Abstract
The thermal behavior of volatile heterometallic CuPdL4 compound and parent β-diketonate complexes CuL2 and PdL2 (L = 2-methoxy-2,6,6-trimethylheptane-3,5-dionate; (CH3)3CCOCHCOC(CH3)2OCH3) were investigated by different methods. It is demonstrated that the combination of data on the gradient sublimation, TG–DTA, the mass transfer in a stream of inert gas and mass spectrometry can determine the composition of the gas phase. The volatility of the heterometallic complex was shown to follow the vapor-phase composition presented by binuclear [CuL2PdL2] molecules, and there was no decomposition into monometallic complexes within the studied temperature range (403–438 K). Temperature dependences of the saturated vapor pressure were obtained, and thermodynamic parameters of the sublimation process were computed for these three compounds for the first time. The thermal decomposition of the bimetallic complex vapor was found to start with the surface reaction of dissociation into monometallic complexes followed by their thermolysis with the formation of Cu/Pd alloy films.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Volatile heterometallic complexes are the ones that can be transferred into the gas phase under heating without the decomposition into monometallic complexes and can be condensed retaining the initial structure and composition. The vast majority of them are hetero- or homoleptic complexes of two different metals with organic ligands such as alkoxides or β-diketonates [1,2,3]. Interest in these compounds is due to their use as single-source precursors (SSPs) of multicomponent inorganic films (mainly oxide) by chemical vapor deposition (CVD). The method is based on the thermolysis of the parent compound vapor on a hot surface; therefore, the successful use of this method requires the knowledge of thermal properties of the precursor. Very often thermogravimetric and differential thermal analyses (TG–DTA) are used to assess the thermal properties: the volatility and thermal stability of precursors in the condensed phase [4,5,6,7]. Data on the saturated vapor pressure are available for a very limited range of heterometallic complexes [8], and reports on the study of their vaporization processes are quite rare [9].
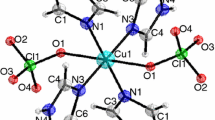
As for SSPs for bimetallic alloys, their number is extremely limited [2], and data on the thermal properties are practically absent. In our recent paper [10], we demonstrated that the heterometallic CuPdL4 complex, formed by β-diketonates of copper(II) and palladium(II) (L = 2-methoxy-2,6,6-trimethylheptane-3,5-dionate; structural formula is presented in Fig. 1), can be used to produce Cu/Pd alloys films with variable metal ratios. These deposition processes are promising to manufacture membrane materials for the separation of pure hydrogen from gas mixtures because the Pd47Cu53 alloy has the best hydrogen conductivity [11]. Moreover, the Cu/Pd membranes are a cheap and feasible alternative to membranes containing precious metals [12]. The effective management of metal ratios in bimetallic film obtained by CVD assumes the knowledge of the volatility and thermal stability of the parent compound (precursor). Quite extensive data on the thermal properties of monometallic copper(II) and palladium(II) β-diketonates are available, including data on the vapor pressure and thermal decomposition processes [13, 14]. But for the complexes of these metals with methoxy-β-diketones, which were obtained previously [15, 16], studies on the thermal behavior have not been reported.
Here, we report the thermal properties of the CuPdL4 complex in the condensed and gas phases, including the study of the evaporation and melting processes of this heterometallic compound and parent monometallic β-diketonates. We discuss the temperature interval of their vapor stability as well as the composition of the gaseous and solid products of vapor thermolysis on a hot surface.
Experimental
Materials
The preparation and identification of PdL2, CuL2 and CuPdL4 were described in refs. [15,16,17], respectively. All compounds were purified by vacuum sublimation (P = 10−2 Torr) prior to use: at = 443, 423 and 413 K for PdL2, CuL2 and CuPdL4, respectively.
Thermogravimetric and differential thermal analyses (TG–DTA) were used to study the thermal properties of the compound in the condensed phase; a TG 209 F1 Iris® (NETZSCH) thermobalance and a standard Al open crucible were used. The measurements were taken under atmospheric pressure in the helium flow (30–40 mL min−1) with a heating rate of 10 K min−1 within the temperature range 323–623 K.
Temperature dependence of the saturated vapor pressure of the complexes was investigated by the flow method when a stream of inert gas (He) carriers the compound vapor under quasi-equilibrium conditions. The flow method makes it possible to obtain reliable data at relatively low vapor pressure values and on the background of the partial thermal decomposition of the tested compound. All complexes under discussion were pre-purified by vacuum sublimation. The measured dry He gas flow (a flow rate measurement error of ±2%) passed through a bulk source with the compound maintained at a predetermined temperature (±0.5 K). The amount of sublimated substances as well as condensed in the cold zone is determined by weighing (error ±0.0005 g). The vapor pressure was calculated according to the formula:
P = P total n/(n + N), where n is the number of moles of the transferred substance; N is the number of gas carrier moles passed through the system; P total is the total pressure in the system. Primary experimental data are available from Table S1 of Supplementary material. In the data processing, it is assumed that the substance passes into the gas phase in accordance with the formula unit. Measurements were taken under quasi-equilibrium conditions, the experimental confirmation of which is the independence of the vapor density upon the gas carrier flow rate in the range of 0.5–5 L h−1 at the same temperature. The results obtained for the mass loss and the amount of a condensate practically coincide. This demonstrates the thermal stability of the complexes in the temperature range studied. The obtained data processing yields the temperature dependence of the saturated vapor pressure in the form ln(P/P°) = B − A/T and thermodynamic characteristics (ΔH(T), ΔS°(T) for the sublimation process, P°—standard pressure in required units. The obtained characteristics are shown in Table 1 and Fig. 1.
Differential scanning calorimetry (DSC) study of the melting process
Calorimetric measurements were taken using Setaram DSC 111 at a heating rate of 1 K min−1. Errors in the heat effect measurements estimated from the calibration experiments (In) were less than 1.2%. The substances were loaded in calorimetric glass ampoules. Sample weights were 9–18.6 mg. After weighing, the ampoules were evacuated and sealed. The samples exhibited the only phase transition (melting) over the temperature range studied (420–470 K). Two calorimetric experiments for each compound were performed with two runs. Repeated heating revealed slight drops of thermal effects obtained (Table 2). A negligible difference with the initial value is observed for CuPdL4 and for CuL2, while for PdL2 it makes ~17%. These differences may indicate partial decomposition of the compounds and especially of palladium complex during melting.
Mass spectrometric study
A specially designed input system for investigating volatile metal–organic compounds was used [18]. This system ensures that the sampling and analysis are performed only on the vapor phase. Approximately 1–2 mg of the compound was placed in an open glass ampoule and maintained at a constant temperature (423, 428 and 423 K for CuPdL4, PdL2 and CuL2, respectively) in the evaporator under dynamic vacuum conditions. The vaporized compound passed through a heated pipeline where it entered into the mass spectrometer ion source directly through a 0.2 mm effusive orifice. The ionization was performed by electrons with energy of ca. 70 eV. These experimental conditions exclude almost all ion–molecule collisions in the mass spectrometer. A time-of-flight mass spectrometer was used to analyze the gas phase. To investigate the thermal stability of the compound in the gas phase, a pipeline section was heated at 5 K min−1. Mass spectra showing the gas-phase composition were recorded every 2 min.
Results and discussion
General considerations
We have previously described a heterometallic CuPdL4 complex and found that in the crystalline state it was a linear coordination polymer of alternating molecules of monometallic complexes. The volatility of CuPdL4 and parent monometallic complexes is revealed by thermal analysis (TG–DTA) and vacuum sublimation. Sublimation tests in vacuum in a gradient tube furnace enabled a qualitative assessment of the volatility and vaporization stability. The heterometallic compound sublimates in one zone and without residue with a noticeable rate at T ~ 403–413 K and P = 10−2 Torr. Its sublimation temperature is lower comparing to that of PdL2 (~443 K) under the same vacuum conditions. A comparison of XRD patterns showed that the starting compound and the sublimate had the same structure. According to TG, the reported heterometallic complex as well as the initial monometallic Cu(II) and Pd(II) complexes completely evaporate in He at atmospheric pressure, exhibiting a single, smooth mass loss that confirms their volatility [17] (Figs. S1–S3). Comparison of TG–DTA data for CuPdL4 and mixture PdL2 + CuL2 1:1 (Figs. S3, S4) revealed that the TG curves are very close at that conditions and the compounds loose mass manly after melting. The heterometallic complex demonstrates one endo peak in DTA for melting process, but for the mixture two peaks are observed. We can assume that when copper complex melts first it dissolves palladium complex producing the heterometallics which exhibits own peak for melting.
However, these results have not answered the question in what form the heterometallic complex passes through the gas phase. The previous mass spectrometric investigation of the thermal behavior of the compounds vapor gave indirect evidence of the existence of heterometallic compounds in the gas phase; however, we were not able to detect bimetallic or oligomeric particles since they were definitely destroyed by electron impact ionization. The results of quantum chemical calculations also indicate that CuPdL4 may sublime as a binuclear complex [17]. If this heterometallic complex can decompose into the mononuclear Cu(II) and Pd(II) components, it is important to study its thermal properties, including the volatility and thermal stability, and compare with those for the parent monometallic complexes. For this purpose, the flow method allowed the measurement of the vapor density at different sublimation temperatures and the computation of the thermodynamic parameters of sublimation for the compounds under discussion. High-temperature mass spectrometry provided data on the gas-phase composition during the heterogeneous decomposition of the compound vapor on the heated surface.
Vaporization and melting processes
Figure 2 compares the experimental vapor density data for the compounds under discussion. The volatility of the copper complex is several times higher than that of the palladium one. It can be seen that the vapor density, calculated for the heterometallic complex, is intermediate in the data for the monometallic complexes. In the case of the CuPdL4 dissociation in the investigated temperature range, its vapor density would be higher than that for CuL2, which contradicts the experimental data from the sublimation test and TG. Thus, the data of the flow method support the assumption that CuPdL4 sublimates in the binuclear form [CuL2PdL2]. Good agreement between the data on the sublimation mass loss and the condensate mass shows that all three substances are thermally stable and do not decompose into smaller volatile products in the investigated range of temperatures.
Table 1 presents the thermodynamic parameters of sublimation resulting from the experimental data processing. The heterometallic complex and the parent copper complex have similar values for the enthalpy of sublimation. It is known that in β-CuL2 and CuPdL4 the molecules of metal complexes are linked in chains by bridging bonds between the copper atom and the oxygen atom of the methoxy group of the neighboring complex [16, 17], whereas the palladium complex PdL2 forms crystals with an island structure [15]. The same type of intermolecular interactions in the crystal can result in close lattice energies of these compounds. In this case, this yields close values for the sublimation enthalpy. It is also interesting to compare the sublimation entropies for these three compounds having a different molecular packing in the crystals. Thus, the ΔS sub value for the heterometallic complex (2D coordination polymer) is intermediate between those for the palladium complex (island structure) and the copper complex (3D coordination polymer). Heats of fusion for the compounds under discussion are compared in Table 2. It is revealed that thermal effects drop down under repeated melting. All complexes under discussion decompose during melting which is typical for these metal β-diketonates, and the reported melting enthalpy is a summary heat effect for melting and decomposition processes. Mean values of MP in K and ΔH melt in kJ mol−1 are: 444.5 ± 0.5 and 57.8 ± 0.9 for CuPdL4; 461.2 ± 0.5 and 23.2 ± 0.5 for PdL2; 436.6 ± 0.5 and 27.8 ± 0.5 for CuL2, respectively. The molar enthalpy of melting for the heterometallic complex is higher than the sum of those values for the monometallic complexes. This may reflect particularly that CuPdL4 is not a simple mixture of the monometallic complexes, and the energy of intermolecular interactions in it is much lower than the intramolecular energy.
Vapor thermolysis
Mass spectrometric monitoring of the gas-phase composition upon the programmed heating (5 K min−1) of the vaporized compounds allowed the study of their thermal stability and the determination of the deposition conditions for metal alloy films. The temperature dependences of the intensity of the selected ion peaks presented in Fig. 3 were derived from the full-range mass spectra recorded every 2 min. The fitting curves are the results of 3-point adjacent averaging smoothing of the experimental points. They are similar to the classical kinetic concentration dependences and characterize the gas phase upon the heterogeneous decomposition (due to the experimental conditions, all conversions occurs on the hot walls of the reactor). When the decomposition onset temperature is reached, the intensities of the metal-containing ion peaks related to the entire compound decrease while peaks related to the decomposition products increase.
The evolution of gaseous products for the thermolysis of CuL2 and PdL2 vapor begins at T = 503 and 513 K, respectively, and a maximum degree of decomposition is achieved at temperatures of 653 and 583 K (Fig. 3a). In the case of CuPdL4, the curves for the peak intensities of Pd- and Cu-containing ions drop symbatically up to ~528 K; then, these curves move apart (diverge) at higher temperatures (Fig. 3b). This observation, combined with a comparison of the curves obtained for the same ions from the mass spectra of heterometallic and monometallic complexes, reveals that the thermal decomposition of the heterometallic complex starts with its dissociation into monometallic complexes. On further heating, they decomposed to form metals (see below) and organic products. This assumption is supported by the character of the temperature dependence of the ion peak with m/z 41. The ion intensity decreases in the range 458–528 K, when it is a fragmentary ion from the heterometallic complex. Then in the range 543–613 K there is a plateau, after which there is an intensity rise, indicating that the ion relates mainly to the thermolysis product. The presence of the plateau with a gradual decrease in the intensity of the metal-containing ions indicates the fragmentation contribution from several sources: CuPdL4, PdL2 and CuL2 to the total intensity of the ion with m/z 41. CVD utilizing the thermolysis of the heterometallic compound vapor on a hot surface resulted in copper–palladium alloys films with a mean ratio Cu/Pd = 55/45 at temperatures 523–623 K [10]. The observed film compositions gives also evidences in favor of the bimetallic complex [CuL2PdL2] being in the gas phase during the evaporation of CuPdL4. Otherwise, a large excess of copper would be in the film due to the higher volatility and a lower thermal stability of the copper complex in comparison with the palladium complex.
Conclusions
The thermal behavior of the volatile heterometallic compound CuPdL4 and the two constituting monometallic complexes were studied by a set of methods: the vacuum sublimation in a gradient oven, TG–DTA, DSC, the flow method and the special mass spectrometric technique. The combination of the data confirms that CuPdL4 sublimes into the gas phase in the form of bimetallic molecules [CuL2PdL2]. The saturated vapor pressure and the thermodynamic parameters of the sublimation and melting of the complexes were measured for the first time. It is found that on heating (after ~528 K) the heterometallic complex vapor the dissociation into monometallic complexes precedes its thermolysis to form bimetallic films. The resulting metal ratio in the alloy films makes them promising material for the engineering of membranes for hydrogen separation.
References
Hubert-Pfalzgraff LG. Some trends in the design of homo- and heterometallic molecular precursors of high-tech oxides. Inorg Chem Commun. 2003;6:102–20.
Gleizes AN. MOCVD of chalcogenides, pnictides, and heterometallic compounds from single-source molecule precursors. Chem Vap Depos. 2000;6:155–73.
Mishra S, Daniele S. Metal-organic derivatives with fluorinated ligands as precursors for inorganic nanomaterials. Chem Rev. 2015;115:8379–448.
Martynova TN, Korchkov VP, Pustovskich II. The volatile tetrakis-chelates of rare-earth-metals containing the cations of alkali-metals. Izvestiya Sibirskogo Otdeleniya Akademii Nauk SSSR Seriya Khimicheskikh Nauk. 1984;5:82–6 (in Russian).
Kuzmina NP, Rogachev AY, Spiridonov FM, Didlovskaya EM, Ketzko VA, Gleizes AN, Battiston G. Heterobimetallic f-d-complexes-β-diketonate derivatives of rare earth elements and N,N′-ethylene-bis-salicylaldiminates of nickel(II) and copper(II). Zh Neorg Khimii. 2000;45(9):1468–75 (in Russian).
Kuzmina NP, Ryazanov MV, Ketzko VA, Gleizes AN. Heterobimetallic f-d-complexes—fluorinated β-diketonate derivatives of lanthanum and gadolinium and N,N′-ethylene-bis-acetylacetoneiminates of nickel(II) and copper(II). Zh Neorg Khimii. 2002;47(1):30–42 (in Russian).
Zhang H, Li B, Dikarev EV. Mn(III) hexafluoroacetylacetonate as an oxidative agent in the synthesis of heterometallic β-diketonates. J Clust Sci. 2008;19:311–21.
Hubert-Pfalzgraff LG, Guillon H. Trends in precursor design for conventional and aerosol-assisted CVD of high-Tc superconductors. Appl Organomet Chem. 1998;12:221–36.
Kuzmina N, Ryazanov M, Malkerova I, Alikhanyan A, Gleizes AN. The heterotrimetallic complex [Ni(acacen)KLa(pta)4]: structural and thermochemical studies. Eur J Inorg Chem. 2001;2001:701–6.
Krisyuk VV, Shubin YV, Senocq F, Turgambaeva AE, Duguet T, Igumenov IK, Vahlas C. Chemical vapor deposition of Pd/Cu alloy films from a new single source precursor. J Cryst Growth. 2015;414:130–4.
Nayebossadri S, Speight J, Book D. Effects of low Ag additions on the hydrogen permeability of Pd–Cu–Ag hydrogen separation membranes. J Membr Sci. 2014;451:216–25.
Burkhanov GS, Gorina NB, Kolchugina NB, Roshan NR, Slovetsky DI, Chistov EM. Palladium-based alloy membranes for separation of high purity hydrogen from hydrogen-containing gas mixtures. Platin Met Rev. 2011;55:3–12.
Igumenov IK, Basova TV, Belosludov VR. Volatile precursors for films deposition: vapor pressure, structure and thermodynamics. In: Mizutani T, editor. Application of thermodynamics to biological and materials science. Rijeka: InTech; 2011. p. 521–46.
Zharkova GI, Stabnikov PA, Sysoev SA, Igumenov IK. Volatility and crystal lattice energy of palladium(II) chelates. J Struct Chem. 2005;46:320–7. doi:10.1007/s10947-006-0047-8.
Krisyuk VV, Tkachev SV, Baidina IA, Korolkov IV, Turgambaeva AE, Igumenov IK. Volatile Pd-Pb and Cu-Pb heterometallic complexes: structure, properties and trans-to-cis isomerization under co-crystallization of Pd(II) and Cu(II) β-diketonates with Pb(II) hexafluoroacetylacetonate. J Coord Chem. 2015;68:1890–902. doi:10.1080/00958972.2015.1035653.
Pisarevsky AP, Yanovsky AI, Struchkov YT, Nichiporuk RV, Snezhko NI, Martynenko LI. Crystal and molecular structure of copper(II) complex with 2-methoxy-2,6,6-trimethylheptane-3,5-dione. Koord Khim. 1994;20:132–5 (in Russian).
Krisyuk V, Baidina I, Turgambaeva A, Nadolinny V, Kozlova S, Korolkov I, Duguet T, Vahlas C, Igumenov I. Volatile heterobimetallic complexes from Pd(II) and Cu(II) β-diketonates: structure, magnetic anisotropy and thermal properties related to CVD of Cu–Pd thin films. ChemPlusChem. 2015;80:1457–64.
Turgambaeva AE, Krisyuk VV, Stabnikov PA, Igumenov IK. Mass spectrometric study of the thermal decomposition mechanism of vapors of 2,2,6,6-tetramethyl-3-iminoheptane-5-one and its copper(II) complex. J Organomet Chem. 2007;692:5001–6.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Krisyuk, V.V., Sysoev, S.V., Turgambaeva, A.E. et al. Thermal behavior of methoxy-substituted Pd and Cu β-diketonates and their heterobimetallic complex. J Therm Anal Calorim 130, 1105–1110 (2017). https://doi.org/10.1007/s10973-017-6469-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6469-z