee DH, Yang M, Min JH, Shin JS. A comparison of some imidazoles in the curing of epoxy resin. J Ind Eng Chem. 2010;16:556–9.
Tan SG, Ahmad Z, Chow WS. Relationships of cure kinetics and processing for epoxidized soybean oil bio-thermoset. Ind Crop Prod. 2013;43:378–85.
Tan SG, Chow WS. Thermal properties, curing characteristics and water absorption of soybean oil-based thermoset. eXPRESS Polym Lett. 2011;5:480–92.
Acknowledgements
The authors would you like to thank the Department of Chemical and Petrochemicals, Government of India, for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, S., Samal, S.K., Mohanty, S. et al. Curing kinetics of bio-based epoxy resin-toughened DGEBA epoxy resin blend. J Therm Anal Calorim 137, 1567–1578 (2019). https://doi.org/10.1007/s10973-019-08080-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08080-4