Abstract
Leather is composed of a three-dimensional weave of collagen fiber bundles. Collagen is a fibrous protein well organized in the formation of skin as building blocks. Leather production involves serial operations where the tanning plays the major role in improving the durability of leather products by stabilizing the triple helical structure of collagen matrix. In the leather-making industry, different tanning agents are used to produce different kind of leather goods. These tanning agents have varied efficiency on the stabilization of collagen. In this study, thermal stability of the leathers tanned with most commonly used tanning agents was evaluated by conventional shrinking test (CST) and differential scanning calorimetry (DSC) methods. The results showed that the thermal stability of leathers varied by the type of tanning agent which were in accordance with theoretical approaches. A distinct correlation was also observed between CST and DSC results of the tanned leathers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The hides or skins which are the main raw materials of leather industry are made up of helix-structured collagen proteins of three polypeptide chains wrapped around each other in a spiral form. Each polypeptide chain carries different functional groups on their side chains that allow various chemical modifications on protein structure [1]. With these modifications, the collagen can be turned into different products which are needed and of benefit in modern life. The leather goes through many stages in the course of this conversion.
Tanning is the major step in leather production giving strength by the addition of cross-links to the collagen, and providing thermal, enzymatic and microbial stability [2]. In the tanning process, the three-dimensional matrix structure is stabilized by various chemical bonds larger than 40 kJ/mol and attraction forces smaller than 40 kJ/mol [3]. The most drastic change in the structure of protein occurs during the tanning process due to the increase in the number and type of bonds between collagen chains and tanning agents. Tanning agents used in leather production have different bond strengths and binding mechanisms [4]. The most effective tanning agents drop into two categories as inorganic tanning agents such as chromium, aluminum, zirconium , and organic agents such as vegetable tannins (i.e., mimosa, tara, valonea, chestnut), phosphonium salts, aldehydes. All these tanning agents give different characteristics (color, firmness, softness, etc.) and thermal stability to the leathers due to their molecular structure and interactions they make with collagen. Among the tanning agents, chromium (III) salts are the most extensively used compounds due to the quality and high stabilization ability they impart to leather [5]. Chromium as a transition element has 3d orbitals which gives possibility to take extra electrons to form coordination complexes. In the practice of tanning, a basic chromium (III) sulfate compound is commonly used. This complex reacts with the ionized carboxyl groups of collagen via covalent bonding and chromium nuclei undergo a self-polymerization via hydroxyl bridges which form in final stable –Cr–O–Cr– bridges between the protein chains (Fig. 1a). Polynuclear chromium–collagen cross-linking is accepted to be the most stable complexes since it gives a hydrothermal stability over 100 °C [6–9].
Another mineral tanning agent used in leather production is the aluminum salts. In comparison with chromium compounds, aluminum salts do not form strong covalent bonds and their stabilization is mostly based on electrostatic interactions (Fig. 1b). Moreover, they are unstable, easily hydrolyzed, and the length of the bond between collagen and aluminum is high. Therefore, the hydrothermal stability of aluminum-tanned leathers is usually lower than others tannages [9].
Zirconium (IV) salts are also used in tanning especially in wet white production. They are acidic and unstable to hydrolysis. The growth of zirconium complex in the fibers is also based on its hydrolysis in the presence of water. They create more stable coordination compounds with collagen (Fig. 1c). However, the hydrophilic nature and the cost of these salts limit their applications [2, 10]. Phosphonium compounds are an alternative metal-free tanning agent and frequently used in the production of orthopedic leathers. Tetrakis (hydroxymethyl) phosphonium sulfate (THPS) is the commercial form used for tanning and forms stable links with collagen. Its hydroxymethyl groups react with the amino groups of collagen and form short and strong cross-valent bonds (Fig. 1d). In addition, the hydroxyl groups present in the complex make extra hydrogen bonding with peptide groups and increase its stability [11, 12].
Another widely used tanning agent is vegetable tannins especially in the production of natural leathers. Their use is known for centuries, and the mechanism of their stabilization is based on multi-hydrogen links between the polyphenols and collagen (Fig. 1e) [9, 13].
The efficiency of the tanning process is usually determined by the thermal stability of leather using various methods. The most commonly used technique is the measurement of hydrothermal stability that is based on the determination of shrinkage temperature of the leather using a special test apparatus. The shrinkage test as a kinetic process is done at elevated temperature in the presence of water where the shrinkage temperature is determined by the decrease in sample dimensions as a result of the breaking of the bonds between fibers. The temperature value of shrinkage depends on the nature of tanning agent which in turn determined by the strength of bonds between the tanning material and collagen. On the other hand, there are critics on this method related to the arbitrary nature of the test. The shrinkage temperature is highly depended on the heating rate which might be difficult to keep constant in some cases. Moreover, the test is not reliable for the leathers having T s over 105 °C and the determination of the T s is highly subjective. Another method that can be used for the determination of thermal stability of leather is differential scanning calorimetry (DSC) where the phase transitions and denaturation process of collagen is followed by the change in heat flow through the sample. The measurement can be done in open or sealed capsules under controlled heating rate within a larger temperature scale. Therefore, it can be a good alternative for the determination of thermal behavior of the leather or pelts. However, the phase changes and test conditions should be well established and compared with the conventional testing. In the literature, there are limited numbers of studies related to the differential scanning calorimetric measurement of leathers. Tang et al. [14] reported a differential scanning calorimetry (DSC) study of the sheepskin collagen samples treated with hydrolyzable tannins, including two commercial tannins’ extracts (chestnut and valonea), two pure ellagitannins (vescalagin and castalagin) and six synthetic gallotannins. They concluded that DSC offered an objective method to detect the stability heterogeneity of collagen matrixes in the solid state, and provided a useful tool for the leather industry to evaluate the uniformity of leather tanning. Hui and Zhi-Hua [15] investigated the changes in hydrothermal stability of collagen with several catechin–metal compounds by using DSC. Krishnamoorthy et al. [16] used DSC analysis for the determination of the denaturation temperatures (T d) of unnatural D-alanin-aldehyde-tanned leathers and compared the results with the conventional shrinkage temperatures (T s). The T d values of the leathers were found to be 10–20 °C higher than T s values; however, there was a correlation between the T d and T s values of the samples. Another DSC study was reported by Wang et al. [17] where the thermal stability of sheepskin collagen cross-linked with chrome sulfate and mimosa (MI)-oxazolidine was examined. They compared the T d and T s values of the tanned leathers with varied moisture content of 20–72%. They found that hydrothermal stability of the leather decreased with the increased moisture content. Until a certain moisture content (55% for chrome leather; 40% for combined tanned leather), T d values were higher than T s values. On the other hand, when the moisture was higher, T d values decreased significantly. They concluded that the relationship between the hydrothermal stability of leather and its containing moisture was very important. Crudu et al. [18] used the titanium metal wastes as tanning agent in leather industry and evaluated the thermal stability of tanned samples by DSC method. Carsote et al. [19] investigated the effect of temperature and relative humidity on vegetable-tanned leather exposed to accelerated aging which the collagen denaturation and shrinkage temperature decreased with the exposure time measured by DSC. In a recent study, Carsote et al. [20] studied the effect of different vegetable tannins and animal species on the thermal stability of leather by differential scanning calorimetry. They reported that the resistance against destabilization and denaturation of the chemically modified collagen is higher than that of unmodified collagen and depends on both the tannin type, i.e., condensed or hydrolyzable, and collagen animal species, i.e., calf or sheep.
There are also studies using DSC technique to investigate the thermal behaviors and phase changes of aged leathers and historical parchment leathers [21–26]. In these studies, the denaturation of collagenous materials under different environmental conditions such as temperature, air, moisture was considered to simulate the storage conditions where DSC was used as a quick technique to determine the changes.
The recent works show that the effect of different and/or new tanning agents on collagen stability has been an important subject of research [27–29]. Differential scanning calorimetry can be used as a versatile tool being a quick, objective and controlled technique to study the thermal behavior of the leathers, skins and/or historical leathers. However, the investigations on the correlation of conventional shrinkage temperature and thermal decomposition of the leathers tanned with widely used tanning agents are still necessary. The aim of the present study was to evaluate the aspects of thermal stability of leathers treated with different types of tanning agents having different bond power with collagenous material. For this purpose, both the shrinkage temperatures from conventional testing and the thermal decomposition temperatures obtained by DSC have been measured and compared. The correlations between results and their relations with the bond power of tanning agents have been discussed.
Experimental
Materials
Commercially pickled domestic sheepskins were used for tanning operations. Tanning agents used in the study were industrially produced, commercially available products: chromium salt from “Sisecam Chemicals,” aluminum salt from “Zschimmer and Schwarz GmbH & Co. KG,” phosphonium and zirconium salts from “Clariant” and tara tannin from “Silvachimica S.r.l.” Other chemicals in the production were provided from various suppliers.
Leather manufacturing processes
Tanning operations with different tanning agents were made in accordance with a production process applied commercially in a leather factory. Depickling process was firstly applied for all leathers in the same way before tanning operations (Table 1). Subsequent to depickling, the skins were tanned with each type of tanning agent using the recipes given in Tables 2–6.
Determination of shrinkage temperature (T s)
The measurement of the shrinkage temperature (T s) of the leathers was performed according to the IUP 16 standard test method. The basic principle of the method is to suspend the leather test sample in water under heating 2 °C min−1 and to note the temperature when it starts to shrink visibly. The temperature at which the collagenous fiber shrunk to one-third of its original length was noted as the shrinkage temperature of the leathers [30]. For each measurement, three parallel samples were used and the average values were presented.
DSC analysis
Differential scanning calorimetry (DSC) measurements were carried out on the tanned leathers to determine the phase transitions and/or decomposition temperatures (T d) using a Shimadzu DSC-60 Plus instrument. DSC analysis of tanned leathers was conducted at a heating rate of 10 °C min−1 under nitrogen atmosphere (purity 99.99%, flow 20 mL min−1). Leather samples were heated from 25 to 250 °C in an aluminum pan, which was covered with an aluminum lid with three small holes. Sample mass was approximately 5 mg in dry form. The reference had a similar empty crucible. For each sample type, the measurements were repeated for 3 times.
Results and discussion
Results of shrinkage temperature
The capacity of tanning agents to form cross-links and the type of bonds are the most important factors affecting the hydrothermal stability of leather [31]. The shrinkage temperature of modified collagen can be noted by the change of macroscopically pattern, such as shortening of length, decrease in volume and induration of the sample. Using the special T s testing instrument, the temperature of heating medium at which the specimen begins to shrink was considered as T s of the sample. T s values of the produced leathers by different tanning agents are given in Table 7.
Larsen et al. [32] showed that shrinkage temperature was an important characteristic for determining collagen stability. Covington [9] stated that the hydrothermal stability of collagen increased according to increase in the number of stable bonds and bond strengths. As it is shown in Table 7, the shrinkage temperature of the pickled leathers significantly increased after tanning. However, T s of the samples showed a variety between 72 and 102 °C due to the type of tanning agent. The highest T s value (102 °C) was obtained at chrome-tanned leathers as expected because of the strong covalent bonding between chromium species and collagen. On the other hand, aluminum-tanned leathers showed the lowest increase in T s values (72 °C) among the other tanning agents since it forms only electrostatic interactions with collagen.
DSC results
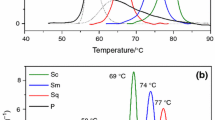
Differential scanning calorimetry (DSC), which was widely used to study various aspects of chemical reactions such as thermally induced polymerizations, decompositions and polycondensations, can also be used to investigate the phase changes or conformational transitions based on the measurements of the specific heat capacity of leathers [14]. DSC curves of different tanned leathers are shown in Fig. 2.
Analyses showed that the leathers processed with different tanning agents had similar thermograms having one endothermic peak. Okamoto and Saeki [33] heated the collagen from room temperature to 225 °C in order to observe the phase changes in collagen. They stated that the endothermic peak occurred between room temperature and 120 °C referred to the breakage of cross-links in the collagen resulting in evaporation of the adsorbed water in fibers. From the DSC curves, it was obvious that the dehydration process of the leathers was strongly affected by the tanning agent and its bond power with the substrate. Therefore, the maximum peak value of the endothermic peak was evaluated as the denaturation temperature of the leather samples. Among the samples, the highest denaturation temperature (T d) was observed in chrome-tanned leathers (95.06 °C), followed by phosphonium (88.01 °C)-, zirconium (72.13 °C)-, vegetable (70.44 °C)- and aluminum (68.84 °C)-tanned leathers, respectively. The decomposition temperatures obtained from DSC were in accordance with the values obtained from conventional shrinkage temperature test, only being slightly lower. Among the tanned leathers, aluminum tanning gave the lowest T d value as it was explained that its tanning effect is based on electrostatic interactions. Although the tanning mechanism of vegetable tannins is associated with only hydrogen bonding that have lower binding energy, vegetable-tanned leathers gave higher T d value than aluminum-tanned leathers. This particular result clearly shows that the number of linkages is also important since polyphenolic tannins make many hydrogen bonding with collagen that increase its stability. All ionic or neutral complexes of zirconium are capable of making electrostatic, co-ordinate covalent and hydrogen bonding with collagen [2]; however, the thermal stability of the zirconium-tanned leathers is much lower than chrome leathers as it was also observed by DSC measurement (T d = 72 °C). Zirconium complexes have many hydroxyl sites on its tetramer structure which makes it as a mineral equivalent of vegetable tanning. Moreover, zirconium complexes linked with hydroxyl groups are not substitution inert as chromium (III) complexes; therefore, the T d value is much lower than chrome-tanned leathers.
The hydroxymethyl groups of phosphonium tanning agents react with collagen amino groups and can form strong covalent bonds. Also, the hydroxyl groups in the hydroxymethyl groups make hydrogen bonds with the peptide groups of collagen. Moreover, it is reported that the phosphonium tanning agent (THPS) is converted into tri-hydroxymethyl phosphonium (TrHP), tri-hydroxymethyl phosphonium hydroxide (TrHPOH) and tri-hydroxymethyl phosphine oxide (TrHPO) species, respectively, during the tanning process. Meanwhile, free formaldehyde is released as a decomposition product which undergoes a nucleophilic substitution with the amine groups of the collagen. The hydroxymethylated amino groups of collagen react with TrHPO and form strong organic covalent bonds which explain the high hydrothermal stability of phosphonium-tanned leathers [11, 12]. On the other hand, in chrome tanning numerous and very stable coordinated covalent bonds are formed between the ionized carboxyl groups of collagen and chromium atom. In addition, the chromium complex goes through a self-polymerization in the form of –Cr–O–Cr– upon deprotonation which results in very stable bridges between the collagen chains. The high cross-linking ability and the stability of the bonds make chromium an unequaled tanning agent with high hydrothermal stability [6–8].
Correlation between CST and DSC test results of the tanned leathers is given in Fig. 3.
From Fig. 3, it is obvious that both shrinkage temperatures and denaturation temperatures obtained by two different methods for the leathers tanned with different agents are similar and have the same trend. As explained in the section above, the number of cross-links and the strength of bonding between the tanning agent and collagen affect the thermal behavior of leathers significantly. Moreover, the denaturation temperatures obtained from relatively dried samples via differential scanning calorimetry analysis showed comparable results with conventional shrinkage testing.
Conclusions
Each type of tanning agents has different effect on the stabilization of hide/skin collagen. The efficiency of tannage is one of the major factors that designate the field of use of the leather product. Therefore, the determination of thermal stability of leather in a controlled, quick and subjective manner is crucial. In the present study, the thermal behavior of the different tanned leathers has been investigated comparatively using conventional shrinkage temperature test and differential scanning calorimetry. The results were correlated with the theoretical binding mechanisms of each type of tanning agent. The findings revealed that both T d and T s values obtained from two methods were comparable and the experimental values were in accordance with the theory since the chrome leathers had the highest T d and T s values, whereas the aluminum tanning gave the lowest increase in thermal stability. Moreover, DSC analysis was found to be good alternative method for the control of the tanning in comparison with the conventional testing. However, more research on the optimization of method is still necessary which is currently ongoing.
References
Cucos A, Budrugeac P, Mitrea S, Hajdu C. The influence of sodium chloride on the melting temperature of collagen crystalline region in parchments. J Therm Anal Calorim. 2013;111:467–73.
Fathima NN, Balaraman M, Rao JR, Nair BU. Effect of zirconium(IV) complexes on the thermal and enzymatic stability of type I collagen. J Inorg Biochem. 2003;95:47–54.
Pauling L. General chemistry. New York: Dover Publications; 1988.
Kronick PL, Cooke P. Thermal stabilization of collagen fibers by calcification. Connect Tissue Res. 1996;33:275–82.
Covington AD. Quo vadit chromium? The future direction of tannage. J Am Leather Chem As. 2008;103:7–23.
Gustavson KH. The chemistry and reactivity of collagen. New York: Academic Press Inc.; 1965.
Bienkiewics K. Physical chemistry of leather making. Melbourne FL: Krieger Pub Co; 1983.
Imer S, Varnali T. Modeling chromium sulfate complexes in relation to chromium tannage in leather technology: a computational study. Appl Organomet Chem. 2000;14:660–9.
Covington AD. Tanning chemistry, the science of leather. Northampton: The University of Northampton; 2009.
Sundarrajan A, Madhan B, Rao JR, Nair BU. Studies on tanning with zirconium oxychloride: part I standardization of tanning process. J Am Leather Chem Assoc. 2003;98:101–6.
Li Y, Shan ZH, Shao SX, Shi KQ. Reaction mechanism of tereakis hydroxymethyl phosphonium with collagen protein. J Soc Leather Technol Chem. 2006;90:214–6.
Shuangxi S, Kaiqi S, Ya L, Lan J, Chun’an M. Mechanism of chrome-free tanning with tetra-hydroxymethyl phosphonium chloride. Chin J Chem Eng. 2008;16:446–50.
Madhan B, Aravindhan R, Ranjithakumar N, Venkiah V, Rao JR, Nair BU. Combination tanning based on tara: an attempt to make chrome-free garment leather. J Am Leather Chem Assoc. 2007;102:198–204.
Tang HR, Covington AD, Hancock RA. Use of DSC to detect the heterogeneity of hydrothermal stability in the polyphenol-treated collagen matrix. J Agric Food Chem. 2003;51:6652–6.
Hui C, Zhi-Hua S. Changes in hydrothermal stability of collagen with several catechin-metal compounds: a DSC study. J Soc Leather Technol Chem. 2008;92:93–5.
Krishnamoorthy G, Sadulla S, Sehgal PK, Mandal AB. Green chemistry approaches to leather tanning process for making chrome-free leather by unnatural amino acids. J Hazard Mater. 2012;215:173–82.
Wang YJ, Guo J, Chen H, Shan ZH. Influence of containing moisture on hydrothermal stability of modified collagen thermal characteristics analysis by DSC. J Therm Anal Calorim. 2010;99:295–300.
Crudu M, Deselnicu V, Deselnicu DC, Albu L. Valorization of titanium metal wastes as tanning agent used in leather industry. Waste Manag. 2014;34:1806–14.
Carsote C, Budrugeac P, Miu L, Yalcin F, Karavana HA, Badea E. Effect of temperature and relative humidity on vegetable tanned leather studied by thermal analysis. In: ICAMS—5th international conference on advanced materials and systems; 2014. p. 505–510.
Carsote C, Badea E, Miu L, Della Gatta G. Study of the effect of tannins and animal species on the thermal stability of vegetable leather by differential scanning calorimetry. J Therm Anal Calorim. 2016;124:1255–66.
Budrugeac P. Phase transitions of a parchment manufactured from deer leather: a calorimetric and kinetic analysis. J Therm Anal Calorim. 2015;120:103–12.
Cucos A, Budrugeac P, Miu L. DMA and DSC studies of accelerated aged parchment and vegetable-tanned leather samples. Thermochim Acta. 2014;583:86–93.
Chahine C. Changes in hydrothermal stability of leather and parchment with deterioration: a DSC study. Thermochim Acta. 2000;365:101–10.
Budrugeac P, Miu L. The suitability of DSC method for damage assessment and certification of historical leathers and parchments. J Cult Herit. 2008;9:146–53.
Ershad-Langroudi A, Mirmontahai A. Thermal analysis on historical leather bookbinding treated with PEG and hydroxyapatite nanoparticles. J Therm Anal Calorim. 2015;120:1119–27.
Sebestyén Z, Czégény Z, Badea E, Carsote C, Sendrea C, Barta-Rajnai E, Bozi J, Miu L, Jaka E. Thermal characterization of new, artificially aged and historical leather and parchment. J Anal Appl Pyrolysis. 2015;115:419–27.
Cai SW, Zeng YH, Zhang WH, Wang YN, Shi B. Inverse chrome tanning technology based on wet white tanned by Al–Zr complex tanning agent. J Am Leather Chem Assoc. 2015;110:114–21.
Santos LMD, Allen SCH, Antunes APM. Optimization of enzyme-assisted phenolic reactions applied to thermal stabilization of collagen using response surface methodology. J Am Leather Chem Assoc. 2016;111:53–61.
Srivatsan KV, Lakra R, Sai KP, Kiran MS. Effect of bimetallic iron: zinc nanoparticles on collagen stabilization. J Mater Chem B. 2016;4:1437–47.
IUP 16. Leather—physical and mechanical tests—determination of shrinkage temperature up to 100 °C; 2002.
Budrugeac P, Cucos A, Miu L. The use of thermal analysis methods for authentication and conservation state determination of historical and/or cultural objects manufactured from leather. J Therm Anal Calorim. 2011;104:439–50.
Larsen R, Vest M, Nielsen K. Determination of hydrothermal stability (shrinkage temperature) of historical leather by the micro hot table technique. J Soc Leather Technol Chem. 1993;77:151–5.
Okamoto Y, Saeki K. Phase transition of collagen and gelatin. Kolloid-Zeitshrift und Zeitshrift fűr Polymere. 1964;194:124–34.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Onem, E., Yorgancioglu, A., Karavana, H.A. et al. Comparison of different tanning agents on the stabilization of collagen via differential scanning calorimetry. J Therm Anal Calorim 129, 615–622 (2017). https://doi.org/10.1007/s10973-017-6175-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6175-x