Abstract
In this paper, propagation and quenching of aluminum dust flame in narrow channels with infinite length and constant width are investigated. Particles are distributed uniformly in three-dimensional space in a quiescent reaction medium. The combustion of a single particle was first studied, and the solution is presented. Each burning/burned particle is considered as a heat source, and the amount of heat loss to the channel walls is assumed as sink source. Based on the superposition principle, the space–time temperature distribution of particles and the heat loss to the walls are estimated based on the generated code. In this study, the amount of heat loss and quenching distance have been investigated as a function of aluminum dust concentration and particle diameter. The effects of preheating of the walls are also studied on quenching distance and heat loss. The estimated results are compared with the experimental data and show a fairly good agreement. The initial wall temperature affects the heat loss, and with the increase in the wall’s initial temperature, the value of quenching distance will be decreased.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays due to fast progress in technology, a new type of energetic materials, nano- and micron-size particles suspended in air with high specific surface are used to make them applicable for many energetic applications such as; propellants, pyrotechnics, and combustion synthesis, there are numerous fine metal powders, such as magnesium, aluminum, iron, boron, titanium, and zirconium and etc. [1].
Dust is an undesirable but inevitable byproduct of many industrial processes, in particular in food processing, mining and metal working, etc. Therefore, combustion of dust particles in air has been a serious safety concern for the mining, manufacturing, and energy production industries [2, 3].
Several accidents have occurred that resulted in the loss of life and substantial property damages, such as: aluminum dust explosion happened in the milling section of the plant located in Modesto California in the year 2000, which was extensive and blowing out practically all the wall panels of the factory building [4]. Such events forced industries to study dust combustion characteristics to better understand this phenomenon. One of the important dust flame characteristics, is the subject of quenching distance which is an important flame feature to prevent and control dust explosions through prevention of dust flame propagation.
Numerous researches were conducted to study combustion of powdered metals (B, Al, Mg, Ti, and etc.). Goroshin et al. [5] compared heat production per unit volume of the fuel for combustion of different metallic elements in air and it was found that boron, beryllium and aluminum have the highest heat production per unit of volume.
Aluminum is widely used because of its high combustion enthalpy, availability, neutralized oxide, and good stability.
Trunov et al. [6] presented a review of experimental measurements of aluminum ignition temperature and different models for aluminum ignition are investigated. In addition, the ignition of aluminum particles is studied experimentally and discussed.
Han et al. [7] developed a numerical three-dimensional Eulerian–Lagrangian two-phase approach for modeling aluminum dust combustion with oxygen as an oxidizer. The model contained detailed combustion characteristics of aluminum particle including melting process, the heterogeneous surface reaction, alumina shell growth, combustion heat distribution and etc. As a result, flame features were calculated in order to discover the behavior of aluminum burning velocity in various conditions and also, temperature variations of the gas and particles were found to be in indirect relation with temperature rise.
Huang, et al. [8] theoretically studied combustion of aluminum dust particles in a laminar air flow under fuel-lean conditions. Particles with the diameters from the nano to micron range were investigated, and the relevant flame speed and temperature distribution were presented. The way of simulation allows for investigation of the effects of particle size, equivalence ratio, etc., on the burning characteristics and flame structures of aluminum particle/air mixtures.
As a result, it was reported that when the particle diameter decreases from the micron to nano range, the flame speed increases and the combustion transits from a diffusion-controlled to a kinetically controlled mode. Bidabadi et al. [9] studied combustion of aluminum dust particles in a quiescent reaction medium under different oxidizers such as carbon dioxide, air, water vapor. It is concluded that for a considered particle diameter and dust concentration, the flame speed in carbon dioxide is the lowest. Many researchers have studied flame propagation and flame velocity of dust particles combustion, but few studies are available regarding quenching distance of metallic dust particles and investigating the effect of different parameters such as the required distance between two plates of a narrow channel that flame would be extinguished or the amount of heat loss on this concept. Quenching distance is defined as the minimum distance between the channel walls that the generated dust flame can propagate through and reducing the distance results in the flame off due to the heat loss between plates. This dynamic flame feature is of importance in the design of flame arresters where the flame propagates through assemblies of equally spaced steel plates installed in the tubes. Ballal [10] experimentally investigated combustion of dust cloud containing different types of materials such as aluminum, magnesium, titanium, carbon. The studies focused on determining the quenching distance and minim ignition energy (MIE) of various dusts.
Jarosinski et al. [11] measured quenching distance of aluminum and coal dust particles. In order to measure quenching distance, then netting steel plate was placed in the middle of the experimental setup to see whether the flame passes through the netting or not. The least quenching distance for Pittsburgh coal dust particles was obtained for a 5 micron particle diameter in the tube. This work was also performed by Proustetal [12] using a similar experimental technique. Bidabadi [13] investigated combustion of aluminum dust particles with a mean diameter of 5.4 micron in different neutralized gasses and different oxidizers. Also, the quenching distances of aluminum dust particles in the air, nitrogen, and helium were measured both experimentally and analytically. In addition, the effect of fuel concentration was studied. It was concluded that the minimum quenching distance in the air was about 5 mm for a considered dust concentration, but if one substitutes nitrogen with helium or increases the concentration of oxygen, quenching distance would be increased to 7 and 15 mm, respectively. Tang et al. [14] presented the results of experiment tests for suspensions of iron dust in the air. The results were obtained for four different uniform dust particles having an average particle sizes in the range 3–27 micron. Bidabadi et al. [15] investigated the propagation and extinction of aluminum dust cloud flame in a narrow channel. They found out that the quenching distance of aluminum dust was reduced by increasing the dust concentration and the channel width. Also, they concluded that the flame propagation speed is lower than the speed in an adiabatic channel.
Palecka et al. [16] experimentally measured quenching of laminar combustion of methane–aluminum particles mixtures in different dust concentrations. The quenching of fuel mixtures versus aluminum dust concentration was studied. In addition, silicon carbide (SiC) powder was used as an inert powder in the fuel mixture which caused a steady increase in the quenching distance.
The aim of the present research is to develop a theoretical thermal model for calculating the quenching distance of aluminum dust particles in a quiescent reaction medium and the amount of heat loss to the narrow channel walls caused by combustion of micron-sized, mono-disperse dust particles under various dust concentrations and sizes. The model employed in the present study is different from the work done by [15]. The governing equations are in Cartesian coordinates and are utilized to suitably simulate combustion of metal particles with abrupt ignition and negligible burning time which is applicable for aluminum and magnesium particles during reaction. The proposed thermal model shows high qualitative accuracy in terms of flame quenching distance compared with different experimental data. The model starts by considering single-particle combustion to obtain a space–time temperature distribution. The ensemble reacting front in the suspended dust combustion is then considered using the superposition principle to include the effects of neighboring particles. All the burned and burning particles are considered as heat sources, and the channel walls are assumed to behave as heat sinks. At the end, flame propagation speed, quenching distance, and the amount of heat losses to the channel walls are obtained.
Dust cloud combustion in narrow channels
Dust cloud combustion is a very complex phenomenon. This complexity is caused by various processes, such as heat transfer in multiphase materials, or blending with an oxidizer, ignition, burning, etc.
Different factors, including the physical and chemical properties of fuel particle, shape, and average particle size, influence the combustion behavior of dust cloud in narrow channels; also, distribution of particles is a significant parameter. Dust clouds are not distributed uniformly in space; however, the assumption of uniform distribution of fuel particles, as considered in this research, still contributes to reasonable predictions. The assumption is only valid early in the dispersal before the effects of gravity/buoyancy influence the dust cloud.
Heat transfer mechanism plays a significant role in dust cloud combustion. Radiation heat transfer has a very complex effect on the combustion process which is still not fully understood.
Bidabadi [13] concluded that the ratio of temperature difference caused by conduction to temperature difference caused by radiation in aluminum particles is 501. This ratio is also known as Stark number which corresponds to the relative role of heat transfer by conduction to radiation. The radiative heat transfer vanishes for the limiting case of a transparent medium. On the other hand, when the aforementioned ratio approaches large numbers, heat transfer within the medium is only by conduction. The opposite extreme, when the above said ratio approaches zero, corresponds to the case in which heat transfer is solely due to radiation. Considering the fact that this ratio has a large value for aluminum, one can neglect the effect of radiative heat transfer in the combustion of aluminum particles.
Therefore, in the present research radiation heat transfer mechanism is neglected, based on the above explanation, and as it is assumed that the reaction medium is quiescent, there is no convection involved in the governing equations. Hence, the main heat transfer mechanism is considered to be conduction. There are two opposite factors in modeling the combustion behavior of dust cloud in narrow channels. The first one is transferring the produced heat from the burning particles to the neighboring particles which gives rise to an increase in neighboring particles temperature and has a positive effect on flame propagation speed. The second and more important issue is transferring the heat released to the channel plates which is considered as heat loss and has a negative effect on flame propagation. The heat loss effect may be so strong that it causes the particles not to reach the ignition temperature.
Governing equations
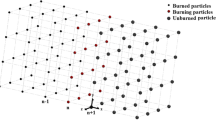
The uniform spatial distribution of fuel particles in a considered cubic control volume in 3D space is illustrated in Fig. 1. In the shown control volume, particles are considered inside the grid nodes and it is assumed that all nodes are filled with particles; therefore, the distribution of particles is uniform. In the present thermal model, particle’s temperature increases and when the temperature reaches the ignition temperature, all particles in a layer burn and act as heat sources that make the temperature of the unburned particles in the neighboring layer to increase.
It is assumed that all particles are spherical and the temperature change in the radial direction is small and negligible; therefore, the Cartesian coordinates is considered here.
The energy equation is written as follows:
where \(\alpha\) is thermal diffusivity(m2 s − 1), k is conductivity coefficient (Wm − 1 k − 1), and \(\dot{q}\) is the rate of heat release from a single particle’s surface during the burning.
The above equation can be made dimensionless by using the parameters below:
where τ is the dimensionless form of time, \(\theta\) is the dimensionless form of temperature, L is the distance between particles, \(T_{\text{in}}\) is the initial temperature, and \(T_{{{\text{ad}} }}\) is the adiabatic flame temperature.
In order to calculate the adiabatic flame temperature, it is assumed that the particles are located in a hypothetical cubic control volume and then energy conservative law is exerted within it.
Energy conservative law:
In Eq. (3), C Pmixture is the specific heat for the mixture of fuel particle and gaseous oxidizer, ρ mixture is the density of the mixture, Q is the total amount of heat released, and B is the mass concentration of fuel.
By utilization of dimensionless form of parameters, the following form of energy law is achieved:
where τ c is the dimensionless form of burning time and defined as below:
Experimental findings [17, 18] show that the burning time of inorganic particles is significantly smaller than the time it takes the particles to reach the ignition temperature; therefore, it can be assumed that inorganic particles would burn immediately so the Dirac delta function can be utilized in the terms of heat source:
In the above equation, the index (i) is the counter variable and (N) is the total numbers of layers. The above equation is solved, and the superposition theorem used for the effects of previous layers on the current layer.
In the above equation, m is the index of the current layer and i is the index of previous layers. The first layer is ignited by igniter, and the burning time of this layer is calculated which is needed for calculation of the ignition temperature and burning time of the second layer. The calculated burning time of the first and second layers are needed for buring time estimation of the third layer. Finally, for the calculation of current layer (m), the burning time of all previous burned layers ranging from first layer to the layer of (m − 1) are vital. The parameter L is the spacing between two adjacent layers and determined from the below equation:
where ρ p and d p are the particle density and diameter, respectively, and B is mass concentration of fuel. The heat loss in narrow channels is considered to be perpendicular to the flame propagation direction and will be discussed further in the following.
Modeling heat LOSS
In the present model, heat loss is considered one dimensional and the heat loss in narrow channels is perpendicular to the direction of flame propagation and the main heat transfer mechanism is conduction heat transfer. Figure 2 shows the schematic of the problem configuration. Also, it is assumed that the wall’s temperature during the heat transfer remains constant at 300 K.
Diagram of the narrow channel and the heat loss of layers to the channel’s plates, adapted with permission from [15]
The heat loss is calculated through the below equation:
In Eq. (10), k a is the conduction coefficient for oxidizer (Wm − 1 k − 1) that in this case is air, A is the heat transfer area, and D is the quenching distance which is unknown.
The overall heat loss is obtained by:
The reduced temperature due to the above heat loss is equal to:
Therefore, the reduced temperature is given by:
Considering the above parameters, dimensionless form of reduced temperature appears as follows:
In the above equation, the dimensionless parameters have been defined below:
where α a and α p are the thermal diffusivity of oxidizer, air in this case, and the thermal diffusivity of particle, respectively. L is the distance between two particles and D defined as a quenching distance.
The final temperature of a current layer is under the influence of two opposite factors: transferring the produced heat from the burning particles to neighboring particles and the transferring of heat to plates which is considered as heat loss. The temperature distribution function is given by:
Results and discussion
In the present research, the quenching distance of combustion of aluminum particles in air is studied, and the physical properties of aluminum and air used in this study is given in Table 1. It should be noted that the thermal diffusivity coefficient in energy equation is sensitive to temperature changes in the reaction medium; therefore, the variation of thermal diffusivity with the temperature of reaction medium is considered and calculated in the generated code.
In the present model, several factors influence quenching distance including the physical properties of the fuel and oxidizer particles, preheat time, the initial temperature of plates, etc.
Figures 3 and 4 show quenching distance as a function of dust concentration for aluminum dust cloud. Figure 3 provides the model’s predictions for particles with 5.4 µm diameter. It shows that with the increase in dust concentration the value of quenching distance will be reduced. This is due to the fact that increasing the amount of dust concentration and the total amount of fuel particles results in higher amount of energy release rate from the burning/burned particles, and this increases the value of flame propagation speed; therefore, the time needed for transferring the generated heat during combustion to the channel plates decreases considerably. Hence, the amount of heat loss to the channel plates would be decreased and it requires smaller quenching distances to quench the propagated dust flame. The results shown in Fig. 3 compared with the Bidabadi experimental data show a fairly good agreement [13]. Figure 4 illustrates the model’s predictions for particles with 17.5 µm diameter and the obtained results compared with Kim’s experimental data [23] generally agree with the experimental data.
Figure 5 shows quenching distance as a function of particle diameter for stoichiometric dust concentration. As it can be concluded, the quenching distance increases with the increase in particle diameter. This is due to the fact that larger particles require much more energy to reach ignition temperature and to be ignited according to the equation T ign = 34.5d p + 789.1 [20]. In other words, small particles burn faster than large particles [24]. As a result, the time it takes larger particles reach ignition temperature increases compared to the smaller ones and sufficient time is available for heating loss, and this causes more heat losses to occur, which leads to larger quenching distance as shown in Fig. 6.
Figures 7 and 8 show the change in the quenching distance in different initial channel wall temperature. Figure 7 shows quenching distance as a function of dust concentration for various initial wall temperatures for 5.4 µm particle diameter. As it is understood, with the increase in the initial temperature of wall channels, the values of quenching distance and heat loss will be reduced. This is due to the fact that as the wall temperature augments, the temperature difference between the wall and the burning/burned layers would be reduced and (according to Eq. (10)) the amount of the heat transferred to the channel plates or heat loss would be decreased.
Figure 8 depicts heat loss as a function of dust concentration for various initial wall temperatures for 5.4 µm particle diameter. It is observed that with the rise of initial wall temperature, the amount of heat loss decreases and as can be seen in both Figs. 6 and 8 the value of heat loss to plates reaches its maximum amount at nearly stoichiometric dust concentration. Because the maximum released energy is generated in the stoichiometric combustion and therefore more energy is dissipated through walls channel.
Conclusions
In the present study, combustion of aluminum dust particles in a quiescent reaction in a medium with spatially discrete sources is studied. The quenching distance in the aluminum–air suspensions will be increased as the particle size increases, since more time is available for heating loss during the ignition of larger particles as compared to the smaller ones. Finally, the estimated quenching distances appear to reasonably obtain the threshold for quenching distances, providing an effective model both for flame propagation and quenching of dust clouds in narrow channels with spatially discrete sources uniformly distributed in a quiescent reaction medium. The effects of burned particles on unburned fuel particles are studied using superstition theorem. The value of the reduced temperature due to overall heat loss is calculated. The governing equations are solved considering heat loss effects. As a result, quenching distance is obtained as a function of dust concentration and particle diameter.
The thermal model presented is an effective tool for the estimation of quenching distance and the amount of heat loss in combustion of aluminum dust particles in narrow channels in various dust concentrations and particle sizes. The present thermal model can be improved to consider the effects of radiation and particle size distributions to enhance the obtained results in comparison with the experimental data.
Abbreviations
- q′:
-
Rate of heat release
- L :
-
Distance between the layers
- c p :
-
Heat capacity at constant pressure
- T :
-
Temperature
- Q :
-
Total heat released
- x, y, z :
-
The dimensional Cartesian coordinate system
- x′, y′, z′:
-
The non-dimensional Cartesian coordinate system
- B :
-
Mass concentration
- t :
-
Time
- d :
-
Particle diameter
- i, m :
-
The counter
- in :
-
Initial temperature
- ad :
-
Adiabatic temperature
- a :
-
Air
- p :
-
Particle
- w :
-
Wall
- ig :
-
Ignition time
- c :
-
Combustion time
- v :
-
Velocity
- α :
-
Thermal diffusivity
- τ :
-
Non-dimensional time
- ψ, γ :
-
Non-dimensional parameters
- θ :
-
Non-dimensional temperature
- ρ :
-
Particle density
- σ :
-
Variance
- η :
-
Non-dimensional velocity
References
Dreizin EL. Metal-based reactive nano materials. Prog Energy Combust Sci. 2009;35(2):141–67.
Eckhoff R. Dust explosions in the process industries: identification and control of dust hazards. Houston: Gulf Professional Publishing; 2003.
Lin-lin L, Pei-jin L, Guo-qiang H. Ignition and combustion characteristics of compound of magnesium and boron. Therm Anal Calorim. 2015;121:1205–12.
Kuriechan SK, Causes and impacts of accidents in chemical process industries and a study of the consequence analysis software. M.Phil. Thesis, Pondicherry University, 2005.
Goroshin S, Higgins AJ, Kamel M. Powdered metals as fuel for hypersonic ramjets. AIAA paper. 2001; 3919.
Trunov MA, Schoenitz M, Dreizin EL. Ignition of aluminum powders under different experimental conditions. Propellants Explos Pyrotech. 2005;30(1):36–43.
Han DH, Shin JS, Sung HG (2016) A detailed flame structure and burning velocity analysis of aluminum dust cloud combustion using the Eulerian–Lagrangian method. In: Proceedings of the combustion institute.
Huang Y, Risha GA, Yang V, Yetter RA. Effect of particle size on combustion of aluminum particle dust in air. Combust Flame. 2009;156(1):5–13.
Bidabadi M, Poorfar AK, Wang S, Bengt S. A comparative study of different burning time models for the combustion of aluminum dust particles. Appl Therm Eng. 2016;105:474–82.
Ballal DR. Ignition and flame quenching of quiescent dust clouds of solid fuels. Proc R Soc Lond A Math Phys Eng Sci. 1980;369(1739):479–500.
Jarosinski JA. Survey of recent studies on flame extinction. Prog Energy Combust Sci. 1986;12(2):81–116.
Proust CH, Veyssiere B. Fundamental properties of flames propagating in starch dust-air mixtures. Combust Sci Technol. 1988;62(4–6):149–72.
Bidabadi M. An experimental and analytical study of laminar dust flame propagation. Montreal: McGill University; 1996.
Tang FD, Goroshin S, Higgins A, Lee J. Flame propagation and quenching in iron dust clouds. Proc Combust Inst. 2009;32(2):1905–12.
Bidabadi M, Zadsirjan S, Mostafavi SA. Propagation and extinction of dust flames in narrow channels. J Loss Prev Process Ind. 2013;26(1):172–6.
Palecka J, Julien P, Goroshin S, Bergthorson JM, Frost DL, Higgins AJ. Quenching distance of flames in hybrid methane–aluminum mixtures. Proc Combust Inst. 2015;35(2):2463–70.
Varma A, Mukasyan AS, Hwang S. Dynamics of self-propagating reactions in heterogeneous media: experiments and model. Chem Eng Sci. 2001;56(4):1459–66.
Mukasyan AS, Rogachev AS, Mercedes M, Varma A. Microstructural correlations between reaction medium and combustion wave propagation in heterogeneous systems. Chem Eng Sci. 2004;59(22):5099–105.
Incropera FP, Dewitt DP. Fundamentals of heat and mass transfer. 5th ed. New York: Wiley; 2002.
Marino TA. Numerical analysis to study the effects of solid fuel particle characteristics on ignition, burning, and radiative emission. Ann Arbor: ProQuest; 2008.
Marion M, Chauveau C, Gökalp I. Studies on the ignition and burning of levitated aluminum particles. Combust Sci Technol. 1996;115(4–6):369–90.
Yetter RA, Frederick LD. Metal particle combustion and classification. In: Microgravity combustion: fire in free fall. 2001: 419–478
Kim SW. Theoretical and experimental studies on flame propagation and quenching of powdered fuels. Ames: Iowa State University; 1989.
Yunlan S, Rong S, Baozhong Z, Keke M, Yuxin W. Thermal reaction mechanisms of nano- and micro-scale aluminum powders dioxide at low heating rate. Therm Anal Calorim. 2016;124(3):1727–34.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bidabadi, M., Biouki, S.A., Afzalabadi, A. et al. Modeling propagation and extinction of aluminum dust particles in a reaction medium with spatially uniform distribution of particles. J Therm Anal Calorim 129, 1855–1864 (2017). https://doi.org/10.1007/s10973-017-6338-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6338-9