Abstract
Vegetable oils can undergo changes in their physical and chemical properties when they are subjected to processes such as extraction, purification, cooking, frying, or chemical modifications that are required depending on their application in the food industry. In the present research, different methods were used to determine physical, chemical, and thermal properties of avocado, sesame, extra virgin flaxseed, extra virgin olive, and grape seed oils, in order to evaluate and compare the compositional effects of each vegetable oil. By using an adiabatic scanning calorimeter, an alternative calorimetric method, the specific heat of five vegetable oils was obtained as a function of the temperature, in the range of 25–90 °C. The fatty acid profile was determined via gas chromatography. Monounsaturated acids present in avocado and extra virgin olive oil cause higher specific heat values in these oils, and the presence of polyunsaturated acids, contained mainly in extra virgin flaxseed oil, reduces the specific heat values. We found an almost linear behavior of vegetable oil specific heats, as a function of the temperature, and the slopes of these linear fits depend on the contained percentage of polyunsaturated fatty acids in the vegetable oil. The unsaturated acids are an important factor to analyze the behavior of specific heat of vegetable oils. The obtained specific heat values were compared with the estimation from the Rowlinson–Bondi equation, for specific heat of vegetable oils, giving a very good adjustment, mainly in a temperature range near to the room temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lipids, proteins, and carbohydrates constitute the principal structural components of foods. Lipids are heterogeneous organic molecules, formed by carbon, hydrogen, oxygen, nitrogen, phosphorus, and sulfur to a lesser extent; due to the formation of its hydrocarbon chain, they are insoluble in water and soluble in organic solvents. The main sources of lipids are animal fats and oils in seeds. The difference between fats and oils lies in the proportion of the present fatty acids [1].
Composition of vegetable oils includes complex mixtures of triacylglycerols (TAGs) which are esters derived from glycerol and three fatty acids. TAGs represent from 95 to 98 % of composition of these oils, which also are composed by minor amounts of diacylglycerols, free fatty acids, phospholipids, carotenoids, chlorophylls, sterols, tocopherols/tocotrienols, phytosterol esters/phytosterols, proteins, resinous and mucilaginous materials, and oxidative products (usually <5 %) [2–4]. All fatty acids are synthesized from palmitic acid through of elongation and desaturation processes. Seeds from some plants contain enzymes capable of stopping the elongation process, resulting in fatty acids of medium or short chain incorporated in idioblasts stored as oil [5].
The fatty acids in nature are classified as saturated, monounsaturated, and polyunsaturated according of the number of double bonds in their fatty acid chains. In turn, the polyunsaturated fatty acids can be classified in omega-3 and omega-6 fatty acids (Table 1), according to the position of the first double bond site or the furthest terminal carbon from the functional group of carboxylic acid (omega-carbon). The most prominent omega-6 fatty acids, in the human diet, are arachidonic acids from animal origin, and also linoleic acid present in vegetables, seeds, and nuts. The main sources of omega-3 fatty acids are fish that contains eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA); as well as nuts, seeds, and vegetable oils containing α-linolenic acid (ALA), which is the precursor of omega-3 fatty acids [5–10].
Other omega fatty acids that are not considered essentials are the omega-7 and omega-9 series or palmitoleic acid; which are monounsaturated fatty acids. The omega-7 can be found in animal oils, marine oils, and vegetable oils [11]. One of the main natural sources of omega-9 is the extra virgin olive oil, rich in oleic acid; other sources of omega-9 are walnuts, hazelnuts, peanuts, mustard seed, canola, and avocado oil [8, 12].
Edible oils extracted from plant and animal sources have a very important role in food products worldwide, because they provide flavor and texture, to taste them, as well as provide energy and vitamins, which help to the growth and keep good health [13–15], also these oils are used in different food industries, cosmetics, pharmaceutical, and lubricants [16, 17]. It is indispensable to know their chemical composition, physical properties, and thermal behavior, in order to evaluate the quality for each specific use of the edible vegetable oils.
Fatty acid composition and minor components of vegetable oils can be characterized by chromatographic technique, which gives good quantitative information about each oil component. Different methodologies have been proposed to know some parameters in order to evaluate quality in vegetable oils such as iodine index, peroxide index, and saponification index, among others [18, 19].
It is essential to know the chemical composition and physical properties of vegetable oils, but the knowledge about their thermal behavior is also very important. In food research, several thermal analysis techniques have been developed to study the vegetable oil components [20] and during several years different calorimetric techniques with varying degrees of accuracy and precision have been developed. Thermal mechanical analysis (TMA), thermogravimetric analysis (TG), differential thermal analysis (DT), and differential scanning calorimetry (DSC) are the most common thermal analysis techniques; the last one is the most usual technique used by researchers to determining thermal parameters of vegetable oils [21–24]. Despite the common use of DSC technique, there are disadvantages that can affect the accuracy of the obtained results [25]. An alternative calorimetric technique is an adiabatic scanning calorimeter (ASC), which is based on the idea of surrounding a measuring cell by a shell exactly the same temperature as the cell, eliminating all unwanted heat exchange with the environment and provides the cell with a system to supply or subtract heat. To maintain exactly the same temperature between the cell and the shell is necessary to take into account the accuracy of the temperature sensors and the fast response of the control system. It is also important to reduce conduction and convection heat transport in order to achieve an adiabatic environment. A typical ASC has one or more additional shields surrounding the first shield, all with their own temperature sensors and control systems. Due to its characteristics are achieved scanning rates of the order of a few mK min−1. For heating runs a constant power is continuously applied to (or extracted from) the cell by an electrical heater. ASC has been applied to study different kind of materials with high temperature resolution and high accuracy, such as liquid crystals, liquid mixtures, and starches, among others [26–28].
In the present study, ASC was used as an alternative method to determine the specific heat, which is one of the main thermal properties of the liquids. Also physical and chemical characterizations, by using different methods including gas chromatography, and determination of peroxide index, iodine index, and saponification index were obtained in order to characterize some edible vegetable oils.
Materials and methods
Five commercial edible vegetable oils, see Table 2, were used in this study (avocado, pure sesame, extra virgin flaxseed, extra virgin olive, and grape seed; AO, PSO, EVFO, EVOO, and GSO, respectively). Then the chemical, physical, and thermal characterizations mentioned above were performed on these oil samples.
Chemical and physical characterization
The fatty acid content was determined via gas chromatography (PerkinElmer, model Autosystem, USA) equipped with a flame ionization detector (FID) and a column Zebron FFAP 25 m × 0.25 mm, d.i. 5 µm after derivatization as methyl esters. The column temperature started at 100 °C for 5 min and then increased to 200 °C at the rate of 5 °C min−1 and held for 3 min and finished at 2 °C held for 10 min. Standards for identification of fatty acids were prepared for myristic, oleic, palmitic, palmitoleic, stearic, oleic, and linoleic. The experimental results are reported in percentage (%), the conventional quantification used for fatty acid contents.
The acid index was determined by titration in accordance to NMX-F-101-1987 [29]; the peroxide index was obtained by NMX-F-154-1987 [30], PANREAC [31], and Qian and Pike [32]; the iodine index was obtained by Hannus method (NMX-F-408-S-1981) [33], and the saponification index was determined using the method described in NMX-F-174-S-1981 [34] and Qian and Pike [32]. All determinations were performed in triplicate.
Thermal characterization
Adiabatic scanning calorimeter (ASC)
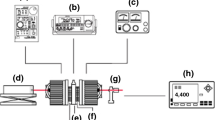
The used adiabatic scanning calorimeter was developed and constructed at KU Leuven, Belgium. The ASC consists of three different stages: (1) the sample cell (holder), (2) inner shield, and (3) the outer shield immersed in a temperature-controlled water bath (Fig. 1). Temperatures of the three stages play an important role to keep adiabatic conditions (temperatures with differences <1 mK). In heating runs, a constant heating power P is continuously supplied electrically to the resistive heater on the sample cell, and the temperature evolution as a function of time T (t) of the sample is measured with a thermistor on the sample holder. The total heating capacity is given by the following equation [35]:
where C s and C h are the heat capacity of the sample and of the sample holder, respectively. dT/dt is the time derivative of the sample temperature T (t).
The specific heat [J (kg K)−1] of each edible vegetable oil was obtained by dividing C s by the total mass of the sample (after subtracting C h). All ASC measurements were done by duplicate, and the mean values are showed.
Results and discussion
Chemical and physical characterization
The fatty acid compositions of vegetable oils are shown in Table 3. The fatty acid with higher content in all samples was oleic acid (C18:1) with 68.94, 49.21, 38.65, 76.73, and 29.87 % for AO, PSO, EVFO, EVOO, and GSO, respectively. Linoleic acid (C18:2) was the second fatty acid in content, which is negatively correlated to the stability, because it is more susceptible to oxidation. The ratio of oleic to linoleic acids has great importance because of the nutritional properties as well as the high oxidative stability, which occurs when the content of oleic acid is higher when compared with linoleic acid content [36]. Therefore, EVOO has greater oxidative stability because it shows a good ratio of 76.73–5.42 %, and the worst oxidative stability comes from GSO with a ratio of 29.87–26.71 % for oleic to linoleic acid, respectively. Flaxseed oil becomes rancid quickly because of its high linolenic acid content (21.71 %), since the polyunsaturated fatty acids (linoleic and linolenic) have lower oxidative stability. The percentage of saturated fatty acids in all oil samples is small when compared with the percentage of unsaturated fatty acids. Palmitic acid was the saturated fatty acid with higher percentage in the samples, ranged from 7.11 (AO) to 11.66 % for GSO. The fatty acid compositions of the AO, PSO, EVFO, EVOO, and GSO analyzed vary slightly from those published in the literature [37–42], which may differ oil to oil, depending on the place of production, latitude, climate, variety, and stage of fruit maturity or seed [43].
Table 4 summarizes the physical–chemical properties of the studied samples. The acid index is a measure of the free fatty acids (FFA) content in oils. All vegetable oils show minimal formation of FFA. The acid values ranged from 0.0659 ± 0.0032 to 1.1112 ± 0.0155 (oleic acid percentage) for AO to PSO, respectively. The high percentage of acid index in PSO (1.1112 ± 0.0155), it could be due to the process to obtain this vegetable oil, through solvent extracting or mechanically pressing, as well as the contain of impurities, moisture, fungal invasion, and other factors that contribute to hydrolysis of TAGs and cause increases in FFA [43]. Higher values of FFA for sesame oil were found by Elleuch et al. [44], ranged from 2.37 to 10.73 as oleic acid percentage; however, in this article is not specified the purity of the samples.
Table 5 shows the standard values established for vegetable oils. The iodine index indicates the degree of oil unsaturation. The higher the iodine value, the greater unsaturation and susceptibility to oxidation, so that AO and EVOO (92.22 ± 0.0550 and 92.30 ± 0.8600, respectively) are more saturated than PSO, EVFO, and GSO [4]. Only two oil samples are within established ranges for iodine index (EVOO and GSO). Lower iodine values, ranged from 119.1 to 119.8 and 81.87 for SO and EVOO, respectively, were reported by Yermanos et al. [45], and Henna Lu and Tan [46].
Elevated peroxide values indicate that lipid oxidation has taken place, and it is measured as reactive oxygen content in terms of milliequivalents per 1000 g of fat. All of vegetable oils studied are within the established range (Table 5), despite the high peroxide value of EVOO (8.069 ± 0.4330), remains within the range published by the International Olive Oil Council (for EVOO, ≤20) and it is similar to that reported by Gutfinger [47].
Saponification index provides the information of the average molecular weight of all fatty acids present. The saponification value is expressed as the amount of potassium hydroxide (KOH) in milligrams required to saponify 1 g of fat or oil under the specified conditions. From molecular point of view, one mole of oil requires three moles of KOH to complete saponification, because there are three ester bonds in one oil molecule, i.e., the oils with high molecular weight have lower saponification values than those with low molecular weight. The obtained saponification values are similar between the studied vegetable oils, 162.942 ± 0.4220, 158.652 ± 0.5840, 160.610 ± 1.1670, 164.283 ± 0.4000, and 160.963 ± 0.1090 for AO, PSO, EVFO, EVOO, and GSO, respectively. Low saponification values were observed in all samples when compared with the established ranges for each type of oil (Table 5).
Unsaponifiable matter includes sterols, hydrocarbons, tocopherols, pigments, and other components of higher molecular weight which are insoluble in water and also cannot be saponified by alkalis. The level of unsaponifiable matter of all studied vegetable oils was higher than the established standards for each kind of vegetable oil (Table 5).
Specific gravity was estimated from saponification and iodine indexes of the different vegetable oils. The method to estimate the specific gravity for vegetable oils was developed by Lund [43]. The relation is:
where sg is the specific gravity of the vegetable oil, compared to water at 15 °C, SV and IV are the oil saponification and iodine indexes, respectively. The estimated sg values for the studied oils are shown in Table 4.
Thermal characterization
From the ASC data, specific heat was obtained for the studied vegetable oils. Figure 2 shows the temperature dependence of the specific heat as calculated from Eq. (1) and divided by the total mass of each vegetable oil in the temperature range from 25 to 90 °C. Table 6 shows the specific heat values at different temperatures.
Figure 2 shows that as oleic acid content (monounsaturated fatty acid) is higher, the specific heat values in the oil samples are higher; i.e., the specific heat increases to a higher degree of unsaturation [55]. AO and EVOO showed a high specific heat related with the degree of unsaturated acid (88.14 and 85.76 %, respectively). Samyn et al. [56] studied the quality of some vegetable oils by modulated DSC, they reported lower values of specific heat than traditional calorimetric measurements (at 50 °C), 1.49, 1.58, 1.63, 1.85, and 3.2 J (g °C)−1 for castor oil, palm oil, canola oil, corn oil, sunflower oil, and soy oil, respectively. Also they concluded that specific heat depends on the unsaturation degree.
A particular case was observed, EVFO that has higher unsaturated fatty acid content (78.84 %) than PSO and GSO (76.05, 69.67 %, respectively), it is expected that its specific heat was higher than PSO and GSO; however, specific heat of EVFO is slightly lower compared with these oils. The low specific heat could be related to the fact that the percentage of polyunsaturated acids (C18: 2, C18: 3; 11.62 %, 21.71 %, respectively) is higher than the percentage of polyunsaturated acids contained in PSO and GSO, since the increase in double bonds in the fatty acid chains causes a decrease in the specific heat of the oil [43]. Another way to observe the unsaturation or presence of double bonds is through the iodine index; a high value indicates a large number of double bonds in the structure of the fatty acids in the vegetable oil, and therefore EVFO and GSO with the higher iodine values, 103.95 ± 3.01 and 104.16 ± 6.09 (gI2/100 g), respectively, show low specific heat values due to percentage of polyunsaturated fatty acids [43].
A similar thermal study of vegetable oils were reported by Pardauil et al. [57], they found that the melting enthalpy is related with the saturation and unsaturation degree of oils studied, and Li et al. [58] obtained by DSC different endothermic and exothermic peaks for camellia oil linked with the content of saturated and unsaturated TAG and fatty acids, which show that thermal behavior is related with fatty acids composition of vegetable oils.
A linear fit to the curves of specific heat for each vegetable oil studied was performed. Figure 3 shows the linear fit to the specific heat curve of AO. The slopes of each linear fit are shown in Table 6. The oils with a higher slope were GSO and EVFO, which could be related to the polyunsaturated fatty acids contained in them. A higher slope indicates that it requires a further increase in energy to raise the temperature of a vegetable oil, in this case in a temperature ranging from 25 to 90 °C. Despite the high percentage of linoleic acid (C18: 2, 21.1 %) for PSO, its slope had the lower value, which could be related to the high content of unsaponifiable matter (5.930 ± 0.0450 %), also is one of the oils with high amount of lignans, such as sesamol, sesamin and sesamolin, which act as natural antioxidants [59, 60].
The specific heat of oils has often been observed to increase linearly, as a function of the temperature, but nonlinear behavior at higher temperatures (T > 70 °C) has been observed by some researchers [13]. Then the oil specific heat (c p) has been estimated by using the mixture properties corresponding to the fatty acid compositions to estimate c p for pure fatty acids by using the Rowlinson–Bondi (RB) equation [61]:
where c 0p is the ideal gas specific heat capacity, T r is the reduced temperature, which was calculated as T r = T/T c (T c, critical temperature), and ω is the acentric factor, a parameter widely used as a measure of the complexity of a molecule with respect to both geometry and polarity. c 0p was calculated using the method of Rihany and Doraiswamy [62]:
The constants a, b, c, and d for several chemical groups were used to calculate the ideal gas capacity for pure fatty acids (FA) [63]. The T c and ω constants values of the FA of oils are listed in Table 7. To estimate c p must be considered the mixture of different fatty acids contained in each vegetable oil. Therefore, any parameter in the equation for oil can be replaced by the sum of the products of fatty acid molar percentages and the corresponding parameter for fatty acid [20].
The estimated values of c p for the oil samples at temperatures between 25 and 90 °C are shown in Table 6. The error, between experimental and estimate values, in the range of 25–30 °C was ±0.3 % for AO, PSO, EVFO, and EVOO. In the case of GSO, this oil has an error of 1 % at 30 °C. The difference between the estimate and experimental values increases for higher temperatures (>30 °C), so the equation Rowlinson–Bondi is adjusted to specific heat of temperatures between 25 and 30 °C approximately. The differences between Rowlinson–Bondi equation of c p and those obtained experimentally by ASC could be due to the differences of heating scan rate; meanwhile, in the ASC the scan rate is around 100 mK min−1, and in the case of DSC, the most common calorimetric technique to obtain c p is of the order of 10 K min−1. Values of specific heat of PSO, EVOO, and GSO are higher than published by Fascina and Colley [15] and lower values for EVOO compared with the reported values of Santos et al. [55].
Conclusions
The physical–chemical properties of AO, PSO, EVFO, EVOO, and GSO vary slightly from those published in the literature, and they are agreed with the values expected for oils with their fatty acid composition. Vegetable oil properties depend on many factors as geographic region, climate, process of oil production, and the characteristics of soil.
It was shown that the ASC technique is an effective tool to study, with high temperature resolution and high accuracy, the specific heat of vegetable oils.
Specific heat increases with the degree of monounsaturated acid (higher chain length; oleic fatty acid) but decreases with the degree of polyunsaturated acid (higher double bonds; Linoleic and Linolenic fatty acids) contained in vegetable oils, and there is a progressive increase in specific heat with increasing temperature.
The slope of specific heat curve fit could be related to the polyunsaturated fatty acids contained in them. The greater the slope the higher content of unsaturated acids present in the oil. The estimation of c p by the Rowlinson–Bondi equation for the studied oils agrees very well with the experimental data obtained by ASC mainly in temperature range of 25–30 °C.
References
Keefe SFO, Pike OA. Fat characterization. In: Nielsen SS, editor. Food analysis. chapter 14, 4th ed. USA: Springer; 2010. p. 239–60.
IUPAC-IUB, Nomenclature C on B. The nomenclature of lipids, chemistry and physics of lipids. Arch Biochem Biophys. 1968;123:409–15.
Potter NN, Hotchkiss JH. Food Science. 5th ed. Berlin: Springer; 1995.
Caballero B, Trugo L, Finglas P. Encyclopedia of food sciences and nutrition. 2nd ed. Amsterdam: Elsevier Science B.V.; 2003.
Bazinet RP, Layé S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. 2014;15:771–85.
Castro González MI. Ácidos grasos omega-3: beneficios y Fuentes. Interciencia. 2002;27:128–36.
DeFilippis AP, Sperling LS. Understanding omega-3’s. Am Heart J. 2006;151:564–70.
FAO. Fats and fatty acids in human nutrition Joint FAO/WHO. Ann Nutr Metab. 2009;55:1–308.
Gogus U, Smith C. n-3 Omega fatty acids: a review of current knowledge. Int J Food Sci Technol. 2010;45:417–36.
Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56:365–79.
Sánchez P, Segundo MJ. Omega 7: su papel en el cuidado de la piel y las mucosas. Offarm: Farmacia y. Sociedad. 2007;26:58–67.
Hernández AG. Tratado de nutrición: Bases fisiológicas y bioquímicas de la nutrición. 2nd ed. Médica Panamericana; 2010.
Coupland JN, McClements DJ. Physical properties of liquid edible oils. J Am Oil Chem Soc. 1997;74:1559–64.
Tan CP, Che Man Y. Differential scanning calorimetric analysis of edible oils: comparison of thermal properties and chemical composition. J Am Oil Chem Soc. 2000;77:143–55.
Fasina OO, Colley Z. Viscosity and specific heat of vegetable oils as a function of temperature: 35 °C to 180 °C. Int J Food Prop. 2008;11:738–46.
Formo MW. Physical properties of fats and fatty acids. Bailey’s Ind Oil Fat Prod. 1979;1:177–232.
Bockisch M. Vegetable fats and oils. Fats and oils handbook. Hamburg: AOCS Press, Urbana II; 1998. p. 174–344.
AOCS. Official methods and recommended practices of the AOCS, 3rd printing. 6th ed. Sallee EM, Hopper TH, Link WE, Walker RO, Firestone D, Mehlenbacher VC, editors. AOCS Press; 2010.
Dieffenbacher A, Pocklington WD. Standard methods for the analysis of oils, fats and derivatives: 1st supplement to the 7th edition. 2nd ed. Oxford: Blackwell Scientific; 1992.
Morad NA, Kamal AAM, Panau F, Yew TW. Liquid specific heat capacity estimation for fatty acids, triacylglycerols, and vegetable oils based on their fatty acid composition. J Am Oil Chem Soc. 2000;77:1001–5.
Bicanic D, Chirtoc M, Dadârlat D, Van Bovenkamp P, Van Schayk H. Direct determination of thermophysical parameter √Kpc in mayonnaise, shortening, and edible oil. Appl Spectrosc. 1992;46:602–5.
Lazzari M, Chiantore O. Drying and oxidative degradation of linseed oil. Polym Degrad Stab. 1999;65:303–13.
Santos JCO, Santos IMG, Conceição MM, Porto SL, Trindade MFS, Souza AG, et al. Thermoanalytical, kinetic and rheological parameters of commercial edible vegetable oils. J Therm Anal Calorim. 2004;75:419–28.
Dweck J, Sampaio CMS. Analysis of the thermal decomposition of commercial vegetable oils in air by simultaneous TG/DTA. J Therm Anal Calorim. 2004;75:385–91.
Cruz-Orea A, Pitsi G, Jamée P, Thoen J. Phase transitions in the starch-water system studied by adiabatic scanning calorimetry. J Agric Food Chem. 2002;50:1335–44.
Tripathi CSP, Losada-Pérez P, Glorieux C, Kohlmeier A, Tamba MG, Mehl GH, et al. Nematic-nematic phase transition in the liquid crystal dimer CBC9CB and its mixtures with 5CB: a high-resolution adiabatic scanning calorimetric study. Phys Rev E. 2011;84:041707.
Losada-Pérez P, Tripathi CSP, Leys J, Glorieux C, Thoen J. Large heat capacity anomaly near the consolute point of the binary mixture nitromethane and 3-pentanol. J Chem Phys. 2011;134:044505.
Contreras-Gallegos E, Dominguez-Pacheco FA, Hernández-Aguilar C, Carballo-Carballo A, Cruz-Orea A, Thoen J, et al. Study of thermal and structural properties of starch granules from different maize genotypes. Food Biophys. 2015;10:19–24.
NMX-F-101-1987. Alimentos. Aceites y grasas vegetales o animales. Determinación del índice de acidez. Foods. Vegetables or animals oils and fats. Acidity index determination. Normas mexicanas. Dirección General de Normas.
NMX-F-154-1987. Alimentos. Aceites y grasas vegetales o animales. Determinación del índice de peróxido. Foods. Vegetables or animals oils and fats. Peroxide index determination. Normas mexicanas. Dirección General de Normas.
PANREAC. Analíticos en alimentaria Métodos oficiales de análisis: Aceites y grasas. Panreac Química, S.A.; 1999.
Qian MC, Pike OA. Fat characterization. In: Nielsen SS, editor. Food analysis laboratory manual. Berlin: Springer; 2010. p. 103–13.
NMX-F-408-S-1981. Alimentos para humanos. Aceites y grasas vegetales o animales. Determinación del índice de yodo por el método de Hanus.
NMX-F-174-S-1981. Alimentos para humanos. Determinación del índice de saponificación en aceites y grasas vegetales o animales. Foods for humans. Determination of the saponification index in oils and vegetal or animal fats. Normas Mexicanas. Dirección General.
Thoen J. Thermal investigations of phase transitions in thermotropic liquid crystals. Int J Modern Phys B. 1995;9:2157–218.
Aparicio R, Roda L, Albi MA, Gutiérrez F. Effect of various compounds on virgin olive oil stability measured by Rancimat. J Agric Food Chem. 1999;47:4150–5.
Ozdemir F, Topuz A. Changes in dry matter, oil content and fatty acids composition of avocado during harvesting time and post-harvesting ripening period. Food Chem. 2004;86:79–83.
NMX-F-002-1985. Alimentos. Aceite comestible puro de ajonjolí. Foods edible pure sesame oil. Normas mexicanas. Dirección General de Normas.
Codex Alimentarius Commission. Codex Standard for Olive Oils, and Olive Pomace Oils, Codex Stan 33-1981. Codex Alimentarius. Roma, Itália: Secretary of the Codex Alimentarius Commission Joint FAO/WHO Food Standards Programme; 2003.
Beare-Rogers J, Dieffenbacher A, Holm JV. Lexicon of lipid nutrition (IUPAC Technical Report). Pure Appl Chem. 2001;73:685–744.
Zarrouk W, Baccouri B, Taamalli W, Trigui A, Daoud D, Zarrouk M. Oil fatty acid composition of eighteen Mediterranean olive varieties cultivated under the arid conditions of Boughrara (southern Tunisia). Grasas Aceites. 2009;60:500–8.
Crews C, Hough P, Brereton P, Godward J, Lees M, Guiet S, et al. Quantitation of the main constituents of some authentic sesame seed oils of different origin. J Agric Food Chem. 2006;54:6266–70.
Gunstone FD. Vegetable oils in food technology. Food technology. Oxford: Blackwell; 2011.
Elleuch M, Besbes S, Roiseux O, Blecker C, Attia H. Quality characteristics of sesame seeds and by-products. Food Chem. 2007;103:641–50.
Yermanos DM, Hemstreet S, Saleeb W, Huszar CK. Oil content and composition of the seed in the world collection of sesame introductions. J Am Oil Chem Soc. 1972;49:20–3.
Henna Lu FS, Tan PP. A comparative study of storage stability in virgin coconut oil and extra virgin olive oil upon thermal treatment. Int Food Res J. 2009;16:343–54.
Gutfinger T. Polyphenols in olive oils. J Am Oil Chem Soc. 1981;58:966–8.
NMX-F-052-SCFI-2008. Aceites y grasas-aceite de aguacate-especificaciones. Fats and oils-avocado oil-specifications.
Firestone D. Physical and chemical characteristics of oils, fats, and waxes. 3rd ed. Champaign, IL: AOCS press; 2006.
Codex Alimentarius Commission. Codex standard for named vegetable oils. Codex Stan 210-1999. Codex Alimentarius. Roma, Itália: Secretary of the Codex Alimentarius Commission Joint FAO/WHO Food Standards Programme; 1999.
World Food Programme. Technical Specifications for the manufacture of: Fortified Refined Flaxseed Oil. 2011.
Shahidi F, editor. Edible oil and fat products: processing technologies. Bailey’s industrial oil and fat products. Wiley-Interscience; 2005. p. 586.
Nykter M, Kymäläinen HR, Gates F, Sjöberg AM. Quality characteristics of edible linseed oil. Agric Food Sci. 2006;15:402–13.
Pardo JE, Fernández E, Rubio M, Alvarruiz A, Alonso GL. Characterization of grape seed oil from different grape varieties (Vitis vinifera). Eur J Lipid Sci Technol. 2009;111:188–93.
Santos JCO, Santos MGO, Dantas JP, Conceição MM, Athaide-Filho PF, Souza AG. Comparative study of specific heat capacities of some vegetable oils obtained by DSC and microwave oven. J Therm Anal Calorim. 2005;79:283–7.
Samyn P, Schoukens G, Vonck L, Stanssens D, Van den Abbeele H. Quality of Brazilian vegetable oils evaluated by (modulated) differential scanning calorimetry. J Therm Anal Calorim. 2012;110:1353–65.
Pardauil JJR, de Molfetta FA, Braga M, de Souza LKC, Filho GNR, Zamian JR, et al. Characterization, thermal properties and phase transitions of amazonian vegetable oils. J Therm Anal Calorim. 2016.
Li R, Huang J, Huang L, Teng J, Xia N, Wei B, et al. Comparison of GC and DSC monitoring the adulteration of camellia oil with selected vegetable oils. J Therm Anal Calorim. 2016.
Hwang LS. Sesame oil, Chapter 12. Bailey’s industrial oil and fat products. Vol 6. 6th ed. 2005.
Yin W, Huixiao L, Guiming F, Xueyang C, Feng C, Xiea M. The relationship of antioxidant components and antioxidant activity of sesame seed oil. Sci Food Agric. 2015;95:2571–8.
Poling BE, Prausnitz JM, O’Connell JP. Properties of gases and liquids. 5th ed. New York: McGraw-Hill; 2000.
Rihani DN, Doraiswamy LK. Estimation of heat capacity of organic compounds from group contributions. Ind Eng Chem Fundam. 1965;4:17–21.
McKetta JJ. Phosphorus to pipeline failure: subsidence strains chemical processing and design. Encyclopedia of chemical processing and design, vol. 36. London: CRC Press; 1990.
Acknowledgements
The authors acknowledge the partial economic support from CONACYT through Project No. 241330, and also the support of Instituto Politécnico Nacional through COFAA, EDI, and BEIFI scholarships. We also thank Ing. Esther Ayala Maycotte, IBQ. Ma. Dolores Díaz Cervantes and Ing. Miguel Márquez Robles for their technical support at the Physics and Biotechnology Department, CINVESTAV-IPN.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Contreras-Gallegos, E., Domínguez-Pacheco, F.A., Hernández-Aguilar, C. et al. Specific heat of vegetable oils as a function of temperature obtained by adiabatic scanning calorimetry. J Therm Anal Calorim 128, 523–531 (2017). https://doi.org/10.1007/s10973-016-5864-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5864-1