Abstract
In the present study, the impacts of nanoparticles volume concentration and temperature on the thermophysical properties and the rheological behavior of water-based CuO/MgO/TiO2 ternary hybrid nanofluids were elucidated. Five types of CuO/MgO/TiO2 aqueous THNFs (ternary hybrid nanofluids) including A (33.4 mass% CuO/33.3 mass% MgO/33.3 mass% TiO2), B (50 mass% CuO/25 mass% MgO/25 mass% TiO2), C (60 mass% CuO/30 mass% MgO/10 mass% TiO2), D (25 mass% CuO/50 mass% MgO/25 mass% TiO2) and E (25 mass% CuO/25 mass% MgO/50 mass% TiO2) were fabricated. All experiments were performed under the temperature range of 15–60 °C in the solid volume concentration range of 0.1–0.5%. The experimental results demonstrated that the rheological and the thermophysical properties of THNFs depend not only on the nanoparticles volume concentration, but also on the temperature of THNFs. All the THNFs demonstrated Newtonian behavior. The dynamic viscosity and the thermal conductivity of THNFs increased with enhancing solid particles volume concentration and temperature. The highest increment in thermal conductivity as compared to distilled water was obtained for the C type of THNFs at 0.5 solid vol% in 50 °C. The specific heat capacity of THNFs first decreased up to 35 °C and then increased with raising temperature. The highest reduction of specific heat capacity of THNFs was found for the C type of THNFs. The surface tension of B and C types of THNFs increased with the particles volume concentration enhancement. In the cases of low particles volume, the surface tension of THNFs was lower than that of the distilled water, for a concentration of the nanoparticles of 1.0%. Four new correlations were developed to predict the viscosity, thermal conductivity, specific heat capacity and density of the THNFs. All the proposed correlations had a satisfactory accuracy of ± 1%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanofluid is a word that was introduced by Choi [1] to create stable colloidal suspensions of nanoparticles in usual base fluids such as oil, water and ethylene glycol. Nanofluids that were made by dispersing nanoparticles into some conventional liquids were suggested as a new heat transfer fluid. Nanofluids offered better potentials to improve the thermo-physical and the rheological properties relative to the conventional liquids. Therefore, nanofluids have attracted great attention due to their potential advantages in many applications such as microelectronics, energy supply and transportation.
Also, low dynamic viscosity of some nanofluids has made them appropriate for the pumping applications, but only a few studies have been conducted on the thermo-physical and rheological properties of the hybrid nanoparticles [2,3,4,5,6,7,8]. The opinion of fabricating hybrid nanofluids is to favor an additional improvement of heat transfer and pressure drop characteristics by trade-off among advantages and disadvantages of individual suspension, attributed to a high aspect ratio, superior thermal network and synergistic impact of nanoparticles. By selecting an appropriate combination of particles manufactured hybrid nanofluid that positive features can be recovered, and infelicities can be hidden owing to their synergistic impact [9, 10].
Baghbanzade et al. carried out a laboratory study on the effective thermal conductivity of SiO2/multiwall carbon nanotubes (MWCNTs)–water hybrid nanofluid at the temperature range of 25–40 °C for the solid volume concentration of 0.1, 0.5 and 1% [11]. Their results showed that the effective thermal conductivity increases with enhancing the particles volume concentration. The percentage of thermal conductivity of hybrid nanofluid enhancement was reported between the percentage of thermal conductivity enhancement of MWCNT–water nanofluid and SiO2–water nanofluid. Fabrication of SiO2/MWCNTs–water hybrid nanofluid improved the thermal properties of water than silica–water nanofluid. Amiri et al. [12] investigated the stability, dynamic viscosity, shear stress and thermal conductivity of multiwall carbon nanotubes/Ag-gum arabic and multiwall carbon nanotubes/cysteine-gum arabic hybrid nanofluids. High thermal conductivity, fairly good stability and appropriate viscosity of MWCNTs/Ag-gum arabic nanofluid make it a suitable nanofluid as a superior cooling agent in thermal systems. Aravind et al. [13] prepared graphene/MWCNT-DW/EG hybrid nanofluid at 25 °C in solid volume concentration of less than 0.4%. Sundar et al. [14] experimentally examined the thermal conductivity of Al2O3–water/EG and CuO–water/EG nanofluid at the temperature range of 15–50 °C with the particles volume concentrations of less than 0.8%. The results showed that the thermal conductivity of CuO–water/EG nanofluid is higher than that of Al2O3–water/EG nanofluid. Additionally, a new empirical correlation was proposed for the thermal conductivity of each of the nanofluids. Sundar et al. [15] fulfilled an experimental work on MWCNT/Fe3O4–water hybrid nanofluid at the temperature range of 20–60 °C with the particles volume fractions of less than 0.003. The results showed that the effective thermal conductivity and the dynamic viscosity of the nanofluids enhanced by 29% and 1.5 times at 0.3 vol% and 60 °C, respectively. Madhesh et al. [16] experimentally performed the thermal conductivity and the friction factor of Cu/TiO2–water hybrid nanofluid at 5–80 °C under the volume concentrations range of 0.1–1 vol%. They concluded that the hybrid nanofluids improved the thermal and rheological properties of the base fluid as compared to the singular nanofluid. In another work, GNP/Ag–water hybrid nanofluid was prepared by Yarmandet et al. [17], and its thermal conductivity and viscosity were measured. The results showed that its thermal conductivity and viscosity enhanced by 22.22% and 1.3 times at 0.1 mass% of nanoparticles in 40 °C, respectively. Soltani et al. [18] studied the dynamic viscosity of MWCNT/MgO-EG hybrid nanofluid. The viscosity of these nanofluids at various particles volume fractions of 0.1–1% was measured at 30–60 °C. The results indicated that the relative viscosity enhances when the solid volume concentration increases from 0 to 1%.
Takabi et al. [19] evaluated the thermal behavior of Al2O3/Cu–water hybrid nanofluid at 30 °C with the solid volume fractions of less than 2%. Hybrid nanofluid indicated better results than Al2O3/water and Cu/water nanofluids, individually. The thermal conductivity of GNP/Pt/water nanofluid was investigated by Yarmand et al. [20]. The results demonstrated an enhancement of 17.7% at 0.1 mass% of nanofluids in 40 °C. An experimental investigation was carried out on ZnO/Ti-EG nanofluid by Toghraie et al. [21]. It could be seen that the thermal conductivity enhancement of hybrid nanofluid at higher volume fractions of nanoparticles is more than that at lower volume fractions.
Eshgarf et al. [22] studied the rheological behavior of MWCNT/SiO2-EG/water hybrid nanofluid functionalized by COOH at the temperature range of 27.5–50 °C, concentration range of 0.0625–2 vol% and the shear rate range of 0.612–122.3 s−1. Their results revealed that the hybrid nanofluid had a pseudo-plastic rheological behavior. Sekhar et al. [23] measured the viscosity and the specific heat capacity of water-based Al2O3 nanofluid at the solid volume concentration of 0.01–1% under the temperature range of 25–45 °C. Their results demonstrated a nonlinear increase in dynamic viscosity with increase in volume fraction; however, the specific heat capacity of this nanofluid declined by enhancing volume fraction. The reasons for viscosity enhancement and heat capacity decrement were the aggregation of nanoparticles and thermal diffusivity enhancement, respectively. The effect of CuO-distilled water nanofluids on the thermo-physical properties of distilled water was experimentally investigated at the solid volume concentration of 0.1–0.5% under the temperature range of 20–80 °C by Kumar et al. [24]. Their results showed that the nanofluid density increases from 0.93 to 1.66% by increasing the volume fraction of nanoparticles and decreases with the temperature enhancement. Also, the viscosity increased from 10.5 to 65.2% with increasing the volume fraction and decreasing the temperature. The heat capacity of nanofluids decreased at first and then increased with increasing temperature. Additionally, it decreased with enhancing the nanoparticles concentration. The dynamic viscosity of CuO/water nanofluids increased about 0.68–2% with decreasing temperature. The results also showed that the thermal conductivity of the nanofluids increased about 22.22% and their viscosity enhanced relative to the base fluid. Zyla [25] experimentally studied the thermo-physical properties of yttrium aluminum garnet-ethylene glycol nanofluid under the temperature range of 0–60 °C and the shear rate of 10 to 1000 s−1 in the volume concentrations of less than 6%. Their results indicated that the nanofluids had Newtonian behavior and their viscosity increased with the nanoparticles volume fraction enhancement and decreased with increasing the temperature.
There are some theoretical studies on the dynamic viscosity and the thermal conductivity of nanofluids as a function of volume fraction and temperature [26,27,28,29]. Some theoretical models have been presented to predict the thermal conductivity and dynamic viscosity of nanofluiuds. However, these models cannot predict exactly the experimental data, so it is essential to peruse about the nanofluids viscosity and thermal conductivity increment mechanisms. Pak and Cho proposed a correlation for the prediction of nanofluid viscosity as a function of nanoparticles concentration and base fluid viscosity as follows [30]:
Batchelor predicted the dynamic viscosity for isotropic suspensions as follows [31]:
Wang et al. [32] obtained a correlation for measuring the dynamic viscosity of nanofluids:
There are several classical approaches like Maxwell’s theory [33] and Hamilton and Crosser approach [34] which are not accurate in foretasting the thermal conductivity of nanofluids. The incidence of convection has to be refrained in base fluids to prosperously evaluate their thermal conductivity. Furthermore, the attendance of dispersed nanoparticles in base fluids can bring a main difficulty for evaluation of the values of viscosity, and thermal conductivity as the uniformity of the medium is to be held.
The Eapen’s mean-field model [35] interference the nanoparticles shapes in parallel or perpendicular to the direction of heat flux. The parallel model was expressed as:
Lu and Li [36] model introduced the correlation for predicting the viscosity of nanofluids by considering near- and far-field pair interaction which is presented as follows:
where μnf, μbf, knf, kbf and φ are the viscosity of the nanofluid and the base fluid, thermal conductivity of nanofluid and base fluid and the volume fraction, respectively. Lu and Li model applied for spherical and non-spherical nanoparticles. These models were not able to predict the thermal properties, accurately.
Considering the main subject, various methods have been suggested to evaluate the values of thermal conductivity of nanofluids over the preceding few years. The most prevalent methods for the evaluation of the values of efficient thermal conductivity of nanofluids are the temperature oscillation procedure [37], transient hot-wire technique [38], cylindrical cell technique [39], steady-state procedure [40], and 3-omega technique [41,42,43,44,45,46]. Sixty-eight percentage of the published literature had used the transient hot-wire method [47]. The researchers suggested some correlations for thermal conductivity and viscosity in accordance with the experimental data [48].
Literature studies showed that a few studies were conducted on the thermo-physical and the rheological properties of the binary hybrid nanofluids, but there was not any work on the rheological and the thermo-physical properties of the ternary hybrid nanofluids. Therefore, the plans of the present study were to investigate the thermophysical and the rheological properties of A, B, C, D and E types of THNFs. The dynamic viscosity, thermal conductivity, specific heat capacity and density of THNFs were experimentally measured at the temperature range of 15–60 °C with the solid volume concentration of 0.1–0.5%. In addition, four new correlations were proposed for the prediction of the viscosity and thermo-physical properties of THNFs in the temperature range of 15–60 °C with the solid volume concentration of 0.1 to 0.5%.
Materials and methods
Synthesis of the hybrid nanocomposite
Five types of hybrid nanocomposites containing A (33.4 mass% CuO/33.3 mass% MgO/33.3 mass% TiO2), B (50 mass% CuO/25 mass% MgO/25 mass% TiO2), C (60 mass% CuO/30 mass% MgO/10 mass% TiO2), D (25 mass% CuO/50 mass% MgO/25 mass% TiO2) and E (25 mass% CuO/25 mass% MgO/50 mass% TiO2) were fabricated by the thermochemical procedure including of spray-drying, oxidation of precursor powder, reduction by hydrogen and homogenization. Copper oxide nitrate, nitrates of magnesium oxide and titanium dioxide were first added to water and mixed the solution vigorously. Afterward, ammonium hydroxide was added into the solution under stirring drop by drop. This solution was filtrated, and the sediment was washed by distilled water. The mixture was then dried at 170 °C by a spray dryer (SPD-P-111, Techno Search Instrument Company, India) to obtain the precursor powder. This powder was then heated at 680 °C for 80 min by SiC electric heating elements (RX2, MHI (Micropyretics Heaters International Company, USA) to form a powder combination of CuO, MgO and TiO2 nanocomposite with different mass percents. Next, these hybrid nanocomposites were heated at 540 °C in the presence of hydrogen gas by a furnace (EX.1700-3L, Extiton Company, Iran). The hybrid nanocomposites were then ball milled in 80 min for obtaining the homogeneous hybrid nanocomposites.
Characterization of CuO/MgO/TiO2 hybrid nanocomposite
TEM image (Transmission Electron Microscope), EDS analysis (Energy-Dispersive X-ray Spectroscopy) and XRD analysis (X-Ray Diffraction, Siemens D-500, 45 kV and 40 mA, θ: 20°–80°) images of the C-type nanocomposite are demonstrated in Fig. 1.
The reflections in the XRD pattern were identified as corresponding to the cubical phases of MgO, TiO2 and CuO nanoparticles using Joint Committee on Powder Diffraction Standards. The average size of the C-type nanocomposite was calculated to be 100 nm using Scherrer correlation. TEM image of the C-type nanocomposite shows that its morphology is somehow spherical and the C type of nanocomposite diameter is 25 nm. The information about the hybrid chemical composition of C type of THNFs was obtained by EDS analysis as shown in Fig. 1 and Table 1. The results confirm the presence of magnesium, titanium, copper and oxygen elements in the C type of nanocomposite combination.
Properties of CuO nanoparticles, MgO nanoparticles, TiO2 nanoparticles and C-type nanocomposite were experimentally measured and are given in Table 2.
Fabrication of hybrid nanofluid
In the present study, all THNFs with different solid particles volume concentrations from 0.1 to 0.5% were fabricated using two-step method by dispersing a specified amount of hybrid nanocomposites in distilled water together with SDS as a surfactant by using an ultrasonic homogenizer (ultrasonic homogenizer UP400A, 400 W, 20 kHz, UTDC, Iran). To get a uniform mixture, the nanofluids were stirred by a magnetic stirrer for 90 min. The solid particles volume concentration could be calculated by the following formula [49].
where φ is the volume concentration of nanocomposite, w denotes the mass of nanocomposite, and ρ is the density.
Thermal conductivity measurement
The thermal conductivity of THNFs was measured using a KD2 Pro thermal properties analyzer (Decagon Devices, Inc., USA) under the temperature range of 15–60 °C and nanoparticles volume concentration of 0.1–0.5 vol%. The measurement technique of KD2 Pro thermal properties analyzer for thermal conductivity of nanofluids is hot-wire method. This device consists of a handheld controller and various sensor needles. The KS1 sensor needle is used for measuring the thermal conductivity of THNFs. The accuracy of the sensor was within 5%. This device was calibrated with distilled water which had good agreement with standard data [50]. The mentioned properties were measured six times for each temperature, and the average values were reported.
Dynamic viscosity measurement
DV-II+ Pro Brookfield viscometer (DV-II+ Pro, Brookfield Company, USA) connected to a water bath was used to measure the viscosity of THNFs at the temperature range of 15–60 °C with the solid volume concentration range of 0.1–0.5%. The Brookfield viscometer was calibrated with distilled water at the room temperature which showed an acceptable accuracy (± 1.0%). All the experiments were carried out in the shear rate range of 10–80 s−1. The dynamic viscosity and shear stress data were recorded to investigate the rheological behavior of THNFs.
Density measurement
Anton Paar digital density meter (DMA 4500M, Anton Paar Company, Austria) was used to measure the amounts of THNFs density at the temperature range of 15–60 °C with the solid volume concentration of 0.1–0.5%. For confidence, the density of DW was measured and compared with the literature data [50]. The accuracy of the instrument was reported about 5 × 10−5 g cm−3.
Specific heat capacity measurement
A differential scanning calorimeter (DSC1 STAR, Mettler Toledo, USA) was used to measure the specific heat capacity of THNFs. The differential scanning calorimeter (DSC) is the most frequently device used for thermal analysis technique. The DSC measures the enthalpy changes of samples due to the changes in specific heat capacity of THNFs as a function of temperature. In order to dry THNFs, water was evaporated by an oven and then the dried samples were placed in the aluminum pans. The specific heat capacity was calculated from heat flow experimental data points using the Stare software of Mettler Toledo DSC1 system. A standard method for measuring the specific heat capacity is Sapphire as a reference. At first, the heat capacity of Sapphire was measured to investigate the validity of this method. The accuracy of temperature for this instrument is ± 0.2 K. The DSC instrument was calibrated with indium, aluminum and melt transition of cyclohexane. In order to estimate the heat capacity of THNFs, the two sample pans were kept empty to reach the baseline heat flux (QO). Then, one of the two pans was filled with the reference sample, and the other one was kept empty. The reference heat flux was measured (Qref), and finally, a pan was filled with the dried THNF. Then, two pans were placed into the calorimeter, and the sample heat flux was recorded (Qsample). The thermal cycle for all steps was fixed. The heat capacity of the sample was calculated from following equation [51]:
where Cp,ref, mref and msample denote the specific heat capacity of sapphire, the mass of sapphire and the mass of sample, respectively.
Results and discussion
Stability of THNFs
Stability is one of the important factors that has an effect on the performance of THNFs. A THNF with low stability tends to deposit due to the agglomeration of nanocomposites in the base fluid [52]. This phenomenon causes to develop challenges such as clogging of pipelines and the decrement of thermal conductivity of nanofluids. Therefore, it is vital to study the stability of the fabricated THNFs. To do so, SDS as a surfactant along with an ultrasonic homogenizer is applied to amend the distribution of suspended nanoparticles. Various amounts of SDS were dispersed in THNFs to attain the optimum value of SDS concentration. Ultrasonication time was one of the important parameters on the stability of THNFs. The stability time of THNFs was determined by means of taking SEM images at various ultrasonication times and also measuring zeta potential. Zeta potential was measured by using the Zetasizer apparatus (Nano ZS90, Malvern Instruments Ltd, UK) working based on the laser Doppler micro-electrophoresis, dynamic light scattering and static light scattering methods. Ghadimi et al. [53] demonstrated that the high zeta potential shows a stable suspension, while THNFs with low Zeta potential make a quick deposition of nanoparticles. Vandsburger [54] reported the stability of nanofluids changes depending on the quantity of zeta potential. If the zeta potential close be to ± 30 mv, the nanofluid is comparatively stable. The stability is favorable, whenever it ranges between ± 30 and ± 45 mv, and higher than this value up to ± 60 mv, an excellent stability for nanofluids can be acceded.
The zeta potential of THNFs for various ultrasonication times with different volume concentrations is given in Tables 3–9. All nanofluids have good stability, except D and E types of THNFs. Also, the influence of ultrasonication time and the mass percent of SDS on the stability of THNFs are demonstrated in Figs. 2 and 3. The optimum values of ultrasonication time for A type, B type, C type, D type and E type of THNFs are found to be about 170, 160, 120, 120 and 150 min, respectively. The optimum values of SDS concentration in mass% and stability time for A type, B type, C type, D type and E type of THNFs are found to be 0.4 mass%, 0.44 mass%, 0.49 mass%, 0.52 mass% and 0.5 mass%, respectively.
The stability and dispersion characteristics of THNFs were investigated by taking SEM images before and after the sonication operation as shown in Fig. 4.
Rheological behavior of THNFs
Effect of temperature and solid volume concentration on dynamic viscosity of THNFs
The dynamic viscosity of all the THNFs with solid volume concentrations of 0.1%, 0.2%, 0.3%, 0.4% and 0.5% was measured at the temperature ranges between 15 and 60 °C. The results are presented in Fig. 5. It is obvious that the dynamic viscosity of THNFs decreases with increasing temperature due to the reduction in intermolecular interactions between the molecules of THNFs. The dynamic viscosity increases with the solid volume concentration enhancement. It is found that the viscosity of THNFs increases as compared to the base fluid at all temperatures and solid volume concentrations. The nano-clusters created based on van der Waals forces between the particles would cause a greater increment in dynamic viscosity due to barrier to the movement of base liquid layers on one another [52]. Nanoparticles in water enhance the viscosity of base fluid due to interactions between water molecules and dispersed nanoparticles. Nanoclusters are created due to the van der Waals forces between the nanoparticles, and the size of nanoclusters would be larger by increasing the nanoparticles volume concentration. The nanoclusters cause a higher enhancement in dynamic viscosity of water due to barrier the movement of base fluid layers on each other. The experimental results showed that the viscosity of nanofluids increased with decreasing temperature. This enhancement percentage was measured by the following formula:
At shear rate of about 12 s−1, enhancements of about 45.22%, 43.3%, 40%, 38.7 and 36.4% for E type, D type, A type, B type, and C type of THNFs were obtained with the solid volume concentration of 0.5 vol% at the temperatures of 15, 55, 40, 60 and 60 °C, respectively.
One of the reasons of the viscosity increment can be due to the existence of great CuO nanoparticles. To the best of our knowledge, no experimental data have been reported for the dynamic viscosity of CuO/MgO/TiO2 THNFs dispersed in water as a base fluid.
Effects of shear rate on shear stress and dynamic viscosity of THNFs
The influence of shear rate and solid particles volume concentration at 55 °C on the dynamic viscosity is shown in Fig. 6. The experimental data demonstrated that the dynamic viscosity of all the THNFs keeps almost constant with increasing shear rate. The experimental data of shear stress at various shear rates and particles volume concentrations at 55 °C are also presented in Fig. 7. It is obvious that the rheological behavior of the THNFs is Newtonian. Because, the variation of shear stress versus shear rate is linear. Chen et al. [55] showed a significant enhancement in heat transfer rate by using the Newtonian fluids. As a result, Newtonian nanofluids are the good choices for the heat transfer characteristics improvement.
Dynamic viscosity correlation for different types of THNFs
Figure 8 compares the experimental viscosity data of THNFs with the estimated values from Pak and Cho [42], Batchelor [43] and Wang [44] models. As can be seen, all the mentioned models underpredict the dynamic viscosity of THNFs. It is clear that Pak and Cho [42], Batchelor [43] and Wang [44] models are not able to estimate the dynamic viscosity of B and C types of THNFs. However, Pak and Cho [42], Batchelor [43] and Wang [44] models treat the experimental data of dynamic viscosity of D and E types of THNFs well. The results show that average absolute relative deviations for the models of Pak and Cho [42], Batchelor [43] and Wang for A, B, C, D and E- types of THNFs are found to be 14.8%, 16.7%, 21.2%, 2.3% and 2.1%, respectively.
The previous theoretical models presented previously were not able to accurately predict the dynamic viscosity of THNFs. Therefore, a correlation was developed to estimate the dynamic viscosity of THNFs as a function of temperature and particles volume concentration. The coefficients of the proposed correlation (Eq. 11) were created by minimization of following objective function using the Levenberg–Marquardt algorithm (LMA), Eq. 10.
where μK is the dynamic viscosity in cp and N is the number of total data points. Subscripts pred and exp represent the predicted dynamic viscosity from the model and experimental dynamic viscosity, respectively. The adjusted R2 of the proposed correlation is 0.992, demonstrating good accuracy of the equation in prediction of viscosity. The absolute deviation between the values obtained from the predicted correlation relative to the experimental data is less than 1.5%. The results are shown in Fig. 9.
Thermal conductivity of THNFs
Thermal conductivity measurement
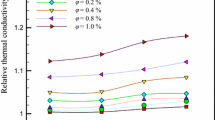
The experimental data of thermal conductivity of THNFs versus temperature and volume fraction are demonstrated in Fig. 10a. The thermal conductivity increases nonlinearly with increasing volume fraction and temperature. The thermal conductivity of THNFs enhancement is calculated from Eq. 12:
where Knanofluid and Kbasefluid denote the thermal conductivity of THNF and distilled water, respectively. The highest thermal conductivity enhancement of A type, B type, D type, E type and C type of THNFs as compared to water are found to be 34.6%, 44.1%, 32.1%, 28.5% and 78.6% at 15 °C and 0.1 vol%, respectively.
The thermal conductivity enhancement of THNFs at low solid volume concentrations is much greater than that of THNFs at high solid volume concentrations as shown in Fig. 10b. A linear relationship between the thermal conductivity and temperature can be attributed to large regions of particle-free liquid with high thermal resistances created by highly agglomerated nanoparticles [52]. The reason for this is that at low solid volume concentrations, the stability and uniformity of nanoparticles in the base fluid would be higher than those at high solid volume concentrations. So, the higher stability at low solid volume concentrations leads to a higher thermal conductivity.
The reversibility of the thermal conductivity of THNFs which showed good stability after 30 days was evaluated. The values of thermal conductivity of THNFs were almost the same values with ± 0.5% error as shown in Fig. 11 and Table 10. It can be concluded that as long as the THNFs are stable, the values of thermal conductivity will remain unchanged.
Proposed correlation
The thermal conductivity of nanofluids depends on important factors such as thermal conductivity of the base fluid, thermal conductivity of individual nanoparticles, solid volume concentration of nanoparticles, shape of nanoparticles, stability of nanofluid, temperature, size of nanoparticles and interfacial layer [56]. The quantities of thermal conductivity of THNFs are greater than those obtained from the Eapen’s mean-field [45] and Lu and Lin [46] models. In their works, the effects of interfacial layer and particles size on the thermal conductivity of nanofluids are not considered. The presence of nanolayers in liquid suspension makes them to act as a thermal bridge between the solid nanoparticles and bulk liquid, which leads to the enhancement of the thermal conductivity of nanofluids [52]. Conventional particle-liquid suspensions do not form such nanolayers in which the typical size of particles is less than 10 nm. Due to the fact that the layered molecules arrange in an intermediate physical state between the bulk liquid and a solid, the molecules in nanolayers lead to a higher thermal conductivity as compared to the bulk liquid. In some cases, nanofluids form clusters over time or when the concentration of nanoparticles is high. The effective surface-area-to-volume ratio, the effective thermal interaction area of particles and thus the thermal conductivity of fluid decrease with the agglomeration of particles [57]. Figure 12 shows the presented theoretical models [45, 46] that are not able to predict the experimental data in a good manner.
The coefficients of the proposed correlation (Eq. 14) were attained by minimization of the following objective function using the Levenberg–Marquardt algorithm (LMA) with an R2 of 0.997, Eq. 13.
where N is the number of total data points and K is the thermal conductivity in Watt per meter per Kelvin. Subscripts est and exp denote the estimated thermal conductivity from the model and experimental thermal conductivity, respectively.
The experimental data and the obtained values from the proposed correlation for thermal conductivity are compared as shown in Fig. 13. As can be seen, the proposed model is in excellent agreement with experimentally measured data. The absolute relative deviation of the obtained values from the proposed model as compared to the experimental data was less than ± 1%.
As earlier mentioned, higher thermal conductivity of nanoparticles in comparison with the base fluid is the most important factor which enhances the thermal conductivity of nanofluid. The thermal conductivity of nanocomposite and the studied nanoparticles were measured at different temperatures as shown in Fig. 14. The results demonstrated that C-type THNF and then CuO nanoparticles have the highest thermal conductivity values. As a result, they can enhance the thermal conductivity of the base fluid. Thermal conductivity data of the studied nanocomposite are also given in Table 11.
Mixing effect
The thermal conductivity of C-type THNF was measured at various ultrasonication times and powers at 60 °C with the solid volume concentration of 0.5% as shown in Fig. 15. The thermal conductivity of nanofluids enhances with the ultrasonication power and time, reaches its maximum value and then reduces. In this case, nanofluids are in a transient state, and nanoparticles are settled in distilled water. The optimal values of ultrasonication power and time of THNFs are found to be 420 W and 140 min, respectively.
The effect of nanoparticles recovery on thermal conductivity coefficient of THNFs was also investigated. At first, the prepared nanocomposites were dispersed in the base fluid under the optimum values of ultrasonication time, ultrasonication power and surfactant concentration in order to measure the thermal conductivity coefficient of nanofluids. The nanoparticles are then detached from the base fluid via the centrifuge (EBA21, Hettich Company, Germany) and dried in an oven at 80 °C. The recovery of nanoparticles is carried out in five times, and their thermal conductivity was measured as shown in Fig. 16. After the first and secondary recovery process of THNFs, the thermal conductivity of THNFs did not significantly change. However, after the third recovery process of THNFs and so on, a great effect on the thermal conductivity of THNFs was observed. The most important reason for variations of thermal conductivity with the recovery process of the nanocomposite is the change of functional groups on the surface of nanoparticles.
Specific heat capacity measurement
Effects of solid volume concentration and temperature
The experimental data of specific heat capacity of THNFs are shown in Fig. 17. The highest reduction in specific heat capacity as compared to distilled water is 2.13% which belongs to C-type THNF. The maximum enhancement for A, B, D and E types of THNFs is found to be 1.68, 2.0, 1.72 and 1.56%, respectively. The experimental data are compared with the values obtained from models 1 and 2 as shown in Fig. 18. Model 1 is the mixing theory for ideal gas mixtures [35], and model 2 is based on the statistical mechanism where a base fluid and nanoparticles are in equilibrium [35]:
where Cp,nf, Cp,np, Cp,bf denote the heat capacity of nanofluid, nanoparticles and base fluid, respectively. Model 2 is adopted as a base model in many investigations, but model 1 gives poor results due to not considering the density of nanoparticles.
Proposed correlation
The coefficients of the proposed correlation were obtained by minimization of the following objective function using the Levenberg–Marquardt algorithm (LMA) [46], Eq. 17.
where N is the number of total data points and CpK is the specific heat capacity in j/g K. Subscripts est and exp denote the estimated specific heat capacity from the model and experimental specific heat capacity, respectively. The absolute deviation of the proposed model is less than ± 1%. The proposed model for the estimation of specific heat capacity of THNFs with an R2 of 0.998 is presented in Eq. 18, and the comparison between its results and experimental data are shown in Fig. 19. The correlation for estimating the specific heat capacity of THNFs is suggested as:
Density measurement
The experimental data of density of THNFs at various temperatures (15–60 °C) in different solid volume concentrations (0.1–0.5 vol%) are demonstrated in Fig. 20. The highest decrement in density of THNFs as compared to distilled water was found to be 2.08% for the C-type THNF at 55 °C with the solid volume concentration of 0.5%. At the same conditions, the greatest decrement for A, B, D and E types are 1.38, 1.88, 1.32 and 1.30%, respectively. The causes for the increase in density of THNFs with enhancing solid volume concentration are the chaotic movement of nanoparticles, particles migration and Brownian motion.
The experimental data of density of THNFs are in good agreement with the values obtained from the mixture density model as shown in Fig. 21. The mixture density values were determined from Pak and Cho correlation [42] as follows:
where ρhnf, ρnp,1, ρnp,2,ρnp,3, ρbf, φ1, φ2 and φ3 denote the density of hybrid nanofluids, the density of nanoparticles 1, 2 and 3 and the solid volume concentration of nanoparticles 1, 2 and 3, respectively. The absolute deviation of experimental data from the mixture density correlation is less than 1%. The main cause of difference between experimental data and Pak and Cho correlation is the non-uniform variations of density in the interfacial region due to having higher van der Waals interactions between the molecules of THNFs.
Proposed correlation
The coefficients of the proposed correlation were fabricated by minimization of the following objective function using the Levenberg–Marquardt algorithm (LMA) [46], Eq. 20.
where N is the number of total data points and ρK is the density in g m−3. Subscripts est and exp denote the estimated density from the model and experimental data, respectively. The absolute deviation of the proposed model is less than ± 1%. The developed model as shown in Eq. 21 can interestingly estimate the density of THNFs with respect to great values of R2, which is 0.999, and comparison between its results and experimental data are shown in Fig. 22. The correlation for estimating the density of THNFs is suggested as:
Surface tension
The quantity of surface tension (σ) was measured between the air–water in different states by applying a surface tension measurement device (DSA-100, Kruss, Germany) at ambient temperature. The results showed that the error is less than ± 1.1%. The surface tension of THNFs was measured at the 0.1, 0.3 and 0.5 solid vol% in 50 °C.
The surface tension decreased from 0.0713 to 0.0431 N m−1 when SDS was added to distilled water. The addition of nanocomposites with various mass percents to the base liquid represents different behaviors. A, C and B types of THNF create a higher decrement in surface tension of the base fluid. However, the surface tension enhanced with enhancing solid volume concentration in the other THNFs as compared to distilled water. The results are demonstrated in Table 12.
The important reason for the increment surface tension with the enhancing solid volume concentration of the nanocomposites is van der Waals forces between nanoparticles at the interface of distilled water/air systems [52]. Therefore, the surface free energy increases and so enhances the surface tension [54]. Sohel et al. [55] showed that nanofluids reduced the surface tension of the water. Also, Chen et al. [56] found that the surfactants had no influence on the nanofluids surface tension. So, the surface tension increment or the surface tension decrement is yet contradictory. The agglomeration of the nanoparticles at the air–base liquid interface and the Brownian motion are the important reasons for this behavior of nanofluids [57, 58]. A possible drag reduction mechanism with adding A, B and C types of nanocomposites in gas/liquid flow is the main reason for the surface tension decrement between air and water systems.
Optimization of the THNF composition
The aim of this paper was to find the optimum mass percent of THNFs leading to the highest heat transfer rate. In other words, finding the best mass percent of THNFs causes the increase in the convective heat transfer. Considering as the Prandtl number increases in value, the THNFs ability in convective heat transfer would be greater. Prandtl number of DHNFs was calculated at different volume concentrations (0.1–0.5 vol%).
where Pr denotes the Prandtl number. The highest and least values of Prandtl number are about 7.11 and 13.76 for the C-type THNF at 0.3 vol% in 50 °C, respectively. Comparison among Prandtl numbers for all the prepared THNFs is shown in Fig. 23. As can be seen, the highest value of Prandtl number belongs to 0.3 vol% of the C-type THNF which can be considered as an optimum concentration in the volume concentration range of 0.1–0.5%.
Generally, the results indicate that the least value of surface tension belongs to the C-type THNF and then belongs to the B-type THNF. Therefore, it is clear that the best nanofluid for drag reduction in heat systems among the nanofluids assessed in this work belongs to the C-type THNF with the highest value of Prandtl number.
Conclusions
CuO–MgO–TiO2 aqueous ternary hybrid nanofluids including A (33.4 mass% CuO/33.3 mass% MgO/33.3 mass% TiO2), B (50 mass% CuO/25 mass% MgO/25 mass% TiO2), C (60 mass% CuO/30 mass% MgO/10 mass% TiO2), D (25 mass% CuO/50 mass% MgO/25 mass% TiO2) and E (25 mass% CuO/25 mass% MgO/50 mass% TiO2) were fabricated at the solid volume concentration range of 0.1 to 0.5 vol%. SDS was added as a surfactant, and the suspensions become homogeneous using an ultrasonic homogenizer and a magnetic stirrer. The thermo-physical properties and rheological behavior of the CuO-MgO-TiO2/DW THNFs were studied at the temperature range of 15–60 °C. Altogether, these findings demonstrate:
-
1.
The synthesized CuO–MgO–TiO2 NPs were characterized using X-ray diffraction (XRD) studies and scanning electron microscopy (SEM). XRD confirms the formation of CuO–MgO–TiO2 NPs, whereas XRD and SEM indicate the sizes in nanometer range. The characterization results showed that the NPs morphology is nearly spherical and its average particles size would be 45–55 nm.
-
2.
The conclusions of measuring stability time illustrated further volume concentration of nanocomposites causing more sedimentation of nanoparticles in the water. Zeta potential measurements of THNFs with low NPs concentrations (0.1 to 0.3) demonstrate good dispersions of A, B, C, D and E types of NPs in distilled water. However, THNFs with high solid volume concentrations (0.4 vol% and 0.5 vol%) tend to aggregate.
-
3.
Thermal conductivity of the recovered THNFs did not vary noticeably at various NPs volume concentrations. The highest reduction in thermal conductivity at the recovery process was earned by 2.7% for 0.5% solid volume concentration.
-
4.
The thermal conductivity of the THNFs was found to enhance monotonically with solid volume concentration and temperature. At low solid volume concentrations (0.1 to 0.5%), the measured thermal conductivity exceeded the predicted values using the classical models. Dynamic contribution arising out of the particles Brownian motion may be attributed to this deviation. At 0.10 mass%, the thermal conductivity of the C-type THNF is improved by 78.6% at a temperature of 50 °C.
-
5.
The viscosity of A, B, C, D and E types of THNFs increased with particles volume concentration and decreased with temperature. The accurate measurements on viscosity of THNFs showed that the least viscosity increment belongs to B and C types of THNFs. In addition, the ternary hybrid nanofluids treat as Newtonian fluids.
-
6.
The experimental density of the THNFs can also be accurately predicted by the mixture law.
The density is lightly enhanced in a linear manner with respect to the loading of NPs. However, the density is linearly decreased with increasing the temperature of the THNFs. Experiments demonstrate that the specific heat capacity of the base fluid may be considerably increased by adding small amounts of different types of NPs.
-
7.
The classical models underpredict the experimental data of the dynamic viscosity and thermal conductivity of THNFs within 6–24% accuracy. Four new correlations were developed for the prediction of thermophysical properties and dynamic viscosity of THNFs with an acceptable accuracy of less than ± 1%.
-
8.
The results obtained from measuring surface tension and calculating Prandtl number revealed that the THNF containing 0.3 vol% C-type NPs was the most proper one to access the highest convective heat transfer rate and drag reduction because of the greatest Prandtl number and the least surface tension, respectively.
Abbreviations
- \( C_{\text{p}} \) :
-
Specific heat capacity, J (g·K)−1
- K :
-
Thermal conductivity, W (m·K)−1
- T :
-
Temperature, °C
- bf:
-
Base fluid
- nf:
-
Nanofluid
- np:
-
Nanoparticles
- DW:
-
Distilled water
- SDS:
-
Sodium dodecyl sulfate
- NPs:
-
Nanoparticles
- THNFs:
-
Ternary hybrid nanofluids
- ρ :
-
Density, g cm−3
- φ :
-
Volume fraction
- μ :
-
Dynamic viscosity, mPa s
References
Lee S, Choi SUS, Li S, Eastman JA. Measuring thermal conductivity of fluids containing oxidenanoparticles. J Heat Transf. 1999;121:280–9. https://doi.org/10.1115/1.2825978.
Aly Wael IA. Numerical study on turbulent heat transfer and pressure drop of nanofluid in coiled tube-in-tube heat exchangers. Energy Convers Manag. 2014;79:304–16. https://doi.org/10.1016/j.enconman.2013.12.031.
Ettefaghi E, Rshidi A, Ghobadian B, Najafi G, Khoshtaghaza MH, Sidik NAC, Yadegari A, Hong WX. Experimental investigation of conduction and convection heat transfer properties of a novel nanofluid based on carbon quantum dots. Int Commun Heat Mass Transf. 2018;90:85–92. https://doi.org/10.1016/j.icheatmasstransfer.2017.10.002.
Kasaeian A, Daviran S, Azarian RD, Rashidi A. Performance evaluation and nanofluid using capability study of a solar parabolic trough collector. Energy Convers Manag. 2015;89:368–75. https://doi.org/10.1016/j.enconman.2014.09.056.
Wen D, Lin G, Vafaei S, Zhang K. Review of nanofluids for heat transfer applications. Particuology. 2009;7:141–50. https://doi.org/10.1016/j.partic.2009.01.007.
Chandrasekar M, Suresh S, Chandra Bose A. Experimental investigations and theoretical determination of thermal conductivity and viscosity of Al2O3/water nanofluid. Exp Therm Fluid Sci. 2010;34:210–6. https://doi.org/10.1016/j.expthermflusci.2009.10.022.
Keblinski P, Eeastman JA, Cahil DG. Nanofluids for thermal transport. J Mater Today. 2005;8:36–44. https://doi.org/10.1016/S1369-7021(05)70936-6.
Baratpour M, Karimipour A, Afrand M, Wongwises S. Effects of temperature and concentration on the viscosity of nanofluids made of single-wall carbon nanotubes in ethylene glycol. Int Commun Heat Mass Transf. 2016;74:108–13. https://doi.org/10.1016/j.icheatmasstransfer.2016.02.008.
Suresh S, Venkitaraj KP, Selvakumar P, Chandrasekar M. Effect of Al2O3–Cu/water hybrid nanofluid in heat transfer. Exp Therm Fluid Sci. 2012;38:54–60. https://doi.org/10.1016/j.expthermflusci.2011.11.007.
Sarkar J, Ghosh P, Adil A. A review on hybrid nanofluids: recent research, development and applications. Renew Sustain Energy Rev. 2015;43:164–77. https://doi.org/10.1016/j.rser.2014.11.023.
Baghbanzadeh M, Rashidi A, Rashtchian D, Lotfi R, Amrollahi A. Synthesis of spherical silica/multiwall carbon nanotubes hybrid nanostructures and investigation of thermal conductivity of related nanofluids. Thermochim Acta. 2012;549:87–94. https://doi.org/10.1016/j.tca.2012.09.006.
Amiri A, Shanbedi M, Eshghi H, Zeinali Heris S, Baniadam M. Highly dispersed multiwalled carbon nanotubes decorated with Ag nanoparticles in water and experimental investigation of the thermophysical properties. Phys Chem. 2012;116:3369–75. https://doi.org/10.1021/jp210484a.
Jyothirmayee Aravind SS, Ramaprabhu S. Graphene wrapped multiwalled carbon nanotubes dispersed nanofluids for heat transfer applications. Appl Phys. 2012;112:123404. https://doi.org/10.1063/1.4769353.
Sundar LS, Hashim Farooky Md, Naga Sarada S, Singh MK. Experimental thermal conductivity of ethylene glycol and water mixture based low volume concentration of Al2O3 and CuO nanofluids. Int Commun Heat Mass Transf. 2013;41:41–6. https://doi.org/10.1016/j.icheatmasstransfer.2012.11.004.
Sundar LS, Singh MK, Sousa ACM. Enhanced heat transfer and friction factor of MWCNT–Fe3O4/water hybrid nanofluids. Int Commun Heat Mass Transf. 2014;52:73–83. https://doi.org/10.1016/j.icheatmasstransfer.2014.01.012.
Madhesh D, Kalaiselvam S. Experimental study on heat transfer and rheological characteristics of hybrid nanofluids for cooling applications. Exp Nanosci. 2015;10:1194–213. https://doi.org/10.1080/17458080.2014.989551.
Yarmand H, Gharekhani S, Ahmadi G, Seyed shirazi SF, Baradaran S, Montazer E, Zubir MNM, Alehashem M, Kazi SN, Dahari M. Graphene nanoplatelets-silver hybrid nanofluids for enhanced heat transfer. Energy Convers Manag. 2015; 100:419-28. https://doi.org/10.1016/j.enconman.2015.05.023.
Soltani O, Akbari M. Effects of temperature and particles concentration on the dynamic viscosity of MgO-MWCNT/ethylene glycol hybrid nanofluids: experimental study. J Physica E: Low-Dimens Syst Nanostruct. 2016;84:564–70. https://doi.org/10.1016/j.physe.2016.06.015.
Takabi B, Gheitaghy AM, Tazraei P. Hybrid water-based suspension of Al2O3 and CuO nanoparticles on laminar convection effectiveness. Thermophys Heat Transf. 2016;30:523–32. https://doi.org/10.2514/1.T4756.
Yarmand H, Gharekhani S, Seyed shirazi SF, Goodarzi M, Amiri A, Sarsam WS, Alehashem M, Dahari M, Kazi SN. Study of synthesis, stability and thermophysical properties of graphene nanoplatelet/platinum hybrid nanofluids. Int Commun Heat Mass Transf. 2016;77:15–21. https://doi.org/10.1016/j.icheatmasstransfer.2016.07.010.
Toghraie D, Chaharsoghi VA, Afrand M. Measurement of thermal conductivity of ZnO–TiO2/EG hybrid nanofluids. Therm Anal Calorim. 2016;125:527–35. https://doi.org/10.1007/s10973-016-5436-4.
Eshgarf H, Afrand M. An experimental study on rheological behavior of non-Newtonian hybrid nano- Coolant for application in cooling and heating systems. Exp Therm Fluid Sci. 2016;76:221–7. https://doi.org/10.1016/j.expthermflusci.2016.03.015.
Sekhar YR, Sharma KV. Study of viscosity and specific heat capacity characteristics of water based Al2O3 nanofluids at low particle concentrations. Exp NanoSci. 2015;10:86–102. https://doi.org/10.1080/17458080.2013.796595.
Kumar S, Sokhal GS, Singh J. Effect of CuO–distilled water based nanofluids on heat transfer characteristics and pressure drop characteristics. Int Eng Res Appl. 2014;4:28–37. https://doi.org/10.1088/1757-899X/225/1/012168.
Zyla G. Thermophysical properties of ethylene glycol based yttrium aluminum garnet (Y3Al5O12-EG) nanofluids. Int Heat Mass Transf. 2016;92:751–6. https://doi.org/10.1016/j.ijheatmasstransfer.2015.09.045.
Abareshi M, Goharshiadi EK, Zebarjad SM, Fadafan HK, Youssefi A. Fabrication, characterization and measurement of thermal conductivity of Fe3O4 nanofluids. J Magn Magn Mater. 2010;322:3895–901. https://doi.org/10.1016/j.jmmm.2010.08.016.
Yang L, Xu J, Du K, Zhang X. Recent developments on viscosity and thermal conductivity of nanofluids. Powder Technol. 2017;317:348–69. https://doi.org/10.1016/j.powtec.2017.04.061.
Hemmat Esfe M, Saedodin S, Biglari M, Rostamian H. An experimental study on thermophysical properties and heat transfer characteristics of low volume concentrations of Ag–water nanofluid. Int Commun Heat Mass Transf. 2016;74:91–7. https://doi.org/10.1016/j.icheatmasstransfer.2016.03.004.
Ganeshkumar J, Kathirkaman D, Raja K, Kumaresan V, Velraj R. Experimental study on density, thermal conductivity, specific heat, and viscosity of water–ethylene glycol mixture dispersed with carbon nanotubes. Thermal Sci. 2017;21:255–65. https://doi.org/10.2298/TSCI141015028G.
Pak BC, Cho YI. Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp Heat Transf. 1998;11:151–70. https://doi.org/10.1080/08916159808946559.
Batchelor GK. The effect of Brownian motion on the bulk stress in a suspension of spherical particles. J Fluid Mech. 1997;83:97–117. https://doi.org/10.1017/S0022112077001062.
Wang X, Xu X, Choi SUS. Thermal conductivity of nanoparticles–fluid mixture. J Thermophys Heat Transf. 1999;131:474–80. https://doi.org/10.2514/2.6486.
Maxwell CA. Treatise on electricity and magnetism. 2nd ed. Cambridge: Oxford University Press; 1904.
Hamilton RL, Crosser OK. Thermal conductivity of heterogeneous two-component systems. Ind Eng Chem Fundam. 1962;1:187–91. https://doi.org/10.1021/i160003a005.
Eapen J, Rusconi R, Piazzo R, Yip S. The classical nature of thermal conduction in nanofluids. J Heat Transf. 2010;132:102402-1. https://doi.org/10.1115/1.4001304.
Lu S, Lin H. Reflective conductivity of composite containing aligned spherical inclusions of finite conductivity. J Appl Phys. 1996;79:6761–9. https://doi.org/10.1063/1.361498.
Roetzel W, Prinzen S, Xuan Y, Cremers CY, Fine HA. Measurement of thermal diffusivity using temperature oscillations thermal conductivity, vol. 21. New York: Plenum Press; 1990. p. 201–7. https://doi.org/10.1007/BF01441907.
Yoo DH, Hong KS, Yang HS. Study of thermal conductivity of nanofluids for the application of heat transfer fluids. Thermochim Acta. 2007;455:66–9. https://doi.org/10.1016/j.tca.2006.12.006.
Kurt H, Kayfeci M. Prediction of thermal conductivity of ethylene glycol–water solutions by using artificial neural networks. Appl Energy. 2009;86:2244–8. https://doi.org/10.1016/j.apenergy.2008.12.020.
Challoner AR, Powell RW. Thermal conductivity of liquids: new determinations for seven liquids and appraisal of existing values. Proc R Soc Lond Ser A. 1956;238:90–106. https://doi.org/10.1098/rspa.1956.0205.
Cahill DG. Thermal conductivity measurement from 30 to 700 K: the 3ωmethod. Rev Sci Instrum. 1990;61:802–8. https://doi.org/10.1063/1.1141498.
Qiu L, Zheng XH, Su GP, Tang DW. Design and application of a freestanding sensor based on 3ω technique for thermal-conductivity measurement of solids, liquids, and nanopowders. Int J Thermophys. 2013;34:2261–75. https://doi.org/10.1007/s10765-011-1075-y.
Qiu L, Zhu N, Zou H, Feng Y, Zhang X, Tang D. Advances in thermal transport properties at nanoscale in china. Int Commun Heat Mass Transf. 2018;125:413–33. https://doi.org/10.1016/j.ijheatmasstransfer.2018.04.087.
Qiu L, Zhang X, Zhu J, Tang D. Note: Non-destructive measurement of thermal effusivity of a solid and liquid using a freestanding serpentine sensor-based 3ω technique. Rev Sci Instrum. 2011;82:086110. https://doi.org/10.1063/1.3626937.
Qiu L, Zhu N, Zou H, Tang D, Wen D, Feng Y, Zhang X. Inhomogeneity in pore size appreciably lowering thermal conductivity for porous thermal insulators. App Therm Eng. 2017;4311:34800–7. https://doi.org/10.1016/j.applthermaleng.2017.11.066.
Qiu L, Schieder K, Radwan SA, Larkin LS, Saltonstall CB, Feng Y, Zhang X, Norris PM. Thermal transport barrier in carbon nanotube array nano-thermal interface materials. Carbon. 2017;6223:30483–9. https://doi.org/10.1016/j.carbon.2017.05.037.
Paul G, Chopkar M, Manna I, Das PK. Techniques for measuring the thermal conductivity of nanofluids: a review. Ren Sust Energy Rev. 2010;14:1913–24. https://doi.org/10.1016/j.rser.2010.03.017.
Ijam A, Saidur R, Ganesan P, Moradi Golsheikh A. Stability, thermo-physical properties, and electrical conductivity of graphene oxide-deionized water/ethylene glycol based nanofluid. Int Heat Mass Transf. 2015;87:92–103. https://doi.org/10.1016/j.ijheatmasstransfer.2015.02.060.
Sundar LS, Sharma KV, Singh MK, Sousa ACM. Hybrid nanofluids preparation, thermal properties, heat transfer and friction factor—A review. Renew Sust Energy Rev. 2017;68:185–98. https://doi.org/10.1016/j.rser.2016.09.108.
Green D, Maloney J. Perry‘s chemical engineers handbook. Lawrence: 7th, Library of Congress Cataloging-in-Publication Data, University of Kansas; 1997.
O’Hanley H, Buongiorno J, McKrell T, Hu L-W. Measurement and model correlation of specific heat capacity of water-based nanofluids with silica, alumina and copper oxide nanoparticles. Int Mech Eng Congr Expos. 2011;10:1209–14. https://doi.org/10.1115/IMECE2011-62054.
Mahian O, Kolsi L, Amani M, Estelle P, Ahmadi G, Kleinstreuer C, Marshall Jeffrey S, Siavashi M, Taylor RA, Niazmand H, Wongwises S, Hayat T, Kolanjiyil A, Kasaeian A, Pop L. Recent advances in modeling and simulation of nanofluid flows-Part I: Fundamental and theory. Phys Rep. 2018. https://doi.org/10.1016/j.physrep.2018.11.004.
Ghadimi A, Saidur R, Metselaar HSC. A review of nanofluid stability properties and characterization in stationary conditions. Int J Heat Mass Transf. 2011;54:4051–68. https://doi.org/10.1016/j.ijheatmasstransfer.2011.04.014.
Vandsburger L. Synthesis and covalent surface modification of carbon nanotubes for preparation of stabilized nanofluid suspensions. Montreal: McGill University; 2009.
Chen H, Ding Y, Tan C. Rheological behaviour of nanofluids. New J Phys. 2007;9:367. https://doi.org/10.1088/1367-2630/9/10/367.
Rashmi W, Ismail AF, Sopyan I, Jameel AT, Yusof F, Khalid M, Mubarak NM. Stability and thermal conductivity enhancement of carbon nanotube nanofluid using gum Arabic. Exp Nano Sci. 2011;6:567–79. https://doi.org/10.1080/17458080.2010.487229.
Machrafi H, Lebon GP. The role of several heat transfer mechanisms on the enhancement of thermal conductivity in nanofluids. Continuum Mech Thermodyn. 2016;28:1461–75. https://doi.org/10.1007/s00161-015-0488-4.
Aybar HS, Sharifpur M, Azizian MR, Mehrabi M, Meyer JP. A review of thermal conductivity models for nanofluids. J Heat Transf Eng. 2015;36:1085–110. https://doi.org/10.1080/01457632.2015.987586.
Acknowledgements
This work was supported by the National Key R&D Program of China (Grant No. 2016YFE0204200) and the National Natural Science Foundation of China (No. 51776170). The authors also would like to express their appreciation to the Shiraz University and the 111 project (B16038) for the support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mousavi, S.M., Esmaeilzadeh, F. & Wang, X.P. Effects of temperature and particles volume concentration on the thermophysical properties and the rheological behavior of CuO/MgO/TiO2 aqueous ternary hybrid nanofluid. J Therm Anal Calorim 137, 879–901 (2019). https://doi.org/10.1007/s10973-019-08006-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08006-0