Abstract
The pyrolysis characteristics and kinetics of lignocellulosic biomass (cotton stalk) and seaweed (Gracilaria lemaneiformis) were studied comparatively. Results of the thermal degradation processes showed that the pyrolysis occurence of G. lemaneiformis is easier than that of cotton stalk. However, G. lemaneiformis released less volatile components and produced more solid residues. As the heating rate increased, the maximum mass loss rates for cotton stalk were decreased, while those for G. lemaneiformis were increased. Results of the kinetic analysis by Popescu method indicated that the pyrolysis mechanism of cotton stalk is three-dimensional diffusion, which can be described by Zhuralev, Lesokin, and Tempelmen (Z–L–T) equation \((G(\alpha ) = \{ [1/(1 - \alpha )]^{1/3} - 1\}^{2} )\), whereas that of G. lemaneiformis is random nucleation and nuclei growth, which can be described by Avrami–Erofeev equation \((G(\alpha ) = [ - \ln (1 - \alpha )]^{1/4} )\). The average activation energy values (192.17 and 146.11 kJ mol−1, respectively) of cotton stalk and G. lemaneiformis obtained by Popescu method are similar with those (189.88 and 153.79 kJ mol−1, respectively) calculated by Flynn–Wall–Ozawa (FWO) method. Moreover, the average activation energy of G. lemaneiformis is lower than that of cotton stalk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fossil fuels (coal, petroleum, and natural gas) are not renewable resources, and their uses contribute significantly to greenhouse gas emission to the environment [1]. As a renewable and environmentally friendly energy resource, biomass has attracted considerable attention all over the world. Biomass, mainly including terrestrial lignocellulosic biomass (woods and crops) and aquatic biomass (freshwater plants and seaweeds), is wide spread on the earth. Lignocellulosic biomass is mainly composed of hemicellulose, cellulose, and lignin. Compared with lignocellulosic biomass, seaweed, primarily composed of carbohydrate, protein, and lipid, has shown several advantages such as fast growth rates, high yields, high efficiency in CO2 capture and photosynthesis. Moreover, seaweed can be cultivated using marine water; therefore, it does not compete with limited arable land [2]. The ocean areas in the word cover nearly three-fourths of the earth’s surface, so seaweed resources are very abundant. However, the energy plants of widespread utilization are mainly the woods and crops.
Biomass conversion routes can be divided into biochemical conversion technologies and thermochemical conversion technologies. Thermochemical conversion processes, mainly including combustion, gasification, and pyrolysis, are thought to have great promise as a means for biomass utilization [3]. The high amounts of alkali and alkali earth metals in biomass can cause some problems such as slagging and fouling during combustion and gasification. An alternative use of biomass to combustion and gasification is the pyrolysis. Pyrolysis is thermal degradation process of biomass in an inert atmosphere to produce char, oil, and gaseous products. A thorough knowledge of the thermal decomposition kinetics of biomass can offer theoretical basis for optimizing the pyrolysis operation and reactor design. Thermogravimetric (TG) analysis is a very effective tool for investigating the thermal decomposition process. The kinetic data acquired from TG analysis are very useful in helping us understand the pyrolysis process and mechanism involved [4].
The kinetic data can be obtained by different methods including model-fitting method and model-free (isoconversional) method [5]. Model-fitting method often assumes that the pyrolysis model is n first-order chemical reaction [6, 7], the kinetic parameters gained by which differ greatly [8]. Model-free method such as Flynn–Wall–Ozawa (FWO) is a simple method for calculating kinetic parameters; it can avoid the errors resulted from selecting the reaction mechanism [9]. Several studies on the thermal decomposition kinetics of different types of lignocellulosic biomass [10,11,12,13,14,15,16] and seaweeds [17,18,19,20,21,22] have been researched. Many researchers employed FWO method to analyze kinetic results. However, the comparative studies on pyrolysis characteristics and kinetics of lignocellulosic biomass and seaweed are scarce [23]. In order to better understand the pyrolysis characteristics and kinetics of different kinds of biomass and develop an efficient pyrolysis technology for biomass utilization, a systematic and comparative study of pyrolysis characteristics of lignocellulosic biomass (cotton stalk) and seaweed (Gracilaria lemaneiformis) was investigated using a thermogravimetric analyzer. The most probable pyrolysis mechanism of two types of biomass was determined by Popescu method. The pyrolysis kinetics parameters of two types of biomass were obtained by Popescu and FWO methods in this study.
Materials and methods
Materials
The lignocellulosic biomass cotton stalk was obtained from the suburban areas of Huainan, China, and the seaweed G. lemaneiformis was collected from Huiquan Bay, Qingdao, China. The samples were air dried, then ground, and sieved to less than 1 mm in size. The proximate analysis of the two types of biomass was carried out based on GB212-91 standard of China. The proximate analysis results are shown in Table 1. The calorific value was determined using an adiabatic calorimeter. It can be seen that G. lemaneiformis has much higher ash content due to its saline growing environment, so it has lower volatile and calorific value than cotton stalk.
Thermogravimetric analysis
The thermogravimetric analysis experiments were carried out using a thermogravimetric analyzer (STD 2960, TA Instruments, USA). In each experiment, approximately 10 mg sample was uniformly placed into the alumina crucible of the thermal analyzer, after then was heated from ambient temperature to 700 °C at different heating rates of 10, 20, 30, 50, and 80 °C min−1 under a high-purity nitrogen atmosphere of 100 mL min−1. The record of mass loss in response to temperature of each sample was collected to determine both TG and derivative thermogravimetric (DTG) curves. All experiments were replicated at least three times to ensure the repeatability.
Kinetics methods
The most probable mechanism and the activation energy and the pre-exponential factor of two types of biomass were determined using Popescu method [24]. The activation energy value can be obtained using FWO method regardless of the pyrolysis mechanism [10, 20, 25, 26]. So, the FWO method can be used to evaluate the validity of pyrolysis mechanism determined by Popescu method.
The decomposition rate of biomass under non-isothermal conditions can be described as:
where k(T) is the reaction rate constant, f(α) is the differential form of mechanism function, the conversion rate α is defined as:
where m 0 is the initial mass of the sample, m is the mass after pyrolysis for a certain time t, and \(m_{\infty }\) is the mass of the final solid residue.
According to Arrhenius equation, k(T) is described as:
where A is the pre-exponential factor, E is the activation energy, R is the universal gas constant, R = 8.314 J mol−1 K−1, and T is the absolute temperature.
The heating rate β is defined as:
Substituting Eq. 4 into Eq. 1 and rearranging gives:
Determination of the most probable pyrolysis mechanism and kinetic parameters by Popescu method
The integral form of Eq. 5 is as follows:
where αm, αn are two different conversion rates, Tm, Tn are their corresponding temperatures, and
If a G(α) is selected properly, a plot of G(α) versus 1/β gives a straight line with an intercept of zero, and the G(α) is then the most probable mechanism function that can describe the true chemical reaction process. In fact, the most probable mechanism function was chosen according to the best correlation coefficient (R).
In order to calculate the activation energy and the pre-exponential factor, substituting Eq. 3 into Eq. 6 gives:
where
where \(T_{\upxi }\) is in the temperature range of Tm–Tn and \(T_{\upxi } = (T_{\text{m}} + T_{\text{n}} )/2\). So,
Rearranging and taking the logarithm, a linear equation is derived as:
A plot of \({ \ln }[\beta /(T_{\text{n}} - T_{\text{m}} )]\) versus \(1 /T_{\upxi }\) yields a straight line with a slope of −E/R and an intercept of \(\ln [A /G(\alpha )]\). Therefore, E and lnA can be determined.
Calculation of the activation energy by FWO method
The integral form of Eq. 5 is generally expressed as:
Generally, the lower limit T0 of the integral on the right side of Eq. 5 is approximately equal to zero, so
where u = E/RT. The term P(u) is the temperature integral form. The temperature integral does not have an exact analytic solution, but it can be approximated via an empirical interpolation formula.
By introducing the approximation P(u) = 0.0048e−1.0516u into Eq. 13, rearranging, and taking the logarithm, the FWO equation is as follows:
For various heating rates and a given value of conversion rate α, plotting of lnβ versus 1/T gives straight line with slopes of − 1.0516E/RT, and then E can be calculated.
Results and discussion
Thermal degradation characteristics
As seen from Fig. 1, the thermal decomposition process of cotton stalk can be divided into three stages. The first stage occurred below 200 °C, which corresponded to the elimination of moisture. A weak peak in DTG curve appeared. The second stage, occurred in the temperature range of 200–400 °C, is the devolatilization process, which is the main decomposition process of cotton stalk. During this stage, various volatile components were released gradually, resulting in a large mass loss. The DTG curve exhibited a strong peak with a shoulder, which attributed to hemicellulose and cellulose degradation [27]. The lignin decomposition occurred between 200 and 500 °C, which was superimposed onto the previous peak, so the characteristic temperature was not be clearly discriminated [28]. The third stage occurred above 400 °C corresponding to the solid residue slow decomposition, which resulted in the formation of a loose porous solid char. A very slight mass loss can be observed. The final solid residues mass was about 23%.
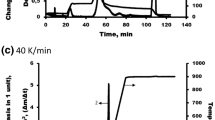
Figure 2 showed three stages in the thermal degradation process of G. lemaneiformis. The first stage (below 180 °C) corresponded to the elimination of cellular water and the external water. A small mass loss appeared. The second stage occurred in the temperature range of 180–500 °C, which attributed to carbohydrate, protein, and lipid decomposition [29] This is the devolatilization process, during which the major thermal decomposition process of G. lemaneiformis occured, bringing about a significant mass loss. Considering the previous studies about the TG experiments with several proteins and carbohydrates [30], it can be concluded that overlap may exist between thermal degradation temperatures of proteins and carbohydrates in the temperature range of 200–350 °C. The shoulder peak appeared in the temperature range of 350–500 °C corresponded to the decomposition of lipid [31]. The third stage (above 500 °C) is the slow decomposition of carbonaceous materials [17]. In this stage, the mass loss was very slight. The final solid residues mass was about 48%.
It can be observed that the TG and DTG curves of two types of biomass are similar. Since the chemical composition of seaweed is different from that of terrestrial lignocellulosic biomass, its thermal decomposition behavior is also different. Compared with cotton stalk, the initial devolatilization temperature and the temperature of maximum mass loss rate of G. lemaneiformis occurred at lower temperature, which indicated that the pyrolysis of seaweed can proceed easier, i.e., the thermal stability of seaweed is lower. However, the main decomposition temperature range of G. lemaneiformis is wider than that of cotton stalk. In addition, the maximum mass loss rate of G. lemaneiformis is relatively lower and the final solid residues are very higher, which suggested that the seaweed released less volatile components in the main decomposition process due to higher ash contents.
As seen from Figs. 1 and 2, heating rate had a significant effect on the thermal behavior of cotton stalk and G. lemaneiformis. As the heating rate increased, the main pyrolysis process all shifted to higher temperature zone, and the temperature at which the maximum mass loss rate occurred all increased. This is a common phenomenon for all non-isothermal biomass pyrolysis experiments. This can be explained on the basis of heat transfer limitation. At lower heating rate, a larger instantaneous thermal energy is provided to the system and a longer time may be required to reach equilibrium between furnace temperature and sample temperature. While at the same time, in the same temperature zone, a higher heating rate need a short reaction time and therefore the temperature needed to decompose is also higher [12]. In addition, with increasing heating rates, the maximum mass loss rates for cotton stalk were decreased; however, those for G. lemaneiformis were increased. These results are in agreement with the previous reports [12, 13, 21, 32].
Kinetic analysis
Determination of the most probable pyrolysis mechanism by Popescu method
The second stage is the main pyrolysis process, so the temperature range of 230–350 and 200–320 °C were chosen to determine the pyrolysis mechanisms of cotton stalk and G. lemaneiformis, respectively. The conversion rate α corresponding to the chosen temperature is calculated. Twenty-two integral functions G(α) [20] most frequently used in reaction mechanism investigations on a solid state process are listed in Table 2. The best correlation coefficients during different temperature intervals are listed in Table 3.
As shown in Table 3, there is different pyrolysis mechanism between cotton stalk and G. lemaneiformis. Function \(6\;(G\left( \alpha \right) = \{ [1/\left( {1 - \alpha } \right)]^{1/3} - 1\}^{2} )\) is the most probable thermal decomposition mechanism of cotton stalk, while function 12 \((G(\alpha ) = [ - \ln (1 - \alpha )]^{1/4} )\) is the most probable thermal degradation mechanism of G. lemaneiformis. These indicated that three-dimensional diffusion was predominant during the main pyrolysis process of cotton stalk, while random nucleation and nuclei growth was predominant during the main pyrolysis process of G. lemaneiformis. For cotton stalk, the pyrolysis mechanism why function 6 is the best one may be ascribed to its compact structure. However, for G. lemaneiformis, the pyrolysis mechanism why function 12 is the best one may be caused by a large amount of inorganic salts in seaweed, which can induce the heterogeneous nucleation of the volatile [23].
Calculation of the kinetic parameters
E and lnA of two types of biomass determined by the Popescu method are listed in Table 4. E of two types of biomass calculated by the FWO method is listed in Table 5.
It can be seen that the activation energy values determined by Popescu method and FWO method for cotton stalk and G. lemaneiformis are 181–206, 170–203 and 107–180, 114–180 kJ mol−1, respectively. The average activation energies obtained by Popescu are similar with those calculated by FWO methods. The average apparent activation energy values of cotton stalk and G. lemaneiformis determined by Popescu method and FWO method are 192.17 and 146.11, 189.88 and 153.79 kJ mo1−l, respectively. These proved that the pyrolysis mechanisms obtained by Popescu method are credible. In this study, the average activation energy of cotton stalk is higher than that of corn straw (129 kJ mol−1) [14] obtained by FWO method. However, the average activation energy of seaweed G. lemaneiformis is lower than that of macro-algae Macrocystis pyrifera residue (219.7 kJ mol−1) [20] calculated by FWO method. These may be caused by the difference in material compositions. Moreover, the activation energies of two types of biomass increased as the conversion rate increased, which suggested that pyrolysis proceeded more easily at lower conversion rate. In addition, the average activation energy of G. lemaneiformis is lower than that of cotton stalk, which may be caused by the low thermal stability of the polysaccharides and the presence of large inorganic salts in G. lemaneiformis. That is to say, G. lemaneiformis is a potential pyrolysis feedstock.
Conclusions
The pyrolysis characteristics and kinetic parameters for lignocellulosic biomass (cotton stalk) and seaweed (G. lemaneiformis) have been investigated comparatively. The thermal stability of G. lemaneiformis is lower than that of cotton stalk. However, G. lemaneiformis released less volatile components and produced more solid residues. As the heating rate increased, the main pyrolysis process of two types of biomass all shifted to higher temperature zone and the maximum mass loss rates for cotton stalk were decreased; however, those for G. lemaneiformis were increased. The most probable mechanism in the main pyrolysis process was deduced by Popescu method. The thermal decomposition mechanism of cotton stalk can be described by Zhuralev, Lesokin, and Tempelmen (Z–L–T) equation \((G(\alpha ) = \{ [1/(1 - \alpha )]^{1/3} - 1\}^{2} )\), which is three-dimensional diffusion model, whereas that of G. lemaneiformis can be described by Avrami–Erofeev equation \((G(\alpha ) = [ - ln(1 - \alpha )]^{1/4} )\), which is random nucleation and nuclei growth model. The activation energy values calculated by Popescu and FWO methods are similar. This proved that the pyrolysis mechanisms determined by Popescu method are credible. Furthermore, the average activation energy of G. lemaneiformis is lower than that of cotton stalk. This indicates that G. lemaneiformis is suitable for pyrolysis.
References
Du ZY, Li YC, Wang XQ, Wan YQ, Chen Q, Wang CG, Lin XY, Liu YH, Chen P, Ruan R. Microwave-assisted pyrolysis of microalgae for biofuel production. Bioresour Technol. 2011;102:4890–6.
Wang N, Tahmasebi A, Yu JL, Xu J, Huang F, Mamaeva A. A comparative study of microwave-induced pyrolysis of lignocellulosic and algal biomass. Bioresour Technol. 2015;190:89–96.
Wang XY, Qin GX, Chen MQ, Wang J. Microwave-assisted pyrolysis of cotton stalk with additives. BioResources. 2016;11:6125–36.
Zou SP, Wu YL, Yang MD, Li C, Tong JM. Pyrolysis characteristics and kinetics of the marine microalgae Dunaliella tertiolecta using thermogravimetric analyzer. Bioresour Technol. 2010;101:359–65.
Vyazovkin S, Wight CA. Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim Acta. 1999;340:53–68.
Damartzis T, Vamvuka D, Sfakiotakis S, Zabaniotou A. Thermal degradation studies and kinetic modeling of cardoon (Carnaracardunculus) pyrolysis using thermogravimetric analysis (TGA). Bioresour Technol. 2011;102:6230–8.
Cao Q, Xie KC, Bao WR, Shen SG. Pyrolytic behavior of waste corn cob. Bioresour Technol. 2004;94:83–9.
Khawam A, Flanagan DR. Complementary use of model-free and modelistic methods in the analysis of solid-state kinetics. J Phys Chem B. 2005;109:10073–80.
Opfermann JR, Kaisersberger E, Flammersheim HJ. Model-free analysis of thermoanalytical data-advantages and limitations. Thermochim Acta. 2002;391:119–27.
Cai JM, Bi LS. Kinetic analysis of wheat straw pyrolysis using isoconversional methods. J Therm Anal Calorim. 2009;98:325–30.
Kim SS, Kim J, Park YH, Park YK. Pyrolysis kinetics and decomposition characteristics of pine trees. Bioresour Technol. 2010;101:9797–802.
Slopiecka K, Bartocci P, Fantozzi F. Thermogravimetric analysis and kinetic study of poplar wood pyrolysis. Appl Energy. 2012;97:491–7.
Gai C, Dong YP, Zhang TH. The kinetic analysis of the pyrolysis of agricultural residue under non-isothermal conditions. Bioresour Technol. 2013;127:298–305.
Wilson L, Yang W, Blasiak W, John GR, Mhilu CF. Thermal characterization of tropical biomass feedstocks. Energy Convers Manag. 2011;52:191–8.
Ceylan S, Topçu Y. Pyrolysis kinetics of hazelnut husk using thermogravimetric analysis. Bioresour Technol. 2014;156:182–8.
Debal M, Girods P. TG-FTIR kinetic study of the thermal cleaning of wood laminated flooring waste. J Therm Anal Calorim. 2014;118:141–51.
Ross AB, Jones JM, Kubacki ML, Bridgeman T. Classification of macroalgae as fuel and its thermochemical behaviour. Bioresour Technol. 2008;99:6494–504.
Li DM, Chen LM, Yi XJ, Zhang XW, Ye NH. Pyrolytic characteristics and kinetics of two brown algae and sodium alginate. Bioresour Technol. 2010;101:7131–6.
Li DM, Chen LM, Zhang XW, Ye NH, Xing FG. Pyrolytic characteristics and kinetic studies of three kinds of red algae. Biomass Bioenergy. 2011;35:1765–72.
Zhao H, Yan HX, Dong SS, Zhang Y, Sun BB, Zhang CW, Ai YX, Chen BQ, Liu Q, Sui TT, Qin S. Thermogravimetry study of the pyrolytic characteristics and kinetics of macro-algae Macrocystis pyrifera residue. J Therm Anal Calorim. 2013;111:1685–90.
Ceylan S, Topcu Y, Eylan Z. Thermal behaviour and kinetics of alga Polysiphonia elongata biomass during pyrolysis. Bioresour Technol. 2014;171:193–8.
Wu KJ, Liu J, Wu YL, Chen Y, Li QH, Xiao X, Yang MD. Pyrolysis characteristics and kinetics of aquatic biomass using thermogravimetric analyzer. Bioresour Technol. 2014;163:18–25.
Wang J, Wang GC, Zhang MX, Chen MQ, Li DM, Min FF, Chen MG, Zhang SP, Ren ZW, Yan YJ. A comparative study of thermolysis characteristics and kinetics of seaweeds and fir wood. Process Biochem. 2006;41:1883–6.
Popescu C. Integral method to analyze the kinetics of heterogeneous reactions under non-isothermal conditions: a variant on the Ozawa–Flynn–Wall method. Thermochim Acta. 1996;285:309–23.
Shen CS, Zhou CR. Investigation of the thermal decomposition kinetics of bezafibrate. J Therm Anal Calorim. 2016;126:959–67.
Wu JZ, Wang BF, Cheng FQ. Thermal and kinetic characteristics of combustion of coal sludge. J Therm Anal Calorim. 2017;129:1899–909.
Yang HP, Yan R, Chen HP, Lee D, Zheng CG. Characteristics of hemicellulose, cellulose and lignin. Fuel. 2007;86:1781–8.
Park HJ, Park YK, Dong J, Kim JS, Jeon JK, Kim SS, Kim J. Pyrolysis characteristics of Oriental white oak:kinetic study and fast pyrolysis in a fluidized bed with an improved reaction system. Fuel Process Technol. 2009;90:186–95.
Yanik J, Stahl R, Troeger N, Sinag A. Pyrolysis of algal biomass. J Anal Appl Pyrol. 2013;103:134–41.
Anastasakis K, Ross AB, Jones JM. Pyrolysis behaviour of the main carbohydrates of brown macro-algae. Fuel. 2011;90:598–607.
Vo TK, Ly HV, Lee OK, Lee EY, Kim CH, Seo JW, Kim J, Kim SS. Pyrolysis characteristics and kinetics of microalgal Aurantiochytrium sp. KRS101. Energy. 2017;118:369–76.
Li DM, Chen LM, Chen SL, Zhang XW, Chen FJ, Ye NH. Comparative evaluation of the pyrolytic and kinetic characteristics of a macroalga (Sargassum thunbergii) and a freshwater plant (Potamogeton crispus). Fuel. 2012;96:185–91.
Acknowledgements
The authors wish to acknowledge the financial support by the Anhui Province Prominent Young Talents Support Program (gxyq2017072) and the National Natural Science Foundation of China (20676002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, X., Wang, X., Qin, G. et al. Comparative study on pyrolysis characteristics and kinetics of lignocellulosic biomass and seaweed. J Therm Anal Calorim 132, 1317–1323 (2018). https://doi.org/10.1007/s10973-018-6987-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-6987-3