Abstract
High thermal conductivity in phase change materials (PCM) is preferred in thermal energy storage (TES) systems. Carbon additives are considered as suitable materials for this purpose; however, some important issues such as price and stability for these materials should be considered. In this study, graphite powder and multiwall carbon nanotubes as an inexpensive and a relatively expensive carbon additives were used to improve the thermal conductivity of a long-chain paraffin (P) with a melting peak point of 64 °C. Also an amine functionalization method was applied to enhance the dispersion of CNTs into the non-polar paraffin medium. At first, the thermal conductivity and stability of the materials, as important and critical properties, were evaluated. In the next step, atmospheric impregnation method was conducted to incorporate modified PCM into the expanded perlite (EP) particles and finally, a lightweight concrete was constructed by these form-stable EP/P composites. Thermal behavior of the final concrete sample was studied by a simple handmade apparatus. Also, DSC analysis was conducted to determine the melting/freezing point and latent heat value for modified paraffin and form-stable PCM composites. The maximum allowable volume percent of EP usage for having a structural concrete was also determined by compression strength analysis. Thermal behavior analysis showed promising results for TES of the concrete block containing modified paraffin as phase change materials; also it was found that in form-stable technique, the stability of the additives is not significantly effective due to the narrow pores and channels, which reduce the effect of particles precipitation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Regarding high consumption rate of non-renewable energy sources, latent heat thermal energy storage (LHTES) has attracted much attention recently. Since a considerable part of energy consumption occurs inside the buildings, it could be a suitable place for using phase change materials. Incorporation of phase change materials in building elements and especially concrete was taken into consideration in recent decades. Preparing a composite PCM by impregnating melted phase change materials into the porosity of lightweight aggregates (LWA) was introduced as a common way to incorporate PCMs into the concrete. Porous aggregates like perlite, diatomite and vermiculate had many advantages in this method; being inexpensive, lightweight, inert and porous are some of the desirable properties of these materials. However, there are some drawbacks like leakage and loss in mechanical strength in using these materials.
Form-stable as a relatively new and effective method was taken into consideration recently. In this method, PCM was incorporated into the porosity of lightweight aggregates (LWAs) including perlite, vermiculate, diatomite. Concretes made by these modified aggregates will operate as thermal energy storage systems. Better mechanical properties in comparison with PCM direct impregnation and lower PCM leakage could be considered as advantages of this method. Lu et al. [1] made a form-stable PCM by impregnating paraffin into the expanded perlite (EP) particles. Prepared form-stable PCM particles were incorporated into different types of concrete with MOC and ordinary Portland cement. The energy storage capacity of the effective sample containing 10 vol% paraffin with MOC cement showed 5804 J latent heat in 14-min heating process; however, thermal conductivity decreased from 1.88 to 0.37 W m−1 K−1. In another study, Memon et al. [2] used Kaolin and ground granulated blast-furnace slag (GGBS) as supporting materials for paraffin (17.5 mass% for Kaolin, 8.8 mass% for GGBS). Form-stable PCM composites were incorporated into the cement paste panel for thermal performance evaluation. They found that the indoor temperature decreased to 4.9 °C and 4.1 °C for panel containing Kaolin and GGBS, respectively. Sun et al. [3] tried to enhance the thermal conductivity of a form-stable paraffin/EP by graphite. They prepared paraffin samples with a different mass fraction of graphite (0, 2, 5 and 10%) and impregnated them into the EP by physical adsorption. They found that thermal conductivity of the paraffin/EP increased with increasing the mass fraction of graphite; however, due to decreasing the latent heat of PCMs by increasing graphite amount, 5% mass% was determined as the optimal mass fraction.
According to the previous researches, paraffin could be a suitable choice as a PCM for embedding in the concrete structure. It has desirable transition temperature range, high latent heat and chemical stability. Also, paraffin has relatively good compatibility with concrete and other building materials [4]. Despite all these advantages, there are some drawbacks like low thermal conductivity. This shortage would be a matter when high rate heat transfer is needed during the transition cycles. However, the thermal conductivity of paraffin could be improved by adding high conductive materials. Among all of these materials, carbon compounds due to their high thermal conductivity and relatively low density have attracted more consideration. Carbon nanotubes (CNTs) play a crucial role in developing field of nanotechnology due to their high potential and unique properties in mechanical and thermodynamic applications [5]. Also various methods of CNT chemical functionalization have been proved quite successful in introducing functional moieties that contribute to better nanotube dispersion in all kinds of matrix [6]. On the other hand, graphite powder is a kind of inorganic mater with excellent thermal conductive property [7]. Fethi et al. [8] succeeded to increase the thermal conductivity of paraffin about four times by using 20% graphite waste in three modes of fibers, fins and foam. However, latent heat of the composite declined about 20% by adding graphite. In another study, Min Li [9] used nano-graphite (NG) to improve the thermal conductivity of paraffin. Test results showed that the NG layers were dispersed randomly in the paraffin and increase the thermal conductivity up to 741% by adding 10 mass% of NG. Cui et al. [10] incorporated the carbon nanofiber (CNF) and carbon nanotube (CNT) into the soy wax and paraffin wax, aiming to improve their thermal conductivities. They found that CNF showed better performance in thermal conductivity (40% improvement by 10 mass%) in comparison with CNT due to its better dispersion in the matrix.
Numerous studies were conducted in preparing form-stable PCM by different PCM materials and LWAs [11,12,13,14,15,16,17]; but most of the time, two major issues are not considered. First, the economical consideration of the construction in real scales and second, less attention has been paid for the final step of these efforts which is constructing a concrete block containing form-stable PCM and evaluating the thermal behavior. For some special buildings in desert areas which the spot temperature rose up to even 60 °C and also for retaining walls around the low temperature furnaces, a layer of PCM-concrete composites between the insulation layers, which contain paraffin with higher melting point which has low cost and also available from industries waste, could be very useful.
In this study, it was tried to improve the thermal conductivity of paraffin as a phase change material, by adding some conductive materials. Also, a functionalization procedure was conducted to enhance the dispersion and stability of these additives in paraffin matrices. Carbon additives were incorporated into the EP as supporting material and consequently used as lightweight aggregates in concrete. Final concrete blocks were tested thermally and mechanically to be evaluated as a promising alternative for building materials.
Materials and methods
Expanded perlite was used as supporting material with the particle size of 2.38–4.78 mm (mesh #8) and provided by Zanjan Perlite Co. Iran (Fig. 1). The thermal conductivity of EP particles was about 0.05 W m−1 K−1 in 24 °C, and also chemical composition of the selected EP is shown in Table 1. Paraffin wax (N-Nonacosane–C29H60) produced by Dr. Mojallali Industrial Chemical Complex Co. Iran, with melting temperature 56–58 °C, was used as PCM. Detailed PCM properties are described in Table 2. Pristine multi-walled carbon nanotubes (MWCNTs) synthesized by chemical vapor deposition process (diameter 20–30 nm and purity > 95%) was purchased from US Research Nanomaterials, Inc. Graphite powder (SG = 2.09–2.23, mesh: 150–200) also was used as a thermal conductivity enhancer purchased from Daejung Company, Korea. All reagents and solvents including ethanol and dodecylamine were obtained from Merck Inc.
Functionalization procedure
In this research, two kinds of carbon materials were applied as thermal conductivity enhancers: MWCNTs as a stable material and graphite powder as an available and also affordable material. A functionalization process was conducted in order to obtain a stable PCM-nanomaterial composite. Following is a brief explanation about the functionalization steps; pristine MWCNTs (0.2 g) were suspended in 100 mL of sulfuric acid/nitric acid solution (3:1 v/v) by sonication and refluxed for 4 h in 140 °C. Then, suspensions were filtered and washed to reach to the neutral pH value then dried at 60 °C. Prepared acid functionalized MWCNTs were suspended in 50 mL of ethanol by sonication. 3 g N,N′-dicyclohexylcarbodiimide (DCC) as an activating agent and finally, 3 g dodecylamine were added to the suspension and stirred for 24 h in 70 °C. The suspension was washed, filtered and dried at 60 °C. Figure 2 shows a schematic design of the functionalization process. At the final stage, different mass fractions of prepared carbon materials were added to the melted paraffin by stirring and sonication process before incorporating into the EP particles.
Preparation of the PCM composite
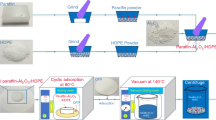
In order to further similarity to the industrial conditions, preparation of EP-modified paraffin composite was made by the atmospheric impregnation method. First, expanded perlite particles have been oven-dried up to 105 °C for 24 h to remove any possible moisture. Paraffin was heated in a container above its melting temperature, and pretreated EPs were added gently and fully soaked in melted paraffin at ambient temperature. The mixture was kept in the oven for 6 h, and the mixing process was repeated every hour until the paraffin was uniformly dispersed and easily penetrated into the expanded perlite porosity. Finally, expanded perlite/paraffin composites (EP/P) were cooled down to ambient temperature. Three samples of form-stable PCM composite were prepared by adding 0.5 mass% graphite, 0.5 mass% DDA-CNT and 10 mass% graphite with a sample without any carbon additives as the control sample (Fig. 3).
Concrete samples are prepared according to the mix design in Table 3 in 15 × 15 × 5 cm molds. Samples were kept in molds for 24 h and then were placed in a water pond for 27 days. Finally, samples were dried at room temperature for 7 days before the test. It is worth mentioning that for samples number 2–4, all expanded perlite particles were soaked in water and used in surface saturated dry (SSD) state in order to prevent any mismatch in the mix design.
Characterization
Mercury intrusion porosimetry (MIP) was used to characterize the porosity and pore size distribution of the expanded perlite particles. The MIP test was conducted on Thermo Finnigan Pascal 140 and Pascal 440 from Thermo Fischer Instruments. Fourier transform infrared spectroscopy (FTIR) was conducted to study the type of functional groups attached to the MWCNTs. The FTIR spectra were recorded by FTIR-Avatar 370 Nicolet spectrometer from 400 to 4000 cm−1, and the samples were placed on KBr pellets. Differential scanning calorimeter (DSC 204, Netzsch Instrument Inc., Germany) is adopted to test the phase change temperatures and latent heat values of the paraffin and composite PCMs. The heating and cooling rates were 5 °C min−1 during the period of 20–110 °C in a nitrogen atmosphere. The morphology and microstructures of the carbon materials, EP and paraffin/EP form-stable PCM were observed by using a TE-SCAN field emission scan electron microscope (FESEM). The thermal conductivities of PCMs were measured by a KD2 Pro Decagon Devices, Inc. with the relative standard uncertainty of ± 3%. The compressive strength of the samples was measured according to ASTM C39 at the loading speed of 2.43 KN S−1; a 200-ton capacity compression jack was used for loading.
Experimental
Porosimetry
The total porosity of the expanded perlite was determined by the following expression:
where ρa is the apparent density which was determined by the principle of Archimedes and ρs is the skeletal density of the expanded perlite particles which was evaluated by weighting and volumetric measurements of the ground expanded perlite particles. In order to reduce the error, three samples with different amount were tested in three replicates. Expanded perlite particles were dried at 110 °C for 24 h before each measurement. The porosity was calculated about 88%, which was a little lower than the amount declared by the manufacturer (90%). Using MIP method helps to determine the percentage of connected porosity and pore size distribution information in specific pore size ranges. The detailed MIP data of the expanded perlite particles are summarized in Table 4.
Figure 4 shows the MIP test results of the expanded perlite before and after paraffin impregnation process in a range of 4–140 µm of pore diameter. It seems that about half of the pores in 4–140 µm range were filled with paraffin by the atmospheric method. The detailed data related to these figures are summarized in Table 5.
Leakage test
Evaluating leakage issue, oozing circle method proposed by Ma et al. [18] was conducted to investigate the leakage condition in EP/P composite. At the second procedure, certain amount of EP/P composite particles dispersed into a circle drawn on a filter paper with a diameter of 70 mm and then it was heated in the oven for half an hour at 80 °C. This step was repeated till no leakage trace can be seen on the paper. Finally, the EP/paraffin composites were weighted in each step and the impregnation amounts are calculated in Table 6. Also the results of the oozing leakage test are shown in Fig. 5.
According to Table 6 and results from oozing circle test, it could be concluded that no leakage occurs in composites containing 31.5 mass% of paraffin and it was determined as maximum impregnation amount of paraffin into the expanded perlite porous structure.
FTIR
FTIR technique was used to prove the functionalization process of chemical groups on CNTs surface. Figure 6 shows the FTIR spectra of pristine MWCNTs and graphite and also MWCNTs-DDA.
Regarding the spectrum of pristine MWCNT, the broad absorption bands at 3650 cm−1 may be assigned to O–H stretching vibrations (due to humidity content). Also, absorption peaks at 1550–1650 cm−1 could be described as vibrations of C=C. For pristine graphite, there was another strong broad spectrum observed at 1030 cm−1 and it could be assigned to C–O stretching vibrations. After acid treatment, the O–H peak at 3650 cm−1 was strengthened due to the presence of the carboxyl group. The spectrum at 1720 cm−1 may be assigned to the C=O stretching vibration and could be a good confirmation of the oxidation process. In the spectrum of DDA-CNT, peaks at 1020 and 1580 cm−1 were observed in the spectrum which may be assigned to the C–N stretching and N–H bending, respectively. The presence of bands in the range of 3300–3500 cm−1 also could be due to the NH2 stretch of the amine group. Finally, last two peaks at 2918 and 2850 cm−1 were assigned to the CH3– and –CH2– bending vibration which could be a further confirmation for attachment of the amine moieties (dodecyl amine) to the MWCNTs.
In the next phase of the experiments which was related to the paraffin impregnation into the expanded perlite porosity, FTIR analysis was conducted for paraffin, expanded perlite and EP/P composite (Fig. 7). All peaks observed for paraffin spectrum were described as follows; the peak at 720 cm−1 may assign to the CH2 bending vibration. Peaks were observed at 1377 cm−1 and 1466 cm−1 corresponded to CH bending vibration. Also, peaks observed at 2918 cm−1 and 2850 cm−1 corresponded to the CH asymmetric and symmetric stretching vibration, respectively. In the expanded perlite spectrum, an observed peak around 450 cm−1 corresponded to the SiO asymmetric stretching vibration [19, 20]. The next peaks at 789 cm−1 and 1092 cm−1 were assigned to the SiO–H and Si–O–Si asymmetric stretching [21]. Finally, a peak observed between 3300 and 3700 cm−1 could be attributed to the –OH asymmetric stretching vibration groups of –Si–OH and also moisture content which may adsorb into the perlite porosity [21]. The spectrum related to the EP/P composite contained all the absorption peaks of the paraffin and EP without any additional band as it was expected. According to the FTIR results, it could be concluded that any chemical reaction has not occurred between paraffin and expanded perlite and all interactions were determined as physical engagements.
DSC tests
The phase change temperatures and latent heats of pure paraffin, paraffin containing 0.5% and 10 mass% of graphite powder were measured by DSC analysis (Fig. 8). Detailed thermal characteristics of the samples are listed in Table 1.
Reviewing curve of the pure paraffin, one relatively weak peak at temperature about 47 °C and another strong peak at 64 °C were observed. The first one could be attributed to the solid-to-solid structure change, and the other one could be considered as solid–liquid phase transition. Two composites of the paraffin also showed relatively similar behavior with some slight differences. Considering Table 1, it is obvious that by incorporating graphite powder both melting/freezing temperature and enthalpy were undergoing changes. As it is clear, decreasing the supercooling temperature which is defined as the difference between melting temperature (Tm) and freezing temperature (Tf) would be desirable in thermal storage systems. From Table 1, it can be observed that by adding 0.5 mass% graphite powder to the paraffin, supercooling temperature decreased about 4.2 °C and also the melting and freezing heat value increased 25 J g−1 and 11.8 J g−1, respectively. These phenomena could be attributed to the nucleation process; graphite powder acted as a nucleation agent and provided a heterogeneous nucleation in comparison with pure paraffin (Table 7). As a result, a mild melting and crystallization process occurred and the supercooling effect reduced significantly. According to this argument, it was predicted that for paraffin sample containing 10 mass% graphite, the similar behavior must be observed. As it was expected, supercooling temperature in this sample decreased to 10.5 °C, However, the melting and freezing heat value decreased about 15.6% and 12.9%. Two possible effects could be involved in this issue; decreasing the ratio of phase change material (paraffin) in the composite and crystallization restriction due to the extra amount of graphite powder [22].
The resulting curves of the paraffin samples containing 0.5 mass% of pristine MWCNT and DDA-CNT are shown in Fig. 9, and the phase change parameters obtained from DSC analysis are summarized in Table 8. Comparing the samples with the same amount of nanoparticles, amine functionalized carbon nanotubes (DDA-CNT) showed lower supercooling temperature and higher latent heat value. Supercooling temperature decreased from 15.5 °C which attributed to the pure paraffin to 11.3 °C by adding 0.5 mass% functionalized MWCNT; however, it did not change significantly by adding the same amount of pristine MWCNT. On the other hand, melting and freezing heat value increased about 5% for the composite containing 0.5 mass% functionalized carbon nanotubes, while these values decreased to 3% and 2.6% for pristine MWCNT, respectively. These behaviors can be explained by better dispersibility of functionalized MWCNTs in paraffin matrix due to their amine moieties. In comparison with pristine MWCNTs, functionalized nanoparticles could form more homogenous nucleation site which is much desirable for the rate of crystallization growth in paraffin phase change process [22].
Figure 10 shows DSC curves of the expanded perlite, paraffin and two kinds of prepared EP/P composites with different paraffin impregnation amounts.
The melting temperature of the both prepared form-stable PCM composites decreased very little for form-stable 1 and much more for form-stable 2 in comparison with pure paraffin. The weak attractive interaction between paraffin and internal surfaces of the perlite porous media which may affect the crystallization process was introduced as a possible reason for this behavior in some literature [23]. Also, the heating value of the composites decreased 37% and 68% for form-stable 1 and form-stable 2, respectively, due to the reduction in the mass fraction of paraffin (Table 9).
Table 10 presents a comparative study of the thermal properties of the prepared samples in this research with other expanded perlite/paraffin composites reported in the literature. It can be seen that the results are in good agreement with the results of previous studies.
Thermal reliability of form-stable composite PCM
Thermal stability and reliability over a large number of melting/freezing cycles are one of the crucial aspects of PCM composites [16]. In this study, thermal cycling tests were performed by applying a heating/cooling program in PID temperature-controlled oven to determine the changes in thermal stability and chemical structure of the composite PCM after repeated 300 melting–freezing cycles.
Figure 11 illustrates the DSC curves of the EP/P composite before and after thermal cycling test. The phase change temperatures of samples were 63.8 and 64.2 °C for EP/P composites before and after thermal cycling, respectively. Also the figures for latent heat values of samples were 125 J g−1 for before and 118 J g−1 for after thermal cycling test (0.056% decrease). This indicates that there was no significant change in the thermal properties of PCM composite after thermal cycling test.
SEM results
The morphology of MWCNTs, graphite, EP and prepared EP/P composites were investigated via SEM analysis, and the results are shown in Fig. 12. Agglomerated CNTs are observed in Fig. 12a due to the van der Waals forces; also, the dimension of the CNTs was in agreement with what announced by the supplier. Figure 12b shows the flaky morphology of graphite particles. As shown in Fig. 12c, EP exhibits a highly porous structure consisting of rough micro-pores, which prepared a suitable media for the absorption of molten paraffin. Physical adsorption and capillary forces prevent the paraffin seepage from expanded perlite porous structure. In the SEM image of EP/P (Fig. 12d), it seemed that by incorporation of paraffin a soft layer uniformly covered the rough surface of expanded perlite and less visible pores were observed. Figure 12e, f is related to the EP/P composites containing CNT and graphite, respectively. The CNTs and graphite particles are seen clearly at SEM images.
Stability in solvent and PCM
Evaluating the dispersion and stability of the carbon additives, the same amount of graphite, MWCNT and DDA-CNT were dissolved in a non-polar solvent (Toluene) by the ultrasonic bath for 30 min. Figure 13 shows the comparisons of the dispersion and stability for all three solutions. After 10 min, most of the graphite powders were sedimented due to their large dimensions but other two samples were still stable. After 24 h, graphite particles were completely sedimented and also MWCNTs agglomerated and partially settled down; however, any changes were not observed in DDA-CNT nanoparticles stability. It was found that the DDA function grafted to CNTs outer surface increased the distance among CNTs and causes the reduction of van der Waals interactions among CNTs; it could be the reason of the improvement in the dispersion and long-term stability of CNT nanoparticles in toluene.
The same scenario was repeated for melted paraffin. Figure 14 shows the results of the dispersion and stability of graphite, MWCNTs and DDA-CNT into the melted paraffin. Immediately after 30-min sonication process, all three samples looked uniform and steady. After 2 h, graphite sample turned to a lighter color due to the partial precipitation of graphite particles. Also, some CNT coagulation and sedimentation were observed in MWCNT sample. After 24 h, paraffin sample containing graphite powder completely divided into two distinct phases and all graphite powders sedimented to the bottom. Also in MWCNT sample, all nanoparticles were sedimented or suspended as agglomerated bulks. However, DDA-CNT sample was completely stable with no precipitate due to the presence of amine functions, even after 24 h. In this case, besides the mentioned reason for the stability, long chains of paraffin grafted into the relatively long chains of DDA-CNTs and prevented the fast sedimentation.
Thermal conductivity
The thermal conductivity of the paraffin with different amount of carbon additives was measured in the solid state at room temperature (25 °C). Each measurement was replicated ten times, and the average was reported. Figure 15 shows the variation of the thermal conductivity of the paraffin as a function of the concentration of carbon additives.
It was found that by increasing the concentration of carbon additives into the paraffin, the thermal conductivity coefficient increased. In a same percentage, graphite powder showed a higher conductivity which could be related to its planar structure. The following equation was developed for thermal conductivity coefficient of paraffin with the mass percentage of graphite powder by R2 = 0.976:
where k represents the thermal conductivity coefficient (W m−1 K−1) and x is the mass percentage of graphite powder.
Thermal behavior of concrete
Thermal behavior of the prepared concrete samples was experimentally investigated via a simple handmade apparatus. The experimental setup which is schematically described in Fig. 16 was able to record the temperature changes of the outer surface, while the inner surface was exposed to a constant heat flux. A flat 100 W electric heater was used as a heat source inside a Teflon box. Temperature changes were recorded via T-type (0.1 °C) thermocouples connected to a data logger. Four-sided surfaces of the samples were insulated by insulating paste and expanded perlites to prevent heat losses.
EP/P particles with 50 mass% paraffin were used as the form-stable composites in the concrete samples. Figure 17 demonstrates the topside temperature variation of the concrete samples with time while electric heater turned on. Room temperature was relatively constant at 19 °C, and the experiment started at the same temperature. The experiment lasted for more than 12 h and approached to the steady state; temperatures were recorded every 30 s during this time period. The temperature related to the concrete sample containing expanded perlite reached 63 °C and it was almost stable. Cement paste sample showed relatively higher temperature as it was expected, and it was stabled at about 68 °C. Expanded perlite particles, due to their highly porous structure, are considered as good thermal insulators. By adding expanded perlite particles to the cement paste, the thermal conductivity of the sample was decreased and consequently, the thermocouple, which was recording the topside temperature, showed lower temperature in comparison with the cement paste sample. For the sample containing EP/P form-stable composite, the temperature was stabled at about 65 °C. By impregnating paraffin into the expanded perlite porosity, the paraffin (solid state) was replaced by trapped air (gas) and as a result, the thermal conductivity increased and heat flow should be passed easier through the expanded perlite matrix; but on the other hand, during heating process, a significant amount of thermal energy was used to melt the impregnated paraffin. As shown in Fig. 17, the temperature difference between cement paste and EP/P form-stable samples was more at first (during phase transition of paraffin), but it was decreased after 8 h in which all paraffin was melted and turned to liquid state and the final temperature differences should just be related to the differences of thermal conductivity coefficient.
After 12-h heating experiment, the heater was turned off and cooling process was started. Figure 18 shows the topside temperature variation of the concrete samples during the cooling experiment. In order to investigate the cooling rate of concrete samples, the average cooling rate of each sample was calculated in each hour and is shown in Table 11.
Topside temperature variation of Fig. 17 concrete samples with time—the cooling process
In cement paste, the cooling rate in the first hour (10.6 °C h−1) was lower than the second hour (13.3 °C h−1), and it is because of the thermal energy which came out from the sample depth and prevents from a sharp drop in temperature. The same behavior was observed in two other samples; however, the difference between the cooling rate of the first and second hour in expanded perlite sample was lower due to the lower thermal conductivity coefficient and much lower for EP/P form-stable sample due to the phase transition process.
Figure 19 represents the topside temperature variation of the concrete samples with time for concrete made by form-stable EP containing paraffin with 0.5% MWCNT, 0.5% and 10% graphite powder. Results for the sample containing 0.5% MWCNT and also 0.5% graphite powder showed approximately similar behavior with insignificant deficiencies; final temperature for both of them reached 66 °C. On the other hand, sample with 10 mass% graphite powder showed a different behavior between times 2–6 h. The temperature in this time period was significantly lower in comparison with other two samples with more fluctuations. Considering the temperature range, this behavior could be attributed to the phase transition of paraffin impregnated into the expanded perlite particles. It seems that by adding 10 mass% graphite powder to the paraffin, phase transition process conducted consistently due to the higher thermal conductivity of the modified paraffin. Finally, it reached to a steady state at about 68 °C. It is worth mentioning that no paraffin leakage was observed in the sample surfaces, even after five times 12-hr heating periods.
Mechanical properties
Evaluating the workability of the prepared energy efficient concretes, density and compression strength were determined as two effective parameters. For this purpose, three volume fraction of expanded perlite (30, 40, 50 and 60%) was added to the cement paste and 10 × 10 × 10 cm lightweight concrete blocks were prepared and mechanically tested. Results of 28-day compression strength and density are shown in Fig. 20.
The density of concrete samples was decreased to 1710, 1540, 1338 and 1272 kg m−3 by increasing the expanded perlite volume percentage to 30, 40, 50, and 60%, respectively. Similarly, the compression strength of the concretes was measured to be 23.5, 15.83, 9.31 and 6.42 MPa on average, respectively; however, these amounts may be increased after paraffin impregnation due to the hydrophobicity properties of the paraffin. Note that only the compression strength of the sample with 30% expanded perlite is more than 18 MPa, indicating that the samples containing less than 30% volume percentage expanded perlite could be used as structural concrete with this mix design.
Conclusions
In the present paper, thermal conductivity enhancement of paraffin and thermal energy storage of concrete containing form-stable PCM were investigated. The major findings of this study are summarized below:
-
Expanded perlite particles are very porous materials; the porosity of the particles (mesh #8) is about 88% in which about 62% of their porosity consists of external macro-pores larger than 140 µm.
-
Paraffin impregnation amount into the expanded perlite porosity could reach 31.5% by the atmospheric method without any leakage. This amount could be much more in vacuum method (about 50%).
-
Adding carbon materials such as MWCNTs and graphite powder could be effective not only in reducing the sub-cooling phenomenon in paraffin, but also in enhancing the thermal conductivity coefficient which leads to better thermal energy storage process.
-
Dodecyl amine due to its long-chain structure and also non-polar properties could be a good option for MWCNT functionalization and enhancing the nanotubes stability in paraffin matrix.
-
Adding 0.5 mass% graphite powder to the paraffin leads to increasing thermal conductivity even higher than MWCNTs; but when it incorporated into the concrete, final behavior showed similar results.
-
Regarding preparing concrete for structural purposes, the volume fraction of the expanded perlite should be less than about 35% and by considering 50 mass% Paraffin impregnation in expanded perlite particles, it would be 7% vol. and about 63 grams in a 10-cm-length cubic block.
Finally, it could be concluded that adding conductive additives to the paraffin could improve the thermal storage behavior of the final concrete; however, it seems that in form-stable technique the stability of the additives is not significantly effective. It could be attributed to the narrow pores and channels in the expanded perlite structure, which reduce the effect of particles precipitation. In the other words, cheap and available materials such as graphite powder generated from wastes of industrial apparatus such as heat exchangers could almost have the same effect as other expensive nanomaterials such as MWCNTs.
References
Lu Z, Zhang J, Sun G, Xu B, Li Z, Gong C. Effects of the form-stable expanded perlite/paraffin composite on cement manufactured by extrusion technique. Energy. 2015;82:43–53. https://doi.org/10.1016/j.energy.2014.12.043.
Memon S, Liao W, Yang S, Cui H, Shah S. Development of composite PCMs by incorporation of paraffin into various building materials. Materials. 2015;8(2):499–518. https://doi.org/10.3390/ma8020499.
Sun D, Wang L, Li C. Preparation and thermal properties of paraffin/expanded perlite composite as form-stable phase change material. Mater Lett. 2013;108:247–9. https://doi.org/10.1016/j.matlet.2013.06.105.
Hawes DW, Banu D, Feldman D. Latent heat storage in concrete. II. Sol Energy Mater. 1990;21(1):61–80. https://doi.org/10.1016/0165-1633(90)90043-z.
Moradi O, Yari M, Zare K, Mirza B, Najafi F. Carbon nanotubes: a review of chemistry principles and reactions. Fuller Nanotub Carbon Nanostruct. 2012;20(2):138–51. https://doi.org/10.1080/1536383X.2010.533312.
Moaseri E, Hasanabadi S, Maghrebi M, Baniadam M. Improvements in fatigue life of amine-functionalized multi-walled carbon nanotube-reinforced epoxy composites: effect of functionalization degree and microwave-assisted precuring. J Compos Mater. 2014;49(16):1961–9. https://doi.org/10.1177/0021998314541306.
Liang J-Z, Qiu Y-L. Thermal conductivity of graphite-filled LDPE composites. Polym Bull. 2015;72(7):1723–34. https://doi.org/10.1007/s00289-015-1366-8.
Fethi A, Mohamed L, Mustapha K, Sassi BN, AmeurTarek B. Investigation of a graphite/paraffin phase change composite. Int J Therm Sci. 2015;88:128–35. https://doi.org/10.1016/j.ijthermalsci.2014.09.008.
Li M. A nano-graphite/paraffin phase change material with high thermal conductivity. Appl Energy. 2013;106:25–30. https://doi.org/10.1016/j.apenergy.2013.01.031.
Cui Y, Liu C, Hu S, Yu X. The experimental exploration of carbon nanofiber and carbon nanotube additives on thermal behavior of phase change materials. Sol Energy Mater Sol Cells. 2011;95(4):1208–12. https://doi.org/10.1016/j.solmat.2011.01.021.
Sarı A, Karaipekli A. Preparation, thermal properties and thermal reliability of capric acid/expanded perlite composite for thermal energy storage. Mater Chem Phys. 2008;109(2–3):459–64. https://doi.org/10.1016/j.matchemphys.2007.12.016.
Zhang J, Guan X, Song X, Hou H, Yang Z, Zhu J. Preparation and properties of gypsum based energy storage materials with capric acid–palmitic acid/expanded perlite composite PCM. Energy Build. 2015;92:155–60. https://doi.org/10.1016/j.enbuild.2015.01.063.
Ramakrishnan S, Sanjayan J, Wang X, Alam M, Wilson J. A novel paraffin/expanded perlite composite phase change material for prevention of PCM leakage in cementitious composites. Appl Energy. 2015;157:85–94. https://doi.org/10.1016/j.apenergy.2015.08.019.
Zhang N, Yuan Y, Yuan Y, Li T, Cao X. Lauric–palmitic–stearic acid/expanded perlite composite as form-stable phase change material: Preparation and thermal properties. Energy Build. 2014;82:505–11. https://doi.org/10.1016/j.enbuild.2014.07.049.
Karaipekli A, Sarı A. Capric–myristic acid/expanded perlite composite as form-stable phase change material for latent heat thermal energy storage. Renew Energy. 2008;33(12):2599–605. https://doi.org/10.1016/j.renene.2008.02.024.
Lorwanishpaisarn N, Kasemsiri P, Posi P, Chindaprasirt P. Characterization of paraffin/ultrasonic-treated diatomite for use as phase change material in thermal energy storage of buildings. J Therm Anal Calorim. 2017;128(3):1293–303. https://doi.org/10.1007/s10973-016-6024-3.
Cui W, Zhang H, Xia Y, Zou Y, Xiang C, Chu H, et al. Preparation and thermophysical properties of a novel form-stable CaCl2·6H2O/sepiolite composite phase change material for latent heat storage. J Therm Anal Calorim. 2018;131(1):57–63.
Ma B, Adhikari S, Chang Y, Ren J, Liu J, You Z. Preparation of composite shape-stabilized phase change materials for highway pavements. Constr Build Mater. 2013;42:114–21. https://doi.org/10.1016/j.conbuildmat.2012.12.027.
Zhang X, Wen R, Huang Z, Tang C, Huang Y, Liu Y, et al. Enhancement of thermal conductivity by the introduction of carbon nanotubes as a filler in paraffin/expanded perlite form-stable phase-change materials. Energy Build. 2017;149:463–70. https://doi.org/10.1016/j.enbuild.2017.05.037.
Karaipekli A, Biçer A, Sarı A, Tyagi VV. Thermal characteristics of expanded perlite/paraffin composite phase change material with enhanced thermal conductivity using carbon nanotubes. Energy Convers Manag. 2017;134:373–81. https://doi.org/10.1016/j.enconman.2016.12.053.
Lu Z, Xu B, Zhang J, Zhu Y, Sun G, Li Z. Preparation and characterization of expanded perlite/paraffin composite as form-stable phase change material. Sol Energy. 2014;108:460–6. https://doi.org/10.1016/j.solener.2014.08.008.
Tang Q, Sun J, Yu S, Wang G. Improving thermal conductivity and decreasing supercooling of paraffin phase change materials by n-octadecylamine-functionalized multi-walled carbon nanotubes. RSC Adv. 2014;4(69):36584–90. https://doi.org/10.1039/c4ra04225e.
Kong X, Yao C, Jie P, Liu Y, Qi C, Rong X. Development and thermal performance of an expanded perlite-based phase change material wallboard for passive cooling in building. Energy Build. 2017;152:547–57. https://doi.org/10.1016/j.enbuild.2017.06.067.
Acknowledgements
This work was supported by Iran Nanotechnology Initiative Council. Financial supports from the research department of Tarbiat Modares University (Grant Number: IG-39710) are gratefully acknowledged by the first and second authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hasanabadi, S., Sadrameli, S.M., Soheili, H. et al. A cost-effective form-stable PCM composite with modified paraffin and expanded perlite for thermal energy storage in concrete. J Therm Anal Calorim 136, 1201–1216 (2019). https://doi.org/10.1007/s10973-018-7731-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7731-8