Abstract
Nanofluids have been introduced as an alternative to conventional fluids to improve energy efficiency in heat transfer systems. However, their stability problems before and after operation cycles can produce inconsistent results in different heat transfer technologies that use them. This review summarizes different experimental results obtained using nanofluids in heat pipes, particularly in two-phase closed thermosyphons, and it focuses on the role of preparation and stability issues of nanofluids before and after their use in these devices. Additionally, the effects of nanofluids on heat pipes’ thermal performance were compiled and compared from available experimental studies in the literature. Nanoparticles’ deposition on the evaporator surface and wick or groove structures were the most common mechanism to explain the reported increase or decrease in the thermal performance of heat pipes. This review also identifies the research problems that need to be solved in order to use nanofluids that outperform conventional fluids in heat pipes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Energy efficiency is a critical issue worldwide, particularly due to climate change and global warming in addition to the ever-increasing consumption. There is a constant demand for new methods and developments that can decrease the consumption of fossil fuels and can also mitigate greenhouse gas emissions. Heat transfer is one of the most important processes in the development of industrial products, and thermal systems have an important role in energy consumption [1, 2]. Energy efficiency improvements in these processes can be achieved by using high-efficiency devices with extended areas or thermal fluids with improved thermophysical properties. Any development or effort regarding the use of said fluids with enhanced properties in efficient thermal transport technologies is significant to improve the performance and efficiency of thermal energy systems.
Nanofluids are suspensions obtained by dispersing nanoparticles (typically < 100 nm) in common fluids. These were presented in 1995 by Choi and Eastman [3] as fluids with high potential to replace the conventional fluids used in heat transfer applications, due to its higher thermal conductivity. Since then, nanofluids are commonly used in heat transfer applications with the aim of increasing the energy efficiency in different devices [3,4,5]. Despite the reported controversy regarding their effect on thermal conductivity and the underlying mechanisms of nanofluids [6], substantial increases in heat transfer technologies with convection and boiling heat transfer of nanofluids have being widely found in the literature [7].
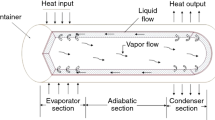
Heat pipes are one of the most effective passive heat transfer technologies, and they are commonly used when efficient heat transfer with small temperature differences is the main purpose. These devices have a very high thermal conductance and are widely used in isothermal applications, temperature control, and (to a lesser extent) waste energy recovery [8]. The addition of heat pipes to heat transfer systems enables efficiently to use them, which is ideal for a number of industries and applications [9]. The main applications of heat pipes are temperature control of central processors in desktop and laptop computers [10], air-conditioning systems, thermal storage, solar heating systems [11], and waste heat recovery from furnaces [12] and boilers using the heat from exhaust gases to preheat the air used for combustion [13]. A heat pipe is composed of a sealed container with a working fluid in their interior. Generally, it comprises three sections, an evaporator, a condenser, and an adiabatic zone, additionally, in their interior can have a wick material that provides a capillary pressure to the liquid movement from the condenser to the evaporator [14]. A special but simple type of gravity-assisted wickless heat pipe is the two-phase closed thermosyphon (TPCT), which has applications in a broader temperature range than conventional heat pipes. In recent years, the number of works on these thermosyphons has risen considerably due to their numerous applications, including their popular use in heating and cooling processes and solar energy recovery [15].
Generally, thermosyphons are either vertically oriented or inclined at about 5° to the horizontal and comprise three main zones, an evaporator, an adiabatic section, and a condenser, with the evaporator below the condenser. The heat supply in the evaporator may be hot exhaust gas from an industrial process, a steam generator, or a preheater for the incoming air or process fluid [16]. Commonly used working fluids in thermosyphons are water, methanol, ethylene glycol, and their mixtures [17]. The working fluid is subjected to film evaporation and pool boiling heat transfer mechanisms in the evaporator section. After the evaporation and condensation processes, gravity-assisted condensed liquid films provide the recirculation of liquid from the condenser to the evaporator section [18]. The working principles of heat pipes as well as thermosyphons are well known [8, 14, 15, 19] and will not be elaborated further.
Nanofluids have been used also in different types of heat pipes, including mesh wick heat pipe, sintered metal wick heat pipe, oscillating heat pipe, and two-phase closed thermosyphon [20]. Literature review about specifically heat pipes and thermosyphons operated with nanofluids was firstly addressed in 2012 by Liu et al. [21]. In 2013, Buschmann et al. [22] realized an critical review and complemented and actualized this in 2015 [23]. Other reviews related to heat transfer associated with the use of nanofluids in heat pipes have been presented by Sureshkumar et al. [24] and Alawi et al. [20]. Recently, Gupta et al. [25] publish a review about the heat transfer mechanisms present in heat pipes using nanofluids. In all these literature reports, it has been concluded that nanofluids as working fluids have the potential to improve the thermal performance of heat pipes; however, all authors agree that deeper research is necessary to understand the real effect of nanoparticles in the heat pipes’ thermal performance.
In this review, we focused on nanofluids’ preparation and characterization and their thermal features when they are used in heat pipes.
However, the results of using nanofluids in heat pipes in the literature are not consistent and sometimes even contradictory. In the majority of cases, heat pipes’ performance was enhanced [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43], while in other cases degradation was apparent [29, 44,45,46,47,48]. The effect of nanofluids on the differences in the performance of heat pipes and TPCTs has commonly been attributed to a porous layer formed on the evaporator surfaces caused by the agglomeration and sedimentation of particle clusters during and after cycles. This porous layer can modify the nucleation sites and the wettability [29] promoting the growth or collapse of bubble [49]. However, the effects of this porous layer in the thermal performance are highly dependent of the nanoparticles’ concentration [50].This porous layer is commonly identified using scanning electron microscopy after heat pipes’ operation; an example of the evaporator surface after TPCT operation can be seen in Fig. 1.
Agglomeration and deposition of nanoparticles on the evaporator surface and differences found in thermosyphons and heat pipes’ performance could be related to different parameters used during nanofluid preparation and difficulties to maintain the particles dispersed over time. Problems related to stability have limited the use of nanofluids in different heat transfer devices, and the formulation of stable nanofluids remains a research challenge [51,52,53,54]. Nanofluids’ preparation and their associated parameters such as sonication type and time, nanoparticle concentration, agglomerates sizes, and chemical additives were not generally studied before they were used in thermosyphons, and only very little information is provided in the experimental studies reported in the literature. Nanofluids’ stability and their thermal properties measured before their use in thermosyphons can suffer modifications during and after operation cycles due to thermal loads, movement of particles, and phase changes [22]. The field of nanofluids is still under development, and further experiments and simulations need to be conducted in order to quantify and validate the effectiveness of nanofluids in different thermal devices such as heat pipes [9].
This review summarizes the reported experimental results of the use of nanofluids in heat pipes, particularly in thermosyphons, and focuses on the preparation and stability issues of nanofluids which can explain the differences found in the literature in terms of their thermal performance. This study also aims to identify the research gaps and problems that need to be solved to achieve a real application of nanofluids in thermosyphons and to verify their thermal performance reproducibility.
Nanofluids as working fluid in TPCTs
Nanofluids’ preparation methods and stability issues
There are two typical methods to obtain nanofluids: one step and two step. The first option combines nanoparticles’ synthesis and nanofluid production simultaneously, using physical and chemical processes; it avoids the drying, storage, transport, and dispersion stages of nanoparticles, but impurities are commonly found and the quality of the nanofluid decreases [55]. In the two-step method, nanoparticles are first obtained by different chemical or physical processes, and after that, they are dispersed in the base fluid [56]; it is commonly preferred due to its low cost and easy implementation. By this method, nanoparticles are synthetized or can be commercially obtained first, and after that, they are dispersed in the chosen base fluid using different dispersion techniques, such as ultrasonication or homogenization.
However, the main drawback of this method is the poor stability of the nanofluid caused by nanoparticle aggregation and sedimentation [57, 58]. Interaction forces between nanoparticles, mainly Van der Waals, are responsible for these phenomena [51,52,53,54, 58, 59]. Therefore, in the two-step method, the use of chemical additives (such as surfactants) or nanoparticle functionalization to minimize nanoparticle agglomeration and improve nanofluid stability is a common practice [39]. Nanofluids’ preparation methods and stability issues can be reviewed more deeply in [60,61,62,63,64,65,66].
Nanofluid stability is one of the principal challenges in the world of nanofluid applications [51,52,53, 61, 67,68,69,70,71,72]. Factors like preparation method [67], nanoparticle concentration [57], nanoparticle type [54], surfactant type [57, 68, 73], surfactant concentration [73,74,75], pH [73, 76], ultrasonic type and time [73, 77], and ultrasonication power [78, 79] greatly affect nanofluids’ stability and thus their performance in applications [72, 80]. Additionally, the stability of nanofluids can change after they are used in different applications due to different thermal loads, movements, phase changes, and other factors that can produce nanoparticle agglomeration and sedimentation that affect their behavior [22, 81].
Different methods and techniques have been employed to evaluate the nanofluids’ stability, including visual inspection, zeta potential, UV–Vis absorbance variation, hydrodynamic diameter size variation and properties such as thermal or electrical conductivity, viscosity, or others over time [74, 75, 82,83,84,85]. However, the methods generally are complementary to complete a most accurate stability characterization [66, 86]. Table 1 resumes ones of the most used methods and the limitations in stability test.
Nanofluids used in heat pipes as working fluids have been commonly prepared via the two-step method. A summary of different experimental studies where the main aspects related to nanofluids’ preparation, such as nanoparticle type, concentration, surfactant type and concentration and dispersion techniques before their use as working fluid in heat pipes is shown in Table 2. Most of them use commercial nanoparticles dispersed in the base fluid by different ultrasonication types, settings, and times. Also, some researchers applied surfactants or functionalized nanoparticles to avoid agglomeration.
It can be seen from Table 2, that some studies did not provide information about the stability of nanofluids after they were prepared [29, 37, 39, 94,95,96,97,98,99], and most of them reported the use of surfactants without detailing with their concentrations, type, selection, or sonication times.
Nanofluids with different types of nanoparticles, such as metal oxides and carbonaceous materials, have been used in heat pipes and produced increases in their thermal performance [17, 36, 37, 100,101,102]. However, there is an information gap regarding the characteristics and use of nanofluids, as well as chemical additives and their effects on thermophysical properties and stability issues. In addition, most reports only provide incipient information about the pre-use state, i.e., before using nanofluids in heat pipes (static mode), and no data after operation cycles.
Surfactant effect on heat pipes
Surfactants and other chemical additives are commonly used to improve nanoparticle dispersion without significantly changing their thermophysical properties over time. However, in the literature, several studies suggest an important relationship between the type and concentration of surfactant and their effect on stability times as well as thermophysical properties [103,104,105,106]. Any research on the effect of the surfactant on the stability of nanofluids must be focused on finding the right type and optimal amount (e.g., concentration) that enables long-term stability without changes in thermal properties before and after nanofluids which are used in thermal systems [107].
Parametthanuwat et al. [98] reported the influence of surfactant on nanofluids’ performance in a thermosyphon. They used different concentrations of oleic acid to disperse Ag nanoparticles in water and found that the highest increase in thermosyphon performance was obtained with 1 w/v% of this surfactant. Nevertheless, the effect of this surfactant on nanofluid stability and heat transfer mechanisms was not reported except for mentioning that the nanofluid was stable for a long time without specifying the length.
Amiri et al. [27] compared the performances of a thermosyphon by using functionalized graphene nanoplatelets and graphene nanoplatelets (GNP) in water with the addition of SDBS as surfactant. They found that the rise in the heat transfer coefficient with functionalized graphene nanoplatelets was 33% greater than the increase with the addition of 0.1 mass% of SDBS surfactant. Additionally, they emphasized that the viscosity was increased by 136% at 80 °C due to the addition of surfactant. Nevertheless, the reasons behind the increment in viscosity caused by adding surfactant at such high temperature were not explained.
Mehrali et al. [49] evaluated the thermal performance of a grooved copper heat pipe using a nitrogen-doped graphene nanofluid, with Triton X100 as surfactant. In their results, they found increases in the thermal performance up to 74% for graphene concentration 0.06 mass% and Triton X100 (0.025 mass%) at 120 W. Additionally, the TX100 solution showed higher heat transfer coefficient compared to water as working fluid. However, the increases in the global thermal performance were superior when nitrogen-doped graphene nanofluid with Triton X100 was used instead of only surfactant solution.
Although few studies were found in the literature with respect to the effect of the surfactant on nanofluids used as working fluids in heat pipes, it is evident that the presence of the former can produce changes in the thermal behavior of the latter. Thermophysical properties such as surface tension, viscosity, and thermal conductivity can change with the surfactant addition, and this can produce changes in different heat transfer mechanism such as convection and boiling [108]. Furthermore, nanofluids are subjected to different transport and heat phenomena, which can produce variations in the surfactant, such as degradation and changes in properties. In this sense, more studies on the relationship between chemical additives, nanofluid stability, and the thermal performance of TPCTs need to be carried out in order to better understand the behavior of TPCTs in the presence of nanofluids.
Nanofluids after operation cycles
Nanofluids in heat pipes are subjected to different thermal loads, phase changes, and movement. Therefore, their stability and thermophysical properties can change significantly after operation cycles. In the reported literature, few studies have evaluated the characteristics of nanofluids after heat pipes’ operation. Some related research studies are briefly discussed below.
Grab et al. [97] investigated the changes in nanoparticle size before (stationary nanofluid, no flow, no phase change conditions) and after operation cycles in a thermosyphon using nanofluids of TiO2 and Au. They found a considerable increase in nanoparticle size as a consequence of their agglomeration. In TiO2 nanofluids, the size increased up to 28%, while in Au nanofluids it was as high as 125%. They also found that when the thermosyphon was not operated for a period of time, nanoparticles sedimented and formed a porous layer on the evaporator, thus affecting the thermal performance. Another study in the same laboratory [37] found that using TiO2–water nanofluids in a thermosyphon enhanced its performance, but when the thermosyphon was left unoperated during 5 weeks it underperformed. This phenomenon was attributed to an increase in agglomerated sedimentation and to the porous layer aging formed in the evaporator.
Contrary results were also found in the literature with respect to stability after thermosyphon operation cycles. Liu et al. [111] used CuO–water nanofluids in a thermosyphon and did not find differences in performances between fresh nanofluids and nanofluids left to rest in the thermosyphon without operation for 2 weeks. Their explanation for this result was that possible sedimented nanoparticles in the evaporator mixed again in the base fluid when the thermosyphon was in operation due to the buoyant forces of the bubbles during boiling. Another study, by Chen et al. [44], used functionalized SiO2 in a thermosyphon and evaluated its behavior a week after the first test. However, they did not observe any porous layer in the evaporator in both experiments, and thus, no difference in the thermal performance was reported. Their results were in accordance with the results found by Yang et al. [114] using functionalized silica nanoparticles and Ozsoy et al. [123] using silver–water nanofluid in a heat pipe evacuated tube solar collector.
Heris et al. [118] used CNT functionalized with different acids in a TPCT. The acids were added to anchor carboxylic groups in the CNT surface. They found that with an increase in carboxylic groups in the CNT surface, thermal performances were superior. This result was associated with the stability of CNT in fluid base and the formation of homogeneous active sites throughout the base fluid in the evaporator. Nevertheless, more extensive research needs to be conducted to draw conclusions about the stability of nanofluids and their relationship with the contradictory results found in the literature regarding TPCT performance. The stability of nanofluids on rest can be changed when they are subjected to different transport and heat phenomena in heat pipes’ operation. Porous layer formation is frequently reported after operation cycles and can change depending on the nanoparticle type, concentration, and morphology. The analyzed literature does not enable to draw conclusions regarding the real effect of nanofluids used as working fluid in heat pipes yet.
Therefore, there are two main questions to address: Is the stability of the nanofluid on rest before using it in TPCTs really important for TPCT performance and can such stability be maintained after discontinuous operation cycles? Can there be reproducibility in the operation of a thermosyphon with nanofluids?
Heat pipes’ performance using nanofluids
Effects of nanofluids on the thermal performance of heat pipes are commonly reported in the literature as variations in thermal efficiency or thermal resistance. Additionally, these variables depend on experimental parameters such as tilt angle, filling ratio, and power [15]. An optimal combination of working fluid and experimental parameters must be found to maximize the thermal behavior of heat pipes.
Kamyar et al. [36] found an increase in the thermal performance of a thermosyphon using nanofluids composed of Al2O3 and TiSiO4 nanoparticles dispersed in water. The thermal resistance of the thermosyphon fell down to 65% with a nanofluid of Al2O3 (0.05 vol%)–water and to 57% with TiSiO4 (0.075 vol%)–water at 40 W. Such an increase in thermal performance was attributed to an increase in base fluid thermal conductivity and a porous layer formed on the thermosyphon evaporator surface.
Buschmann et al. [37] evaluated the effect of TiO2–water nanofluids on the thermal performance of a thermosyphon. They reported a 24% decrease in thermal resistance when they used concentrations between 0.2 and 0.3 vol% of TiO2. This decrease in thermal resistance was also attributed to a porous layer formed in the evaporator and not to the thermophysical properties of the nanofluids.
An increase in thermal efficiency of up to 14.7% in a thermosyphon using Al2O3–water (2% v/v) nanofluids with a power of 97.1 W was reported by Noie et al. [17]. However, they did not attribute this increase to a specific phenomenon, and in their analysis, they established several possibilities such as the formation of a porous layer, an increase in thermal conductivity, and Brownian motion. Nonetheless, no explicit cause for such an increase in thermal efficiency was specified.
Azizi et al. [101] observed an increase in the thermal efficiency of a TPCT using graphene nanofluids as a working fluid compared to water. The maximum thermal efficiency was 90.2% for a graphene concentration of 0.1 mass%. They attributed the thermal efficiency increase to Brownian motion, and its effects were associated with microconvection that could cause the energy exchange rate around the wall, and thus, the heat transfer between the evaporator wall and fluid increases considerably.
Grab et al. [97] were able to improve the thermal performance of a thermosyphon by lowering thermal resistance by up to 10% when using TiO2–water nanofluids (0.2 and 1 vol%) with power variations between 150 and 300 W. While Au–water nanofluids (0.4 and 0.59 vol%) showed a fall between 10 and 20% up to 150 W, they did not show any change beyond 200 W. This reduction of thermal resistance was also considered due to the formation of a porous layer of nanoparticles in the evaporator, which was identified by scanning electron microscopy. These authors emphasized that the porous layers were different with the two types of nanoparticles and that the layer shows agglomerates bigger than the initial size of the nanoparticles.
Tong et al. [102] used graphene oxide (GO)–water and silver oxide (SO)–water nanofluids to evaluate their effects on TPCT performance. The thermal resistance of the TPCT with GO nanofluids was overall lower than when charged with deionized water, while the SO nanofluid caused a minor increase even at high concentrations of SO. They associated these reductions in thermal resistance with the increase in evaporation strength as a consequence of the fast water permeation of GO deposition.
Experimental research to determine of temperature profile and heat transfer of a thermosyphon charged with Fe2O3–water nanofluids was carried out by Huminic et al. [94]. Their results showed a maximum 42% increase in the heat transfer rate when using this nanofluid at 5.3 vol% concentration. This behavior was attributed to the bombardment of vapor bubbles by the dispersed nanoparticles in the base fluid, which makes them smaller and promotes heat transfer by boiling.
Shanbedi et al. [39, 100] conducted experimental studies to evaluate the performance of thermosyphons with multiwall carbon nanotubes MWCNT–water and MWCNT + Ag–water nanofluids. They found a decrease in the thermal resistance of the thermosyphon, which was attributed to the increase in the thermal conductivity of water caused by the addition of these nanoparticles. In another study using MWCNT–water nanofluids as a working fluid in a thermosyphon, a drop in evaporator thermal resistance and a rise of 150% in the heat transfer coefficient were reported by Liu et al. [96]. This improvement in thermosyphon performance was also attributed to the formation of a porous layer, which facilitated the enhancement of the boiling heat transfer mechanism.
Menlik et al. [95] evaluated the performance of a thermosyphon using MgO–water (5 vol%) nanofluids as working fluid. They reported a 26% improvement in thermal efficiency and a reduction in thermal resistance of 18% for a power of 200 W. Hung et al. [5] found a 22.7% increase in the thermal efficiency of a thermosyphon using Al2O3–water nanofluids (0.5 mass%). Notwithstanding, none of these researchers reported or provided a complete explanation of the real phenomenon behind such increase in the efficiency of the thermosyphon.
Soleymaniha et al. [121] in a recent work used an stable water-based quantum dots (GQD) in a thermosyphon. In their work, in order to obtain nanofluids’ stability, graphene was treated with acids and an amidation reaction to include carboxyl and amine groups attached to their surface. They found an increase of 11.5% with water-based A-GQD at a concentration of 0.002 mass% was used at a power of 150 W compared with DI water.
Liu et al. [99] found increases in the condenser and evaporator heat transfer coefficients of a mesh heat pipe using CuO–water nanofluids. The maximum thermal performance enhance was found at concentration of 1 mass%, and after operation, almost no sedimentation of nanoparticles was found on the mesh surface. The increases in the thermal performance were attributed to modifications in the thermal properties of nanofluids. In the same way, Ramachandran et al. [29] used an hybrid nanofluid Al2O3/CuO–water in a cylindrical screen mesh heat pipe. They found decreases in the thermal resistance up to 44.25% compared with deionized water. This was attributed to the formation of a porous coating on the wick surface which enhanced the wettability and surface roughness, creating more nucleation sites.
In oscillating heat pipes, Goshayeshi et al. [122] used a Fe2O3/Kerosene nanofluid as working fluid in the presence of a magnetic field. They found increases in the thermal performance up to 16% compared with kerosene. In another study, Zhou et al. [34] used graphene nanofluids and they reported a reduction in the thermal resistance up to 83.6% for a graphene concentration 2.0 mass%, at filing ratio 62%, and heating power 80 W. This improvement in the thermal performance was attributed to increased thermal conductivity of the nanofluid compared with water and the enhanced surface wettability. In a similar way, in a vertical closed pulsating heat pipe, Xing et al. [124] reported a reduction in the thermal resistance by 34% compared to water using hydroxylated MWCNT nanofluid as working fluid.
As mentioned before, deteriorated heat pipes’ thermal performance or without changes was found in some studies using nanofluids. For example, using carbon nanotubes (1 vol%)–water nanofluids in a thermosyphon, Xue et al. [45] reported a large increase: 3.3 times the thermal resistance of water as a working fluid. They attribute this deterioration in performance to the altered interfacial properties between deposited nanoparticles and the evaporator surface, which produce a reduction in active nucleation sites for the boiling mechanism.
In another study, Chen et al. [44] found increases in thermal resistance between 22 and 25% of their functionalized SiO2–water nanofluids. They attributed their results to a more viscous working fluid due to added nanoparticles. Nevertheless, the way the viscosity of nanofluids contributed to the increase in thermal resistance was not explained. They also used SiO2 nanoparticles without functionalization, and likewise, they found deterioration in thermosyphon performance. With the nanoparticles without functionalization, the authors found a layer of nanoparticles deposited in the evaporator surface and suggested that the reduced roughness of this layer was progressively responsible for the decrease in thermosyphon performance. Yang et al. [114] also used functionalized silica nanoparticles in a thermosyphon. They report that their nanofluids were stable and no sedimentation was observed due to a steric stabilization effect. They found that the evaporation heat transfer coefficient grew by 17% at the operating temperature of 40 °C, and this improvement was attributed to changes in thermophysical properties. However, no significant increases in thermosyphon heat transfer were found. Additionally, these authors used silica nanoparticles without functionalization, and a porous layer was identified in the evaporator surface, which resulted in heat transfer coefficient deterioration.
Khandekar et al. [46] found a worsening thermosyphon performance when they used nanofluids of CuO–water, Al2O3–water, and laponite clay–water. The maximum rise in thermal resistance was found for laponite. The results were attributed to less nanoparticle interaction with the nucleation cavities in the evaporator surface. Active sites for nucleation were blocked by nanoparticles deposited, and the density of sites for boiling was reduced.
Kyung et al. [48] found that the thermal performance of a screen mesh wick heat pipe using SiC nanofluids as working fluid was not enhanced compared to the heat pipe using water. The evaporator thermal resistance was increased due to increases in the nanoparticles–deposition layer, large viscosity, and interruption of phase change.
A summary of the experimental results with nanofluids in heat pipes found in the literature is presented in Table 3. It lists nanoparticle type, concentration, base fluid used, filling ratio, inclination angle, and the most relevant results. From this table, contradictions in reported thermal performance results can be observed regarding the most commonly used nanofluids.
Additionally, big differences among experimental parameters can be noticed. These dissimilarities can influence the results of using nanofluids in heat pipes; different experimental parameters and nanofluids must be combined to thoroughly evaluate heat pipe performance.
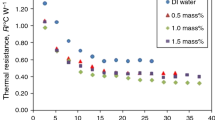
Finally, Fig. 2 summarizes the relationship between the thermal resistance of nanofluids and water (red dashed line) as working fluids in TPCTs and the applied power in the evaporator. It can be seen from this figure that most experimental studies reported a decrease in thermal resistance (i.e., positive results) when nanofluids replaced water as working fluid. Notwithstanding, other studies describe higher thermal resistance.
It can be observed from Table 3 and Fig. 2 that no conclusions can be drawn from different experimental studies using nanofluids as a working fluid in TPCTs. Different nanoparticles, nanofluid preparations, chemical additives, and experimental parameters make the comparison difficult. Interestingly, in most cases any increase or even decrease in the performance of the thermosyphon using nanofluids was generally attributed to a porous layer formed in the evaporator surface and its effect on the boiling mechanism. In that sense, the thermophysical properties of nanofluids were set aside as a secondary factor.
The study of such porous layer can be very complex because its formation and structure are affected by nanoparticle type, size, morphology, concentration, agglomerate sizes, and chemical additives used during their preparation. Moreover, several experimental parameters produce variations in the transport and thermal phenomena that could have different effects on nanofluid behavior during TPCT operation. In this regard, more theoretical and experimental studies are necessary to better understand the different phenomena involved in the thermal behavior of heat pipes using nanofluids as working fluid before they are introduced at the industrial level.
Heat transfer mechanisms in heat pipes affected by nanofluids
In TPCTs, the heat transfer mechanism has been established to be directly related to the heat flux in the evaporator. In specific, at low flux, the main heat transfer mechanism reported is natural convection, while at a high flux nucleate boiling is the associated mechanism [15, 46]. However, from the literature, the most common analyzed mechanism to explain the effect of nanofluids is nucleate boiling [26, 46, 94, 96, 97, 119]. The boiling heat transfer characteristic of the TPCTs is related with the thermophysical properties, stability of the nanofluids, and the surface characteristics of the heater [21]. Accordingly, the increase in the nanofluid thermal conductivity and the nanoparticle deposition on the heated surface is considered to be responsible for the modifications of the thermal performance in the TPCT [102].
Nanoparticles’ deposition on the evaporator surfaces forms an artificial layer. This was reported as the main responsible for the thermal performance variations in heat pipes [30,31,32,33,34,35, 48, 102, 125]. Reduction in the bubble size during bubble formation at the solid–liquid interface and reduction in the nucleate sites by blocking of nucleation cavities has been used to explain the increase or reduction of the thermal performance, respectively.
Do et al. [41] and Liu et al. [99] observed in their work a thin porous coating layer formed by nanoparticles on the wick structures. Both authors concluded that this is the primary mechanism to enhance the heat pipes’ thermal performance, because this layer can not only extend the evaporation surface but also improve the surface wettability and capillary wicking performance. In the same way, Putra et al. [42] argued that this coating layer in a screen mesh wick improves the surface wettability by reducing the contact angle and increasing the surface roughness, which in turn increases the critical heat flux. This observation was complemented by Asirvatham et al. [43] who showed evidence of the formation of a thin porous silver nanoparticles coating layer at screen mesh wick at the evaporation region. The coating layer on the mesh wick surface provided an additional evaporating surface where high heat transfer rates occur. This drastically reduces the thermal resistance of the mesh wicked heat pipe and increases the capillary pumping ability.
A mathematical description of the heat pipes performance enhancement with the use of nanofluids was attempted by Qu et al. [126], who described that the thermal resistance due to nucleate boiling at the evaporator can be written as shown in Eq. 1:
where \(k_{\text{nf}}\) is the thermal conductivity, \(\rho_{\text{nf}}\) is the density, \(C_{\text{p,nf}}\) is the specific heat, \(N_{\text{b}}\) is the active nucleation site, \(D_{\text{b}}\) is the bubble release diameter, and \(f\) is the bubble release frequency. In nanofluids with low concentration, the product \(k_{\text{nf}} \rho_{\text{nf}} C_{\text{p,nf}}\) is less changed compared to the base fluid. It is rather the increase of the active nucleation site density \(N_{\text{b}}\), the bubble release diameter \(D_{\text{b}}\), and the bubble release frequency \(f\) that decreases the thermal resistance at the evaporator.
High wettability and roughness increases also were used by Ramachandran et al. [29] to explain the reduction of 44.25% of the thermal resistance of the hybrid Al2O3/CuO nanofluid compared to deionized water in a cylindrical screen mesh heat pipe, and by Sadeghinezhad et al. [30] who found a maximum reduction in the thermal resistance of 48.4% with 0.1 mass% of graphene nanoplatelets nanofluid compared with distilled water in a sintered wick heat pipe. On the other hand, the thermal performance decrease has been explained based on the reduction in the nucleation sites density due to the nanoparticles’ deposition [46, 47].
A possible explanation to the contradictory results was exposed in the review presented by Fang et al. [127], who claim that there is an optimum value of nanoparticles’ concentrations to enhance the heat transfer with nanofluids. The authors proposed that at concentration under the optimum value, the nanoparticles deposited layer may create active cavities, increase nucleation sites, and modify the surface wettability. On the other hand, at concentrations higher than the optimal value, nanoparticles deposited may block nucleation sites.
Summary and conclusions
Most experimental studies about heat pipes using nanofluids provided a few details about the preparation and dispersion of nanofluids, whose stability was reported only before being used, under rest conditions. Furthermore, during nanofluid preparation, it is common to use chemical additives such as surfactants to improve nanoparticle dispersion. However, the effects of the type of chemical additive and its concentration on the thermophysical properties of nanofluids and heat pipes performance are not commonly studied.
In addition, stability issues of nanofluids after operation cycles are not studied in depth. Some researchers reported that the hydrodynamic diameter of agglomerates was increased and that a porous layer was formed on the evaporator surface during heat pipe operation. This suggests that nanofluid stability on rest changed during heat pipe operation due to different thermal loads, phase changes, movement, and maybe functionality loss of chemical additives used during their preparation. The main issue to resolve is the reproducibility of the operation of a thermosyphon using nanofluids for a long time.
The reported performances of nanofluids in heat pipe have commonly been attributed to a porous layer formed on the evaporator surface during cycle operation. Increases or decreases in nucleation sizes and modifications in wettability during boiling on the evaporator surfaces, wicks, and grooves have been presented as responsible for the performance changes of heat pipes. Nevertheless, additional studies on the formation of the porous layer must be conducted in order to determine the effects of nanoparticle type, morphology, size, base fluid, concentration, and experimental conditions during heat pipe operation.
References
Godson L, Raja B, MohanLal D, Wongwises S. Enhancement of heat transfer using nanofluids—an overview. Renew Sustain Energy Rev. 2010;14:629–41. https://doi.org/10.1016/j.rser.2009.10.004.
Das SK, Choi SUS, Patel HE. Heat transfer in nanofluids—a review. Heat Transf Eng. 2006;27:3–19. https://doi.org/10.1080/01457630600904593.
Choi J, Eastman JA. Enhancing thermal conductivity of fluids with nanoparticles. ASME Int Mech Eng Congr Expos. 1995;231:99–105.
Sms M, Nieto de Castro CA. Nanofluids: synthesis, properties and applications. New York: Nova Science Publishers Inc; 2014.
Murshed SMS, De Castro CAN. A critical review of traditional and emerging techniques and fluids for electronics cooling. Renew Sustain Energy Rev. 2017;78:821–33. https://doi.org/10.1016/j.rser.2017.04.112.
Sohel Murshed SM. Correction and comment on “thermal conductance of nanofluids: is the controversy over? J Nanoparticle Res. 2008;11:511–2. https://doi.org/10.1007/s11051-008-9553-2.
Murshed SMS, Nietode Castro CA, Lourenço MJV, Lopes MLM, Santos FJV. A review of boiling and convective heat transfer with nanofluids. Renew Sustain Energy Rev. 2011;15:2342–54. https://doi.org/10.1016/j.rser.2011.02.016.
Faghri A. Heat pipes: review, opportunities and challenges, front. Heat Pipes. 2014. https://doi.org/10.5098/fhp.5.1.
Jouhara H, Chauhan A, Nannou T, Almahmoud S, Delpech B, Wrobel LC. Heat pipe based systems—advances and applications. Energy. 2017;128:729–54. https://doi.org/10.1016/j.energy.2017.04.028.
Reay D, Harvey A. The role of heat pipes in intensified unit operations. Appl Therm Eng. 2013;57:147–53. https://doi.org/10.1016/j.applthermaleng.2012.04.002.
Hung Y-H, Teng T-P, Lin B-G. Evaluation of the thermal performance of a heat pipe using alumina nanofluids. Exp Therm Fluid Sci. 2013;44:504–11. https://doi.org/10.1016/j.expthermflusci.2012.08.012.
Srimuang W, Amatachaya P. A review of the applications of heat pipe heat exchangers for heat recovery. Renew Sustain Energy Rev. 2012;16:4303–15. https://doi.org/10.1016/j.rser.2012.03.030.
Leong KY, Saidur R, Mahlia TMI, Yau YH. Performance investigation of nanofluids as working fluid in a thermosyphon air preheater. Int Commun Heat Mass Transf. 2012;39:523–9. https://doi.org/10.1016/j.icheatmasstransfer.2012.01.014.
Reay D, Kew P, McGlen R. Heat pipes theory, design and applications. Amsterdam: Elsevier; 2014.
Jafari D, Franco A, Filippeschi S, Di Marco P. Two-phase closed thermosyphons: a review of studies and solar applications. Renew Sustain Energy Rev. 2016;53:575–93. https://doi.org/10.1016/j.rser.2015.09.002.
ESDU 81038. Heat pipes performance of two phase closed thermosyphons. In: ESDU International PLC; 1983. p. 38.
Noie SH, Heris SZ, Kahani M, Nowee SM. Heat transfer enhancement using Al2O3/water nanofluid in a two-phase closed thermosyphon. Int J Heat Fluid Flow. 2009;30:700–5. https://doi.org/10.1016/j.ijheatfluidflow.2009.03.001.
Poplaski LM, Benn SP, Faghri A. International journal of heat and mass transfer thermal performance of heat pipes using nanofluids. Int J Heat Mass Transf. 2017;107:358–71. https://doi.org/10.1016/j.ijheatmasstransfer.2016.10.111.
Reay D (2014) Heat transfer and fluid flow theory. In: Heat pipes: theory, design and applications, 6th edn, Butterworth Heinemann; 2014, pp. 15–64.
Alawi OA, Sidik NAC, Mohammed HA, Syahrullail S. Fluid flow and heat transfer characteristics of nanofluids in heat pipes: a review. Int Commun Heat Mass Transf. 2014;56:50–62. https://doi.org/10.1016/j.icheatmasstransfer.2014.04.014.
Liu Z-H, Li Y-Y. A new frontier of nanofluid research—application of nanofluids in heat pipes. Int J Heat Mass Transf. 2012;55:6786–97. https://doi.org/10.1016/j.ijheatmasstransfer.2012.06.086.
Buschmann MH. Nanofluids in thermosyphons and heat pipes: overview of recent experiments and modelling approaches. Int J Therm Sci. 2013;72:1–17. https://doi.org/10.1016/j.ijthermalsci.2013.04.024.
Buschmann MH. Heat pipes and thermosyphons operated with nanofluids. In: Bianco V, Manca O, Nardini S, Vafai K, editors. Heat transfer enhancement of nanofluids. New York: Taylor & Francis; 2015. p. 391–407.
Sureshkumar R, Mohideen ST, Nethaji N. Heat transfer characteristics of nanofluids in heat pipes: a review. Renew Sustain Energy Rev. 2013;20:397–410. https://doi.org/10.1016/j.rser.2012.11.044.
Gupta NK, Tiwari AK, Ghosh SK. Heat transfer mechanisms in heat pipes using nanofluids—a review. Exp Therm Fluid Sci. 2018;90:84–100. https://doi.org/10.1016/j.expthermflusci.2017.08.013.
Sarafraz MM, Hormozi F, Peyghambarzadeh SM. Thermal performance and efficiency of a thermosyphon heat pipe working with a biologically ecofriendly nanofluid. Int Commun Heat Mass Transf. 2014;57:297–303. https://doi.org/10.1016/j.icheatmasstransfer.2014.08.020.
Amiri A, Sadri R, Shanbedi M, Ahmadi G, Chew BT, Kazi SN, Dahari M. Performance dependence of thermosyphon on the functionalization approaches: an experimental study on thermo-physical properties of graphene nanoplatelet-based water nanofluids. Energy Convers Manag. 2015;92:322–30. https://doi.org/10.1016/j.enconman.2014.12.051.
Kamyar A, Ong KS, Saidur R. Effects of nanofluids on heat transfer characteristics of a two-phase closed thermosyphon. Int J Heat Mass Transf. 2013;65:610–8. https://doi.org/10.1016/j.ijheatmasstransfer.2013.06.046.
Buschmann MH, Franzke U. Improvement of thermosyphon performance by employing nanofluid. Int J Refrig. 2014;40:416–28. https://doi.org/10.1016/j.ijrefrig.2013.11.022.
Hajian R, Layeghi M, AbbaspourSani K. Experimental study of nanofluid effects on the thermal performance with response time of heat pipe. Energy Convers Manag. 2012;56:63–8. https://doi.org/10.1016/j.enconman.2011.11.010.
Shanbedi M, Heris SZ, Amiri A, Baniadam M. Improvement in heat transfer of a two-phased closed thermosyphon using silver-decorated MWCNT/water. J Dispers Sci Technol. 2014;35:1086–96. https://doi.org/10.1080/01932691.2013.833101.
Tsai CY, Chien HT, Ding PP, Chan B, Luh TY, Chen PH. Effect of structural character of gold nanoparticles in nanofluid on heat pipe thermal performance. Mater Lett. 2004;58:1461–5.
Do KH, Jang SP. Effect of nanofluids on the thermal performance of a flat micro heat pipe with a rectangular grooved wick. Int J Heat Mass Transf. 2010;53:2183–92. https://doi.org/10.1016/j.ijheatmasstransfer.2009.12.020.
Putra N, Septiadi WN, Rahman H, Irwansyah R. Thermal performance of screen mesh wick heat pipes with nanofluids. Exp Therm Fluid Sci. 2012;40:10–7. https://doi.org/10.1016/j.expthermflusci.2012.01.007.
Asirvatham LG, Nimmagadda R, Wongwises S. Heat transfer performance of screen mesh wick heat pipes using silver–water nanofluid. Int J Heat Mass Transf. 2013;60:201–9. https://doi.org/10.1016/j.ijheatmasstransfer.2012.11.037.
Ghanbarpour M, Nikkam N, Khodabandeh R, Toprak MS. Improvement of heat transfer characteristics of cylindrical heat pipe by using SiC nanofluids. Appl Therm Eng. 2015;90:127–35. https://doi.org/10.1016/j.applthermaleng.2015.07.004.
Ramachandran R, Ganesan K, Rajkumar MR, Asirvatham LG, Wongwises S. Comparative study of the effect of hybrid nanoparticle on the thermal performance of cylindrical screen mesh heat pipe. Int Commun Heat Mass Transf. 2016;76:294–300. https://doi.org/10.1016/j.icheatmasstransfer.2016.05.030.
Sadeghinezhad E, Mehrali M, Rosen MA, Akhiani AR, Tahan Latibari S, Mehrali M, Metselaar HSC. Experimental investigation of the effect of graphene nanofluids on heat pipe thermal performance. Appl Therm Eng. 2016;100:775–87. https://doi.org/10.1016/j.applthermaleng.2016.02.071.
Karthikeyan VK, Ramachandran K, Pillai BC, BruslySolomon A. Effect of nanofluids on thermal performance of closed loop pulsating heat pipe. Exp Therm Fluid Sci. 2014;54:171–8. https://doi.org/10.1016/j.expthermflusci.2014.02.007.
Kim KM, Bang IC. Effects of graphene oxide nanofluids on heat pipe performance and capillary limits. Int J Therm Sci. 2016;100:346–56. https://doi.org/10.1016/j.ijthermalsci.2015.10.015.
Nazari MA, Ghasempour R, Ahmadi MH, Heydarian G, Shafii MB. Experimental investigation of graphene oxide nanofluid on heat transfer enhancement of pulsating heat pipe. Int Commun Heat Mass Transf. 2018;91:90–4. https://doi.org/10.1016/j.icheatmasstransfer.2017.12.006.
Zhou Y, Cui X, Weng J, Shi S, Han H, Chen C. Experimental investigation of the heat transfer performance of an oscillating heat pipe with graphene nanofluids. Powder Technol. 2018;332:371–80. https://doi.org/10.1016/j.powtec.2018.02.048.
Aly WIA, Elbalshouny MA, Abd El-Hameed HM, Fatouh M. Thermal performance evaluation of a helically-micro-grooved heat pipe working with water and aqueous Al2O3 nanofluid at different inclination angle and filling ratio. Appl Therm Eng. 2017;110:1294–304. https://doi.org/10.1016/j.applthermaleng.2016.08.130.
Chen Y, Wang P, Liu Z. Application of water-based SiO2 functionalized nanofluid in a loop thermosyphon. Int J Heat Mass Transf. 2013;56:59–68. https://doi.org/10.1016/j.ijheatmasstransfer.2012.09.048.
Xue HS, Fan JR, Hu YC, Hong RH, Cen KF. The interface effect of carbon nanotube suspension on the thermal performance of a two-phase closed thermosyphon. J Appl Phys. 2006;100:104909. https://doi.org/10.1063/1.2357705.
Khandekar S, Joshi YM, Mehta B. Thermal performance of closed two-phase thermosyphon using nanofluids. Int J Therm Sci. 2008;47:659–67. https://doi.org/10.1016/j.ijthermalsci.2007.06.005.
Sarafraz MM, Hormozi F, Peyghambarzadeh SM. Role of nanofluid fouling on thermal performance of a thermosyphon: Are nanofluids reliable working fluid? Appl Therm Eng. 2015;82:212–24. https://doi.org/10.1016/j.applthermaleng.2015.02.070.
Kim KM, Jeong YS, Kim IG, Bang IC. Comparison of thermal performances of water-filled, SiC nanofluid-filled and SiC nanoparticles-coated heat pipes. Int J Heat Mass Transf. 2015;88:862–71. https://doi.org/10.1016/j.ijheatmasstransfer.2015.04.108.
Mehrali M, Sadeghinezhad E, Azizian R, Akhiani AR, TahanLatibari S, Mehrali M, Metselaar HSC. Effect of nitrogen-doped graphene nanofluid on the thermal performance of the grooved copper heat pipe. Energy Convers Manag. 2016;118:459–73. https://doi.org/10.1016/j.enconman.2016.04.028.
Alhuyi Nazari M, Ahmadi MH, Ghasempour R, Shafii MB. How to improve the thermal performance of pulsating heat pipes: a review on working fluid. Renew Sustain Energy Rev. 2018;91:630–8. https://doi.org/10.1016/j.rser.2018.04.042.
Farzaneh H, Behzadmehr A, Yaghoubi M, Samimi A, Sarvari SMH. Stability of nanofluids: molecular dynamic approach and experimental study. Energy Convers Manag. 2016;111:1–14. https://doi.org/10.1016/j.enconman.2015.12.044.
Babita S, Sharma SK, Mital GS. Preparation and evaluation of stable nanofluids for heat transfer application: a review. Exp Therm Fluid Sci. 2016;79:202–12. https://doi.org/10.1016/j.expthermflusci.2016.06.029.
Devendiran DK, Amirtham VA. A review on preparation, characterization, properties and applications of nanofluids. Renew Sustain Energy Rev. 2016;60:21–40. https://doi.org/10.1016/j.rser.2016.01.055.
Anushree C, Philip J. Assessment of long term stability of aqueous nanofluids using different experimental techniques. J Mol Liq. 2016;222:350–8. https://doi.org/10.1016/j.molliq.2016.07.051.
Zhang H, Cui H, Yao S, Zhang K, Tao H, Meng H. Ionic liquid-stabilized non-spherical gold nanofluids synthesized using a one-step method. Nanoscale Res Lett. 2012;7:583. https://doi.org/10.1186/1556-276x-7-583.
Kamatchi R, Venkatachalapathy S. Parametric study of pool boiling heat transfer with nanofluids for the enhancement of critical heat flux: a review. Int J Therm Sci. 2015;87:228–40. https://doi.org/10.1016/j.ijthermalsci.2014.09.001.
Xia G, Jiang H, Liu R, Zhai Y. Effects of surfactant on the stability and thermal conductivity of Al2O3/de-ionized water nanofluids. Int J Therm Sci. 2014;84:118–24. https://doi.org/10.1016/j.ijthermalsci.2014.05.004.
Witharana S, Palabiyik I, Musina Z, Ding Y. Stability of glycol nanofluids—the theory and experiment. Powder Technol. 2013;239:72–7. https://doi.org/10.1016/j.powtec.2013.01.039.
Karthikeyan R, Mahalakshmi NV. Performance and emission characteristics of a turpentine-diesel dual fuel engine. Energy. 2007; 32:1202–1209. http://ST–performance and emission character.internal-pdf://performance-a-1527260677/Performance-and-emission-characteristics-of-a-turpentine-diesel-dual-fuel-engine_Karthikeyan_2007_Energy.pdf.
Rashmi W, Khalid M, Ong SS, Saidur R. Preparation, thermo-physical properties and heat transfer enhancement of nanofluids. Mater Res Express. 2014;1:032001. https://doi.org/10.1088/2053-1591/1/3/032001.
Yu W, Xie H. A review on nanofluids: preparation, stability mechanisms, and applications. J Nanomater. 2012;2012:1–17. https://doi.org/10.1155/2012/435873.
Rao Y. Nanofluids: stability, phase diagram, rheology and applications. Particuology. 2010;8:549–55. https://doi.org/10.1016/j.partic.2010.08.004.
Dey D, Kumar P, Samantaray S. A review of nanofluid preparation, stability, and thermo-physical properties. Heat Transf Res. 2017. https://doi.org/10.1002/htj.21282.
Mukherjee S, Paria S. Preparation and stability of nanofluids—a review. IOSR J Mech Civ Eng. 2013;9:63–9. https://doi.org/10.9790/1684-0926369.
Sidik NAC, Mohammed HA, Alawi OA, Samion S. A review on preparation methods and challenges of nanofluids. Int Commun Heat Mass Transf. 2014;4:1. https://doi.org/10.1016/j.icheatmasstransfer.2014.03.002.
Yu F, Chen Y, Liang X, Xu J, Lee C, Liang Q, Tao P, Deng T. Dispersion stability of thermal nanofluids. Prog Nat Sci Mater Int. 2017;27:531–42. https://doi.org/10.1016/j.pnsc.2017.08.010.
Pang C, Lee JW, Kang YT. Review on combined heat and mass transfer characteristics in nanofluids. Int J Therm Sci. 2015;87:49–67. https://doi.org/10.1016/j.ijthermalsci.2014.07.017.
Yang L, Du K, Zhang XS, Cheng B. Preparation and stability of Al2O3 nano-particle suspension of ammonia–water solution. Appl Therm Eng. 2011;31:3643–7. https://doi.org/10.1016/j.applthermaleng.2010.11.031.
Sarkar J, Ghosh P, Adil A. A review on hybrid nanofluids: recent research, development and applications. Renew Sustain Energy Rev. 2015;43:164–77. https://doi.org/10.1016/j.rser.2014.11.023.
Sidik NAC, Mohammed HA, Alawi OA, Samion S. A review on preparation methods and challenges of nanofluids. Int Commun Heat Mass Transf. 2014;54:115–25. https://doi.org/10.1016/j.icheatmasstransfer.2014.03.002.
Philip J, Shima PD. Thermal properties of nanofluids. Adv Colloid Interface Sci. 2012;183–184:30–45. https://doi.org/10.1016/j.cis.2012.08.001.
Fedele L, Colla L, Bobbo S, Barison S, Agresti F. Experimental stability analysis of different water-based nanofluids. Nanoscale Res Lett. 2011;6:300. https://doi.org/10.1186/1556-276x-6-300.
Menbari A, Alemrajabi AA, Ghayeb Y. Investigation on the stability, viscosity and extinction coefficient of CuO-Al2O3/water binary mixture nanofluid. Exp Therm Fluid Sci. 2016;74:122–9. https://doi.org/10.1016/j.expthermflusci.2015.11.025.
Nabati Shoghl S, Jamali J, Keshavarz Moraveji M. Electrical conductivity, viscosity, and density of different nanofluids: an experimental study. Exp Therm Fluid Sci. 2016;74:339–46. https://doi.org/10.1016/j.expthermflusci.2016.01.004.
Khairul MA, Shah K, Doroodchi E, Azizian R, Moghtaderi B. Effects of surfactant on stability and thermo-physical properties of metal oxide nanofluids. Int J Heat Mass Transf. 2016;98:778–87. https://doi.org/10.1016/j.ijheatmasstransfer.2016.03.079.
Zhu D, Li X, Wang N, Wang X, Gao J, Li H. Dispersion behavior and thermal conductivity characteristics of Al2O3–H2O nanofluids. Curr Appl Phys. 2009;9:131–9. https://doi.org/10.1016/j.cap.2007.12.008.
Mahbubul IM, Saidur R, Hepbasli A, Amalina MA. Experimental investigation of the relation between yield stress and ultrasonication period of nanofluid. Int J Heat Mass Transf. 2016;93:1169–74. https://doi.org/10.1016/j.ijheatmasstransfer.2015.10.046.
Mahbubul IM, Saidur R, Amalina MA, Elcioglu EB, Okutucu-Ozyurt T. Effective ultrasonication process for better colloidal dispersion of nanofluid. Ultrason Sonochem. 2015;26:361–9. https://doi.org/10.1016/j.ultsonch.2015.01.005.
Nguyen VS, Rouxel D, Hadji R, Vincent B, Fort Y. Effect of ultrasonication and dispersion stability on the cluster size of alumina nanoscale particles in aqueous solutions. Ultrason Sonochem. 2011;18:382–8. https://doi.org/10.1016/j.ultsonch.2010.07.003.
Li Y, Zhou J, Tung S, Schneider E, Xi S. A review on development of nanofluid preparation and characterization. Powder Technol. 2009;196:89–101. https://doi.org/10.1016/j.powtec.2009.07.025.
Hassan MI, Alzarooni IA, Shatilla Y. The effect of water-based nanofluid incorporating Al2O3 nanoparticles on heat pipe performance. Energy Procedia. 2015;75:3201–6. https://doi.org/10.1016/j.egypro.2015.07.674.
Li X, Zhu D, Wang X. Evaluation on dispersion behavior of the aqueous copper nano-suspensions. J Colloid Interface Sci. 2007;310:456–63. https://doi.org/10.1016/j.jcis.2007.02.067.
Lee JH, Kam DH, Jeong YH. The effect of nanofluid stability on critical heat flux using magnetite-water nanofluids. Nucl Eng Des. 2015;292:187–92. https://doi.org/10.1016/j.nucengdes.2015.05.026.
Ismay MJL, Doroodchi E, Moghtaderi B. Effects of colloidal properties on sensible heat transfer in water-based titania nanofluids. Chem Eng Res Des. 2013;91:426–36. https://doi.org/10.1016/j.cherd.2012.10.005.
Zafarani-Moattar MT, Shekaari H, Munes-Rast R, Majdan-Cegincara R. Stability and rheological properties of nanofluids containing ZnO nanoparticles, poly(propylene glycol) and poly(vinyl pyrrolidone). Fluid Phase Equilib. 2015;403:136–44. https://doi.org/10.1016/j.fluid.2015.06.013.
Ghadimi A, Saidur R, Metselaar HSC. A review of nanofluid stability properties and characterization in stationary conditions. Int J Heat Mass Transf. 2011;54:4051–68. https://doi.org/10.1016/j.ijheatmasstransfer.2011.04.014.
Haghighi EB, Nikkam N, Saleemi M, Behi M, Mirmohammadi SA, Poth H, Khodabandeh R, Toprak MS, Muhammed M, Palm B. Shelf stability of nanofluids and its effect on thermal conductivity and viscosity. Meas Sci Technol. 2013;24:105301. https://doi.org/10.1088/0957-0233/24/10/105301.
Bhattacharjee S. DLS and zeta potential—What they are and what they are not? J Control Release. 2016;235:337–51. https://doi.org/10.1016/j.jconrel.2016.06.017.
Angayarkanni SA, Philip J. Review on thermal properties of nanofluids: recent developments. Adv Colloid Interface Sci. 2015;5:1. https://doi.org/10.1016/j.cis.2015.08.014.
Sadeghy R, Haghshenasfard M, Etemad SG, Keshavarzi E. Investigation of alumina nanofluid stability using experimental and modified population balance methods. Adv Powder Technol. 2016;27:2186–95. https://doi.org/10.1016/j.apt.2016.08.003.
Tantra R, Schulze P, Quincey P. Effect of nanoparticle concentration on zeta-potential measurement results and reproducibility. Particuology. 2010;8:279–85. https://doi.org/10.1016/j.partic.2010.01.003.
Li X, Chen Y, Mo S, Jia L, Shao X. Effect of surface modification on the stability and thermal conductivity of water-based SiO2-coated graphene nanofluid. Thermochim Acta. 2014;595:6–10. https://doi.org/10.1016/j.tca.2014.09.006.
Simonsen MCAJ. The importance of suspension stability for the hot-wire measurements of thermal conductivity of colloidal suspensions. In: 16th Australasian fluid mechanics conference (AFMC); (2007), p. 1154–57.
Huminic G, Huminic A, Morjan I, Dumitrache F. Experimental study of the thermal performance of thermosyphon heat pipe using iron oxide nanoparticles. Int J Heat Mass Transf. 2011;54:656–61. https://doi.org/10.1016/j.ijheatmasstransfer.2010.09.005.
Menlik T, Sözen A, Gürü M, Öztaş S. Heat transfer enhancement using MgO/water nanofluid in heat pipe. J Energy Inst. 2015;88:247–57. https://doi.org/10.1016/j.joei.2014.10.001.
Liu Z, Yang X, Wang G, Guo G. Influence of carbon nanotube suspension on the thermal performance of a miniature thermosyphon. Int J Heat Mass Transf. 2010;53:1914–20. https://doi.org/10.1016/j.ijheatmasstransfer.2009.12.065.
Grab T, Gross U, Franzke U, Buschmann MH. Operation performance of thermosyphons employing titania and gold nanofluids. Int J Therm Sci. 2014;86:352–64. https://doi.org/10.1016/j.ijthermalsci.2014.06.019.
Parametthanuwat T, Rittidech S, Pattiya A, Ding Y, Witharana S. Application of silver nanofluid containing oleic acid surfactant in a thermosyphon economizer. Nanoscale Res Lett. 2011;6:315. https://doi.org/10.1186/1556-276X-6-315.
Liu ZH, Zhu QZ. Application of aqueous nanofluids in a horizontal mesh heat pipe. Energy Convers Manag. 2011;52:292–300.
Shanbedi M, Zeinali Heris S, Baniadam M, Amiri A. The effect of multi-walled carbon nanotube/water nanofluid on thermal performance of a two-phase closed thermosyphon. Exp Heat Transf. 2013;26:26–40. https://doi.org/10.1080/08916152.2011.631078.
Azizi M, Hosseini M, Zafarnak S, Shanbedi M, Amiri A. Experimental analysis of thermal performance in a two-phase closed thermosyphon using graphene/water nanofluid. Ind Eng Chem Res. 2013;52:10015–21. https://doi.org/10.1021/ie401543n.
Tong WL, Ong W, Chai S, Tan MK, Hung YM. Enhanced evaporation strength through fast water permeation in graphene-oxide deposition. Nat Publ Gr. 2015;5(11896):1–13. https://doi.org/10.1038/srep11896.
Sahooli M, Sabbaghi S, Niassar MS. Preparation of CuO/water nanofluids using polyvinylpyrolidone and a survey on its stability and thermal conductivity. Int J Nanosci Nanotechnol. 2012;8:27–34.
Saleh R, Putra N, Wibowo RE, Septiadi WN, Prakoso SP. Titanium dioxide nanofluids for heat transfer applications. Exp Therm Fluid Sci. 2014;52:19–29. https://doi.org/10.1016/j.expthermflusci.2013.08.018.
Zawrah MF, Khattab RM, Girgis LG, El Daidamony H, Abdel Aziz RE. Stability and electrical conductivity of water-base Al2O3 nanofluids for different applications. HBRC J. 2015. https://doi.org/10.1016/j.hbrcj.2014.12.001.
Ghadimi A, Metselaar IH. The influence of surfactant and ultrasonic processing on improvement of stability, thermal conductivity and viscosity of titania nanofluid. Exp Therm Fluid Sci. 2013;51:1–9. https://doi.org/10.1016/j.expthermflusci.2013.06.001.
Haddad Z, Abid C, Oztop HF, Mataoui A. A review on how the researchers prepare their nanofluids. Int J Therm Sci. 2014;76:168–89. https://doi.org/10.1016/j.ijthermalsci.2013.08.010.
Hetsroni G, Zakin J, Lin Z, Mosyak A, Pancallo E, Rozenblit R. The effect of surfactants on bubble growth, wall thermal patterns and heat transfer in pool boiling. Int J Heat Mass Transf. 2001;44:485–97. https://doi.org/10.1016/s0017-9310(00)00099-5.
Sarafraz MM, Hormozi F. Experimental study on the thermal performance and efficiency of a copper made thermosyphon heat pipe charged with alumina–glycol based nanofluids. Powder Technol. 2014;266:378–87. https://doi.org/10.1016/j.powtec.2014.06.053.
Yang X-F, Liu Z-H. Flow boiling heat transfer in the evaporator of a loop thermosyphon operating with CuO based aqueous nanofluid. Int J Heat Mass Transf. 2012;55:7375–84. https://doi.org/10.1016/j.ijheatmasstransfer.2012.07.026.
Liu ZH, Yang XF, Guo GL. Effect of nanoparticles in nanofluid on thermal performance in a miniature thermosyphon. J Appl Phys. 2007;102:013526. https://doi.org/10.1063/1.2748348.
Sözen A, Menlik T, Gürü M, Irmak AF, Kılıç F, Aktaş M. Utilization of fly ash nanofluids in two-phase closed thermosyphon for enhancing heat transfer. Exp Heat Transf. 2016;29(3):337–54. https://doi.org/10.1080/08916152.2014.976724.
Parametthanuwat T, Rittidech S, Pattiya A. A correlation to predict heat-transfer rates of a two-phase closed thermosyphon (TPCT) using silver nanofluid at normal operating conditions. Int J Heat Mass Transf. 2010;53:4960–5. https://doi.org/10.1016/j.ijheatmasstransfer.2010.05.046.
X. Yang, Z. Liu, Application of functionalized nanofluid in thermosyphon, (2011) 1–12.
Huminic G, Huminic A. Heat transfer characteristics of a two-phase closed thermosyphons using nanofluids. Exp Therm Fluid Sci. 2011;35:550–7. https://doi.org/10.1016/j.expthermflusci.2010.12.009.
Shanbedi M, Heris SZ, Baniadam M, Amiri A, Maghrebi M. Investigation of heat-transfer characterization of EDA-MWCNT/DI-water nanofluid in a two-phase closed thermosyphon. Ind Eng Chem Res. 2012;51:1423–8.
Heris SZ, Mohammadpur F, Shakouri A. Effect of electric field on thermal performance of thermosyphon heat pipes using nanofluids. Mater Res Bull. 2014;53:21–7. https://doi.org/10.1016/j.materresbull.2014.01.030.
ZeinaliHeris S, Fallahi M, Shanbedi M, Amiri A. Heat transfer performance of two-phase closed thermosyphon with oxidized CNT/water nanofluids. Heat Mass Transf. 2015. https://doi.org/10.1007/s00231-015-1548-9.
Asirvatham LG, Wongwises S, Babu J. Heat transfer performance of a glass thermosyphon using graphene—acetone nanofluid. J Heat Transf. 2016;137:1–9. https://doi.org/10.1115/1.4030479.
Mohideen ST, Suresh Kumar R. An experimental investigation of the thermal performance of two-phase closed thermosyphon using zirconia nanofluid. Therm Sci. 2016;20:1565–74. https://doi.org/10.2298/tsci140403116t.
Soleymaniha M, Amiri A, Shanbedi M, Chew BT, Wongwises S. Water-based graphene quantum dots dispersion as a high-performance long-term stable nanofluid for two-phased closed thermosyphons. Int Commun Heat Mass Transf. 2018;95:147–54. https://doi.org/10.1016/j.icheatmasstransfer.2018.05.009.
Goshayeshi HR, Goodarzi M, Dahari M. Effect of magnetic field on the heat transfer rate of kerosene/Fe2O3 nanofluid in a copper oscillating heat pipe. Exp Therm Fluid Sci. 2015;68:663–8. https://doi.org/10.1016/j.expthermflusci.2015.07.014.
Ozsoy A, Corumlu V. Thermal performance of a thermosyphon heat pipe evacuated tube solar collector using silver-water nanofluid for commercial applications. Renew Energy. 2018;122:26–34. https://doi.org/10.1016/j.renene.2018.01.031.
Xing M, Yu J, Wang R. Performance of a vertical closed pulsating heat pipe with hydroxylated MWNTs nanofluid. Int J Heat Mass Transf. 2017;112:81–8. https://doi.org/10.1016/j.ijheatmasstransfer.2017.04.112.
Gupta NK, Tiwari AK, Ghosh SK. Heat transfer mechanisms in heat pipes using nanofluids: a review. Exp Therm Fluid Sci. 2017;90:84–100. https://doi.org/10.1016/j.expthermflusci.2017.08.013.
Qu J, Ying Wu H, Cheng P. Thermal performance of an oscillating heat pipe with Al2O3-water nanofluids. Int Commun Heat Mass Transf. 2010;37:111–5. https://doi.org/10.1016/j.icheatmasstransfer.2009.10.001.
Fang X, Chen Y, Zhang H, Chen W, Dong A, Wang R. Heat transfer and critical heat flux of nanofluid boiling: a comprehensive review. Renew Sustain Energy Rev. 2016;62:924–40.
Acknowledgements
The authors gratefully acknowledge the financial support of COLCIENCIAS—Colombia (1118-669-46092 CTO: 126-2015), Universidad Nacional de Colombia, and Thermal Sciences Lab at Instituto Tecnológico Metropolitano de Medellín—Colombia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cacua, K., Buitrago-Sierra, R., Herrera, B. et al. Nanofluids’ stability effects on the thermal performance of heat pipes. J Therm Anal Calorim 136, 1597–1614 (2019). https://doi.org/10.1007/s10973-018-7787-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7787-5