Abstract
Nucleated polylactide (PLA) blend films with various types and contents of nucleating agent were prepared in a twin-screw extruder. The influences of type and level of nucleating agent on the tensile, thermal, and morphological properties of the blend films were investigated. Furthermore, effects of different cooling rates (1–10 °C min−1) on non-isothermal processes and various crystallization temperatures (T c) (100–125 °C) on isothermal conditions were used to evaluate the crystallization behaviors and kinetics of these films by differential scanning calorimeter (DSC) and polarized light microscope. Nanoprecipitated calcium carbonate (NPCC) and talc were used as a nucleating agent at different concentrations from 0 to 2 phr. The results showed that the tensile properties, thermal stability, spherulitic morphology, and crystallization behaviors of the nucleated PLA blends significantly depended upon the addition of nucleating agent. Tensile properties of the blends were improved with increasing of nucleating agent contents; in contrast, its thermal stability decreased. These behaviors were similarly observed in both nucleated PLA blends with NPCC and talc. Furthermore, DSC curves revealed that NPCC and talc could be a proficient nucleating agent for PLA, resulting in the increments of T c, crystallization rate, degree of crystallinity (χ c), and spherulitic density of nucleated PLA films.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polylactide (PLA), a linear aliphatic polyester, is derived from renewable resources such as corn and sugar cane [1]. PLA becomes one of the most popular biodegradable plastics in the present because it offers many benefits in terms of biodegradation, high mechanical properties, easy processability, transparency, and gas permeability [2–6]. These are important reasons why PLA can be an excellent and promising bio-based material over the other biodegradable polymers for replacing commodity plastics, i.e., polyolefins, polystyrene (PS), and poly(ethylene terephthalate) (PET). These advantages make PLA being applicable in various applications not only in medical and pharmaceutical fields, tissue engineering, and drug delivery system but also in agricultural and food packaging products [7–9]. Unfortunately, low crystallinity and slow crystallization rate of PLA still restrict its usages in some applications [10]. Thus, crystallization improvement of PLA should be focused and carried out for extending PLA applications.
Practically, there are several methods to enhance the PLA crystallinity, e.g., (1) mixing PLA with a nucleating agent or a plasticizer for initiating a great number of nuclei or increasing chain mobility in melted PLA (2) adjusting the molding conditions during processing in terms of cooling rate, time, and temperature [11]. Nevertheless, the limitation of blending PLA with plasticizer is a blooming effect which is occurred from the migration of plasticizer molecules to polymer surface. This phenomenon leads to blooming or separation between plasticizer and polymer matrix after shelf life. Severe blooming might cause a lot of droplets on material surface and progressively affect to overall properties of the products [12]. So, this route is quite complicate to manage and hard to control the product quality in long term. Using a nucleating agent still becomes one of the most potential methods to enhance the polymer crystallinity and has been investigated with various plastics such as high-density polyethylene (HDPE) [13], poly(ethylene terephthalate) (PET) [14], polypropylene (PP) [15, 16], including PLA [17–19].
Calcium carbonate (CaCO3) and talc, the most common inorganic filler, are often used for increasing the overall crystallinity of polymer because they are inexpensive and widely used in industries [15, 20]. Several studies have been reported that using of CaCO3 and talc into the composites not only increases the crystallization rate and χ c but also improves successfully the mechanical properties, e.g., impact strength, impact modulus, strain at break, and toughness of the composites [15, 18, 21–23]. These might be significantly associated with the crystal size, spherulitic density, and crystallinity resulting from the presence of nucleating agent and/or the adjustment of molding conditions. In order to clearly understand the changes of these properties, the investigation of crystallization behaviors and kinetics of the composites under isothermal and non-isothermal conditions is crucial.
As a result, in this research, the influences of type and amount of nucleating agent on the tensile, thermal, and morphological properties of the PLA blend films were focused. The effects of different cooling rates on the non-isothermal crystallization processes and various T c’s on the isothermal crystallization conditions were used to estimate the crystallization behaviors and kinetics of PLA and its blends.
Experimental
Materials
PLA resin (PLA 4042D) purchased from NatureWork LLC (Cargill-Dow, Minneapolis, MN) was used as a polymer matrix. The PLA pellets were transparent with a density reported by the manufacturer of 1.24 g cm−3. Weight average molecular weight and polydispersity (PDI) of PLA characterized by gel permeation chromatography (GPC) in tetrahydrofuran (THF) were 130 kDa and 1.46, respectively. The glass transition temperature (T g) and melting temperature (T m) determined by differential scanning calorimetry (DSC) were about 60 and 153 °C, respectively. Talc and NPCC (nanoprecipitated calcium carbonate) were used as nucleating agent. Talc with a volume mean diameter of 19.03 μm was obtained from Siam Cement Group (SCG Chemicals) Co., Ltd., Rayong, Thailand. NPCC with a volume mean diameter of 3.36 μm was supplied from Behn Meyer Chemical Co., Ltd., Bangkok, Thailand.
Material preparation
The PLA resin, talc, and NPCC were dried in vented oven at 60 °C overnight before processing. Talc and NPCC were used as a nucleating agent for accelerating crystallization rate. The amount of nucleating agent was varied from 0.25 to 2.0 phr. The mixtures of PLA and nucleating agent were pre-mixed in zip-lock plastic bag before extrusion process. The nucleated PLA blends were hot-melted in a corotating twin-screw extruder (PRISM TSE 16TC, Thermo Electron, Staffordshire, UK) having a screw diameter of 15.6 mm and L/D ratio of 40. The extrusion temperature was controlled on five zones along the extrusion barrel and a strand die to achieve a temperature profile ranging from 120 to 190 °C with a screw speed of 60 rpm. The extruded strand was cooled in water and pelletized in the line. The pellets were dried in vented oven overnight and then compression-molded using a hydraulic press (Scientific, Labtech Engineering, Samutprakarn, Thailand) under optimum conditions; holding temperature, pressure, and time of 180 °C, 1500 psi, and 20 min, respectively. The neat PLA was also taken in the same way in order to be used as a reference material. The sample formulations are listed in Table 1.
Testing methods
Particle size distribution
Particle size distribution of talc and NPCC was investigated by Mastersizer (S long bed Ver. 2.19, Malvern Instruments Ltd., Worcestershire, UK). The relative volume distribution of both wet and dry samples can be characterized, based on a diffraction of a laser beam under a particle size ranging from 0.01 to 3500 µm. Sample solution prepared by dissolving 1 mg of sample in ethanol (100 mL) was homogenized and deagglomerated in ultrasonic instrument for 10 min before analyzing the particle size distribution.
Gel permeation chromatography (GPC)
The gel permeation chromatography systems (GPC; 10A series, Shimadzu, Japan) were composed of the following units: LC-10ATVP Shimadzu solvent delivery system, a SIL-10ADVP Shimadzu auto-injector, a column set which consisted of a PL 5.0 µm bead size guard column and a set of 5.0 µm PL linear columns (103, 104, 105 Å) kept at a constant temperature of 40 °C inside a CTO-10AC VP Shimadzu Column Oven, and a RID-10A Shimadzu Refractive Index Detector. The GPC was used to determine molecular weight and polydispersity index (PDI) of neat PLA. This process was done by first analyzing a series of known molecular weight standards. The retention time for these standards was then used to create a calibration curve. Polystyrene (PS) was used for calibration, whereas tetrahydrofuran (THF) was utilized as the continuous phase. Test specimen was prepared by dissolving 50 mg of sample powder in THF. The temperature and flow rate were used as 25 °C and 1 mL min−1, respectively.
Tensile properties
Tensile test of rectangular film specimens with the size of 15 mm wide, 150 mm long, and about 250 μm thickness was conducted on a universal testing machine (LR 100 k, LLOYD, Fareham, UK) using a crosshead speed of 10 mm min−1 and a gauge length of 100 mm, according to the ASTM D882-02. A 1kN load cell was employed for neat and nucleated PLA blend films testing. At least five specimens of each film were tested and the results were averaged to obtain a mean value.
Thermal analysis
The thermal behaviors of neat PLA and its blends were evaluated using a thermogravimetric analyzer (TG; TGA/SDTA851e, Mettler-Toledo, Greifensee, Switzerland) and a differential scanning calorimeter (DSC; DSC7, PerkinElmer, Waltham, MA). TG technique was used to determine thermal stability of the PLA films before and after the addition of various amount of nucleating agent. Samples (about 4 mg) were cut from the neat and nucleated PLA blend films and placed in a crucible (50 μL) to use in each TG experiment. The operation was occurred under nitrogen atmosphere using heating rate of 20 °C min−1 from 50 to 600 °C. For each DSC analysis, approximately 6–8 mg of samples was encapsulated in a hermetically sealed aluminum pan (30 μL). A first heating scan was operated from room temperature to 200 °C at a heating rate of 10 °C min−1 and held at 200 °C for 3 min to eliminate any thermal history of all samples. Then, it was cooled down to 50 °C with various cooling rates from 1 to 10 °C min−1. A second heating scan was finally heated to 200 °C at a heating rate of 10 °C min−1 to determine the non-isothermal crystallization behaviors of the films. All experiments were carried out under nitrogen atmosphere. The glass transition temperature (T g), crystallization temperature (T c), cold crystallization temperature (T cc), melting temperature (T m), specific cold crystallization enthalpy (ΔH cc), and specific melting enthalpy (ΔH m) of the samples were recorded. The degree of crystallinity (χ c) of neat and nucleated PLA blend films was calculated by using Eq. (1):
where ΔH 0m is the melting enthalpy of the 100 % crystalline PLA which is equal to 93.6 J g−1 [24].
Isothermal crystallization experiment
The effects of nucleating agent types on the isothermal crystallization behaviors and kinetics of the neat and nucleated PLA blend films were studied by DSC technique. For each experiment, about 6–8 mg of sample was cut from the films and sealed in an aluminum pan (30 μL). It was then heated to 200 °C and held for 3 min to remove the thermal history of all samples. To evaluate isothermal crystallization behaviors and kinetics of the neat and nucleated PLA films, the melted sample was quenched to a determined isothermal crystallization temperature (T c) and remained there until completely crystallization. The indicated T c’s were varied from 100 to 125 °C. This T c range for isothermal crystallization was considered from our previous work [25]. The isothermal crystallization behaviors and kinetics of neat PLA and blend films with different types of nucleating agent were estimated from these parameters: relative crystallization (X t), crystallization time (t), crystallization half-time (t 1/2), crystallization rate constant (K), Avrami exponent (n), and activation energy (ΔE a).
Morphological studies
Scanning electron microscope (SEM; JSM 6480, JEOL, Tokyo, Japan) was employed to characterize the fracture surface of the nucleated PLA blend films. For SEM analysis, the fractured surface of the PLA blend films was coated with a thin layer of gold before being scanned. The SEM was operated at 15 kV to image the films. In addition, Energy Dispersive Spectroscopy (EDS; 7573 INCAx-sight EDS, Oxford Instruments, Concord, MA) on SEM was used to identify the particle elements and disperse state of the nucleating agent.
Polarized light microscope
The nucleation and isothermal crystallization behaviors of neat and nucleated PLA blend films were investigated by a polarized light microscope (POM; DFC295, Leica Microsystems Ltd., Wetzlar, Germany) equipped with a hot stage (FP82HT, Mettler-Toledo, Greifensee, Switzerland). The polarized light micrographs can be used to follow the transformation of nucleation density, spherulitic growth, crystal size, and crystallization behaviors of the films influenced by various types and levels of nucleating agent. First, a test specimen was placed between cover and glass slides. The sample was then moved to the hot stage which was set at 200 °C for melting the films and kept at that temperature for 3 min to destroy thermal history of the films. After that, it was quenched to a determined T c (100–125 °C) and maintained at that T c in order to investigate the isothermal crystallization behaviors as a function of crystallization time.
Results and discussion
Non-isothermal crystallization behaviors
The non-isothermal crystallization behaviors of neat and nucleated PLA blend films at various cooling rates (ϕ) from 1 to 10 °C min−1 are listed in Table 2. The DSC data showed that crystallization temperature (T c) of neat PLA obviously depended upon the influence of cooling rate. The T c of neat PLA was not appeared at high cooling rate (10 °C min−1); in contrast, this was clearly observed with the decreasing of cooling rate. It was because the cooling rate of 10 °C min−1 was too fast for crystallization process of neat PLA. Hence, the PLA polymeric chains were re-crystallized during second heating step leading to an exothermic peak of cold crystallization. This result supported that the neat PLA had a slow crystallization rate. Furthermore, it can be seen that the T c values of neat PLA increased almost 10 °C when the cooling rate was depressed from 5 to 1 °C min−1. These results revealed that PLA crystallization process was easily carried out under slow cooling rate as evidenced from the increment of χ c of neat PLA from 4.6 to 32.9 % at cooling rates of 10 and 1 °C min−1, respectively. On the other hand, the crystallization half-time (t 1/2), which is defined as time at which the degree of crystallization reached to 50 %, was increased with the decreasing of cooling rate, indicating a slower crystallization rate of PLA under slow cooling rate. The other thermal transitions such as glass transition temperature (T g), cold crystallization temperature (T cc), and melting temperature (T m) were slightly affected from the different cooling rates.
These similar behaviors could be noticed in both nucleated PLA blend films with NPCC and talc. The T c and t 1/2 of the blend films increased with slower cooling rate. Furthermore, non-isothermal crystallization behaviors of nucleated PLA blend films were significantly affected from various types and amounts of nucleating agent. The exothermic peak of the blend films (Fig. 1a, c) was shifted toward higher temperature when the nucleating agent levels were increased comparing to that of neat PLA under the same conditions. In contrast, the t 1/2 values of the nucleated blends were progressively reduced with the addition of NPCC and talc. These results implied that both NPCC and talc can accelerate the crystallization rate and could be a good nucleating agent for PLA. For example, at a cooling rate of 1 °C min−1, T c values of nucleated PLA blend films with 1 phr of NPCC and talc were shifted to 105.8 and 110.1 °C, while the t 1/2 values were reduced to 6.9 and 3.5 min, respectively, comparing to those of neat PLA (T c = 97.1 °C and t 1/2 = 11.7 min).
The addition of nucleating agent (NPCC and talc) into the PLA blend films led to the significant increment of χ c from both of first and second heating curves under the same conditions. From Table 2, these data indicated that the addition of lower levels (0.25 and 0.5 phr) NPCC into the blend films hardly affected to non-isothermal crystallization parameters of PLA comparing to the effect from higher NPCC contents (1 and 2 phr). In contrast to the nucleated PLA blend films with talc, the obvious changes in terms of T c, t 1/2, and χ c were recorded when only a small amount of talc was added into the blend films. These results are clearly observed in Fig. 1. The DSC cooling curves in Fig. 1a, c indicated that the T c of nucleated blend films with lower amount of NPCC was not observed, but was obviously appeared at higher NPCC content. In contrast, the outstanding T c was clearly noticed when a small level of talc was blended with PLA films. However, the PLA blend films with 2 phr of NPCC showed the appearance of the both of T c and T cc. It might be resulting from some agglomeration of the overloading NPCC particles into the nucleated PLA blend films affecting to the reduction of its nucleation efficiency. Hence, the optimal level of NPCC should not be more than 2 phr being in agreement with the results of tensile properties. These results indicated that talc could be more effective nucleating agent for PLA than NPCC at the same level.
From the non-isothermal crystallization studies, it could be summarized that crystallization behaviors of neat PLA and its blends evidently depended upon the type and amount of nucleating agent as well as cooling rate. The slower cooling rate led to the increasing T c and t 1/2, indicating easy crystallization but prolonged crystallization rate. Furthermore, the addition of either NPCC or talc in PLA blend films prompted to easier crystallization (higher T c), faster crystallization rate (lower t 1/2), and greater crystallinity (higher χ c) compared to the neat PLA. The increment of nucleating agent level into the blend films led to an increase in T c and χ c of PLA but decreasing t 1/2 values. Thus, both NPCC and talc can be used as a powerful nucleating agent for PLA which is confirmed by tensile properties, spherulitic morphology, and crystallization kinetic studies. Moreover, 1 phr of NPCC and talc into the nucleated PLA blend films should be an optimum level for PLA crystallization with high tensile properties.
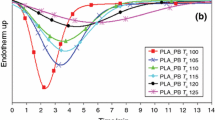
The influence of type and level of nucleating agent on the T cc and double melting behaviors of PLA at a cooling rate of 5 °C min−1 is displayed in Fig. 1. The DSC cooling (Fig. 1a, c) and second heating (Fig. 1b, d) curves led to the understanding of melting and crystallization behaviors of PLA with and without nucleating agent. At cooling rate of 5 °C min−1, DSC curves showed that neat PLA did not crystallize under cooling cycle but can form the T cc peak during heating process at about 116 °C, indicating that crystallization of neat PLA was not completed upon cooling process. Hence, recrystallization of PLA lamellae was taken place in the second heating scan leading to the obvious T cc peak. Double melting temperature of neat PLA was clearly observed at 147 and 154 °C, respectively.
Generally, the influence of T c, crystallization time, molecular weight, heating and cooling rates significantly affects to the double melting behavior of PLA [11]. The lower melting peak (T m1) probably relates to the crystallization of PLA upon heating cycle, while the higher melting peak (T m2) should be correspond to the crystallization of PLA upon cooling process [11, 26]. As shown in Fig. 1a, b, these similar behaviors could be observed in the nucleated PLA blend films with low levels (0.25 and 0.5 phr) of NPCC. Their T c peaks were disappeared and led to increasing of T cc upon heating curves. The double melting characteristics were still similar to those of neat PLA. In contrast, with the presence of high amounts of NPCC, the blend films were partially crystallized in both cooling and heating processes. On the one hand, the T m2 height of the blend films with high NPCC level was increased when T c peak was clearly observed, but on the other hand, the T m1 height was reduced with the depression of T cc peak. It means that the addition of 1 and 2 phr of NPCC into the blend films could enhance the crystallinity and accelerate the rate of crystallization upon cooling of PLA. The percentage of perfect crystals during cooling increased which was in agreement with the increment of T m2 height. It can be implied that the crystallization upon cooling process was directly related to the fusion of PLA perfect crystals in T m2. In other words, the results indicated that the crystallization upon cooling cycle was associated with the higher T m (T m2).
DSC curves of the blend films with talc (Fig. 1c, d) revealed the similar thermal characteristics with those with high NPCC content. These results showed that the PLA crystals were completely formed during cooling at all compositions and led to the obvious T c peak but disappearance of T cc exotherm. The addition of talc into the PLA blend films affected to the decreasing T m1 and increasing T m2 heights. These behaviors confirmed that T m2 was significantly related to the perfect crystal forming in cooling processes.
Tensile properties
Tensile strength, elongation at break, and Young’s modulus of neat and nucleated PLA blend films with various amounts of nucleating agent (0.25–2.0 phr) are displayed in Fig. 2. Neat PLA showed a brittle behavior with very high tensile strength of 52 MPa and Young’s modulus of 2.4 GPa but low elongation at break of only about 4.7 %. The results revealed that the tensile strength and modulus of the nucleated PLA blend films with NPCC and talc slightly decreased compared to neat PLA, as shown in Fig. 2a, c. Nevertheless, the elongation at break of the nucleated blend films gradually increased with the increment of NPCC levels between 0.25 and 1 phr and the increasing of talc contents in range of 0.25 and 2 phr, respectively. This could be explained that the addition of nucleating agent might lead to the increases of χ c as evidenced from first heating run and smaller spherulitic size, resulting in the enhancement of elongation at break of the nucleated PLA blend films. This result was in agreement with the χ c from DSC data (as shown in Table 2). Nevertheless, at higher level of NPCC (over 1 phr), the elongation at break of the nucleated blend films were apparently reduced. It might be occurred from some agglomeration of overloading nucleating agent particles and thus leading to more defects and cracks into the blend films. This is a possible reason why the tensile properties of the blends at high level of nucleating agent were significantly decreased. Furthermore, the presence of NPCC could enhance the elongation at break of the nucleated PLA blend films better than the addition of talc. It might be due to smaller crystal size and well dispersion of NPCC particles, as evidenced by the EDS/SEM results. In addition, the smaller spherulite size of the blend films with NPCC might affect on the decreasing of tensile strength compared to that with talc and neat PLA. These results showed that the nucleated PLA films with talc had higher tensile strength and modulus but lower elongation at break than those with NPCC at the entire composition ranges. The PLA blend films with 1 phr of nucleating agent should be an optimum concentration for nucleation of PLA. To investigate the dispersion and the presence of nucleating agent into the blend films, the EDS/SEM with element mapping was used. Figure 3 displays the EDS/SEM of the fracture surface of the nucleated PLA blend films with NPCC (3a and 3b) and talc (3c and 3d) under element mapping mode. The well dispersion and distribution of NPCC and talc particles on the PLA matrix were observed. It can be claimed that the good mixing was occurred under this study which significantly affected to the properties and crystallization behaviors of the blend films.
Thermal stability
TG technique was used to evaluate the thermal stability and decomposition temperature of neat PLA and its blends. The effect of type and content of nucleating agent on the thermal properties of these films was characterized. TG curves of (a) neat PLA and nucleated PLA blend films with NPCC and (b) neat PLA and nucleated PLA blend films with talc at different levels from 0.25 to 2.0 phr are displayed in Fig. 4. The neat PLA showed only one step of decomposition from about 320 to 360 °C which was attributed to thermal degradation of PLA backbone. Surprisingly, TG curves revealed that thermal stability of the nucleated PLA blend films with NPCC was obviously reduced with the increasing of the nucleating agent content comparing to that of the neat PLA. For example, thermal decomposition temperature (T d) of nucleated PLA blend films with 1 phr NPCC decreased almost 10 °C comparing to neat PLA. It might be because the presence of nucleating agent could obviously enhance a large number of nuclei leading to a greater amount of tiny and imperfect crystals in the PLA matrix. These PLA crystals with very small size and imperfection might play an important factor on the depression of thermal stability of the nucleated PLA blend films. These behaviors could be observed in both nucleated PLA blends with NPCC and talc as shown in Fig. 4a, b, respectively. However, it seems that the thermal stability of the blend films with talc was slightly higher than that with NPCC.
Isothermal crystallization behaviors and kinetics
The DSC analysis was used to determine isothermal crystallization behaviors and kinetics of the neat and nucleated PLA blend films with various crystallization temperatures (T c) from 100 to 125 °C. The DSC results revealed several important parameters based on Avrami and Arrhenius models: relative crystallization (X t), crystallization time (t), crystallization half-time (t 1/2), crystallization rate constant (K), Avrami exponent (n), and activation energy (ΔE a).
Figure 5 represents the DSC curves of isothermal crystallization exotherm of neat PLA and nucleated PLA blend films with NPCC and talc in the indicated T c range of 100–125 °C. The isothermal crystallization exotherms of neat PLA are displayed in Fig. 5a. It can be seen that the exothermic peaks were broader when the neat PLA films were isothermally crystallized at higher T c, suggesting that the isothermal crystallization rate of neat PLA was slower with the increasing of T c. Similar behaviors could be observed in both nucleated PLA blend films with NPCC and talc (Fig. 5b, c). On the other hand, the exothermic curves of PLA blend films were significantly narrowed with the addition of NPCC and talc at all conditions. These results revealed that crystallization behaviors of PLA were accelerated with the presence of nucleating agent into the blend films, leading to a significant reduction of crystallization time under isothermal crystallization of PLA at various T c’s. Furthermore, crystallization rate of nucleated PLA blend films with NPCC seems to be slightly prolonged than that of the nucleated PLA blend films with talc. The isothermal crystallization rate of neat and nucleated PLA blend films at each T c can be obviously evaluated by the value of crystallization half-time (t 1/2). The details of this parameter will be explained shortly.
The plots of relative crystallinity (X t) as a function of time for neat and nucleated PLA blend films at different T c’s are displayed in Fig. 6. The relationship of relative crystallinity (X t) and time was used to evaluate the rate of crystallization. The X t was calculated by Eq. (2) [27].
where t 0 and t ∞ are the beginning and ending crystallization times, respectively. The slope of S-like shaped graphs appeared in Fig. 6 indicated the crystallization rate of the neat and nucleated PLA blends. The S-like shaped graphs with higher slope revealed a faster change of relative crystallization with time. It can be seen that the slope of S-like shaped graphs depended apparently on the increasing of T c and the presence of nucleating agent. For example, at X t value of 0.4, the crystallization time at T c of 100 °C for neat PLA, nucleated PLA with NPCC, and that with talc were 3.8, 1.6, and 1.0 min, respectively. These experimental values showed that the faster crystallization of PLA was associated with the addition of NPCC and talc. These behaviors could be explained in the same way of Fig. 5.
The plots of t 1/2 as a function of T c for neat PLA and nucleated PLA blend films with 1 phr of nucleating agents are revealed in Fig. 7 in order to compare the crystallization rate for each sample. The t 1/2 which is defined as time at which the degree of crystallization is 50 % can be inversely used to determine the rate of crystallization [14]. The t 1/2 data of neat PLA and its blends are listed in Table 3. The t 1/2 values of neat PLA increased significantly with the increment of T c especially at 125 °C, whereas the addition of 1 phr of NPCC and talc in the blend films led to the depression of t 1/2 values. For example, the t 1/2 values at T c of 100 °C of neat PLA, PLA/NPCC, and PLA/talc blend films were 4.3, 1.7, and 1.2 min, whereas those at T c of 125 °C were 6.7, 2.4, and 2.2 min, respectively. These results indicated that the crystallization rate at T c of 100 and 125 °C of nucleated PLA blend films with talc was almost 3.5 and threefold faster than those of neat PLA, respectively. These behaviors might be explained that both NPCC and talc acted as an effective nucleating agent of PLA driven a great number of nuclei and spherulitic site into melt PLA and led to higher nucleation density and crystal formation. As a result, the crystallization rate of PLA was increased with the presence of nucleating agent. Interestingly, although the particle size of NPCC (3.36 μm) was almost sixfold smaller than that of talc (19.03 μm), the addition of talc into the blend films can accelerate crystallization rate more than NPCC does, as evidenced obviously by morphological results of polarized light micrographs. It could be implied that nucleation effect of PLA might significantly depend not only upon particle size but also on geometric characteristics of nucleating agent. From these results, it can be summarized that (1) the crystallization rate of neat PLA and its blends was prolonged with the increasing T c, (2) NPCC and talc played an important role for accelerating isothermal crystallization rate of PLA, and (3) Talc is more effective nucleating agent than NPCC.
The Avrami equation which explains the time evolution of overall crystallinity can be applied to any types of crystallization and not restricted to polymers [28]. As a result, in this work, the isothermal crystallization kinetics of neat and nucleated PLA blend films were estimated by using Avrami model according to the following Eq. (3):
where X t is the relative crystallinity depended on time, t is crystallization time, K is crystallization rate constant, and n is Avrami exponent which related to nucleation and crystal growth geometry [27]. The Avrami Eq. (3) can be rearranged into the following Eq. (4).
The plots of log[−ln(1 − X t)] as a function of log t can be used to analyze the important kinetic parameters, n and K from slope and intersection point with Y axis according to linear expression. Figure 8 illustrates the plots of log[−ln(1 − X t)] as a function of log t of neat PLA and nucleated PLA blend films with NPCC and talc. All crystallization kinetic parameters and t 1/2 of neat PLA and its blends are listed in Table 3.
From Table 3, the average n value of neat PLA was 2.32, while those of nucleated PLA blends with NPCC and talc were 2.92 and 2.63, respectively. These results indicated that the presence of NPCC and talc led to the increment of n value of PLA. The n value of neat PLA was closed to 2 representing that homogeneous nucleation of neat PLA with circular disk shape growth was occurred under isothermal crystallization, whereas those of nucleated PLA blends were about 3 suggesting that heterogeneous nucleation of nucleated PLA blends with spherical growth could be observed. These results implied that the chain folding crystallization mechanism of PLA was affected by the addition of NPCC and contributed to the increasing of n values [29].
The K values are the crystallization rate constant which can be used to evaluate the rate of crystallization of the neat PLA and its blends. The K values were affected from the various T c’s and the presence of NPCC and talc into the blends. The K values of the neat and nucleated PLA blends were clearly reduced with the increment of T c from 100 to 125 °C at all cases, indicating that the rate of crystallization was slower with higher T c for isothermal crystallization. Moreover, these results displayed the increasing K values in the following orders from neat PLA, PLA/NPCC, and PLA/talc blend films, respectively. For instance, at T c of 100 °C, the K value of neat PLA was approximately 3 and 11-folds lower than those of nucleated PLA blend films with NPCC and talc, respectively, which indicated that the addition of nucleating agent can enhance the isothermal crystallization of PLA. Furthermore, NPCC and talc can be used as an effective nucleating agent for PLA. These experimental values were in consistent with the results of t 1/2, crystallization rate, and polarized light micrographs.
The n, K, and T c values from crystallization kinetic studies were used to estimate the activation energy of isothermal crystallization processes according to the Arrhenius equation form [30].
where K 0 is the temperature-dependent pre-exponential factor, ΔE a is the activation energy, R is the gas constant, and T is the absolute temperature. Figure 9 shows the plots of (1/n) ln K as a function of 1/T c which can be used to determine the ΔE a for each sample. For isothermal crystallization at T c range of 100–125 °C, ΔE a values of neat PLA, and nucleated PLA blend films with NPCC and talc were about −595, −178, and −166 kJ mol−1, respectively. Generally, the minus signal of energy value means the exothermic reaction which indicates the crystal forming in crystallization processes. These kinetic data revealed that the crystallization of nucleated PLA blend films with a nucleating agent was easier than that of neat PLA. These results were in consistent with t 1/2 results, suggesting that the NPCC and talc acted as an excellent nucleating agent of the PLA blend films and could significantly accelerate the crystallization process.
Spherulitic density and crystallization
To confirm the nucleation and crystallization behaviors of neat PLA and its blend films, the spherulitic density and crystallization were isothermally followed at 115 °C by polarized light microscope technique. Table 4 illustrates the polarized light micrographs of neat PLA and nucleated PLA blend films with 1 phr of NPCC and talc after isothermal crystallization at 115 °C as a function of time. These results showed that the nucleation growth and spherulitic density of neat PLA and its blends were evidently observed with the increasing of isothermal crystallization time. However, the neat PLA films had the lowest crystallinity and slowest crystallization rate as clearly observed from its polarized light micrographs. After isothermal crystallization time for 5 min, the spherulitic growth on neat PLA was just slightly formed, whereas the outstanding nucleation growth of nucleated PLA films with both NPCC and talc was appeared within 3 min of isothermal crystallization time, confirming the faster growth rate and denser nucleation of crystallization compared to neat PLA films. These polarized light micrographs exhibited that both NPCC and talc were a key factor to develop a great number of nuclei into melted PLA matrix leading to tiny and more spherulites. It could be claimed that the influence of nucleating agent as NPCC and talc explicitly affected to the crystallization behaviors and kinetics of PLA isothermal crystallization.
Moreover, the polarized light micrographs of the nucleated blend films also indicated that talc could play more effective and powerful roles as a nucleating agent of PLA films than NPCC at all conditions. These results were in agreement with the previous isothermal crystallization kinetic discussions (Topic 3.1) in terms of X t, t 1/2, K, n, and ΔE a, respectively. For example, at 3 min of isothermal crystallization, the polarized light micrographs of the nucleated PLA blend films with talc revealed a smaller crystal size and a denser spherulitic nucleation than those of nucleated PLA with NPCC under the same conditions. In addition, nucleated PLA blend films with talc showed a slightly faster crystallization rate compared to those with NPCC as well.
Conclusions
Both tensile strength and elongation at break of the blend films were improved over the whole composition ranges with the presence of NPCC and talc. However, the thermal stability of the blend films progressively decreased when the nucleating agent content increased. For non-isothermal crystallization, the results showed that type and content of nucleating agent and cooling rate obviously affected to T c, χ c, and crystallization rate of neat PLA. Furthermore, the experimental data indicated that NPCC and talc could be a proficient nucleating agent for PLA. The presence of both NPCC and talc into the PLA blend films can help increasing the χ c and accelerate the crystallization rate of PLA. The optimal level of NPCC and talc for PLA should be at 1.0 phr.
Under isothermal crystallization conditions, the DSC curves illustrated that the crystallization rate of PLA was accelerated with the depression of T c from 125 to 100 °C and the addition of nucleating agent. These are in consistent with the decreasing of t 1/2 values. Besides, the polarized light micrographs implied the faster rate of crystallization and displayed the denser spherulitic nucleation into the melted PLA blend films, comparing to the neat PLA. Based on the Avrami and Arrhenius equations, the kinetic parameters showed that n and K values of PLA were evidently improved with the addition of NPCC and talc. In contrast, the ΔE a values of these blends were reduced, suggesting that the crystallization process of the nucleated PLA was faster and easier under isothermal conditions. Moreover, based on our kinetic data, it seems that talc is more effective and successful nucleating agent than NPCC.
References
Xiao H, Lu W, Yeh JT. Crystallization behavior of fully biodegradable poly(lactic acid)/poly(butylene adipate-co-terephthalate) blends. J Appl Polym Sci. 2009;112:3754–63.
Nam BU, Min KD, Son Y. Investigation of the nanostructure, thermal stability, and mechanical properties of polylactic acid/cellulose acetate butyrate/clay nanocomposites. Mater Lett. 2015;150:118–21.
Kelnar I, Kratochvil J, Kapralkova L. Crystallization and thermal properties of melt-drawn PCL/PLA microfibrillar composites. J Therm Anal Calorim. 2016;124:799–805.
Wang Y, Chiao SM, Hung TF, Yang SY. Improvement in toughness and heat resistance of poly(lactic acid)/polycarbonate blend through twin-screw blending: influence of compatibilizer type. J Appl Polym Sci. 2012;125:E402–12.
Shi X, Zhang G, Phuong TV, Lazzeri A. Synergistic effects of nucleating agents and plasticizers on the crystallization behavior of poly(lactic acid). Molecules. 2015;20(1):1579–93.
Li C, Dou Q, Bai Z, Lu Q. Non-isothermal crystallization behaviors and spherulitic morphology of poly(lactic acid) nucleated by a novel nucleating agent. J Therm Anal Calorim. 2015;122:407–17.
Akos NI, Wahit MU, Mohamed R, Yussuf AA. Preparation, characterization, and mechanical properties of poly(ε-caprolactone)/polylactic acid blend composites. Polym Compos. 2013;34:763–8.
Blanco I, Siracusa V. Kinetic study of the thermal and thermooxidative degradations of polylactide-modified films for food packaging. J Therm Anal Calorim. 2013;112:1171–7.
Arrieta MP, Lopez J, Hernandez A, Rayon E. Ternary PLA–PHB–Limonene blends intended for biodegradable food packaging applications. Eur Polym J. 2014;50:255–70.
Henricks J, Boyum M, Zheng W. Crystallization kinetics and structure evolution of a polylactic acid during melt and cold crystallization. J Therm Anal Calorim. 2015;120:1765–74.
Battegazzore D, Bocchini S, Frache A. Crystallization kinetics of poly(lactic acid)-talc composites. Express Polym Lett. 2011;5:849–58.
Wypych G. Handbook of plasticizers: chapter 7. In: Wypych G, editor. Plasticizer motion and diffusion. Toronto: ChemTec Publishing; 2004. pp. 159–160.
Ali I, Elleithy R, Al-Zahrani SM, Ali Mohsin ME. Viscoelastic, thermal, and morphological analysis of HDPE/EVA/CaCO3 ternary blends. Polym Bull. 2011;67:1961–78.
Jiang XL, Luo SJ, Sun K, Chen XD. Effect of nucleating agents on crystallization kinetics of PET. Express Polym Lett. 2007;1:245–51.
Hanim H, Ahmad Fuad MY, Zarina R, Mohd Ishak ZA, Hassan A. Properties and structure of polypropylene/polyethylene-octene elastomer/nano CaCO3 composites. J Thermoplast Compos. 2008;21:123–40.
Zhang YF. Comparison of nucleation effects of organic phosphorous and sorbital derivative nucleating agents in isotactic polypropylene. J Macromol Sci, Phys. 2008;47:1188–96.
Huang JW, Hung YC, Wen YL, Kang CC, Yeh MY. Polylactide/nano- and micro-scale silica composite films. II. Melting behavior and cold crystallization. J Appl Polym Sci. 2009;112:3149–56.
Fowlks AC, Narayan R. The effect of maleated polylactic acid (PLA) as an interfacial modifier in PLA-talc composites. J Appl Polym Sci. 2010;118:2810–20.
Xiao H, Yang L, Ren X, Jiang T, Yeh JT. Kinetics and crystal structure of poly(lactic acid) crystallized nonisothermally: effect of plasticizer and nucleating agent. Polym Compos. 2010;31:2057–68.
Cai Y, Yan S, Yin J, Fan Y, Chen X. Crystallization behavior of biodegradable poly(L-lactic acid) filled with a powerful nucleating agent: N, N′-Bis (benzoyl) suberic acid dihydrazide. J Appl Polym Sci. 2011;121:1408–16.
Wilbrink MWJ, Argon AS, Cohen RE, Weinberg M. Toughen-ability of Nylon 6 with CaCO3 filler particles: new findings and general principles. Polymer. 2001;42:10155–80.
Bartczak Z, Argon AS, Cohen RE, Weinberg M. The morphology and orientation of polyethylene in films of sub-micron thickness crystallized in contact with calcite and rubber substrates. Polymer. 1999;40:2367–80.
Huda M, Drzal L, Misra M. A study on green composites from recycled newspaper fiber reinforced poly(lactic acid). Ind Eng Chem Res. 2005;44:5593–601.
Wang H, Sun XZ, Seib PJ. Strengthening blends of poly(lactic acid) and starch with methylenediphenyl diisocyanate. Appl Polym Sci. 2001;82:1761–7.
Phetwarotai W, Aht-Ong D. Properties and nonisothermal crystallization behavior of nucleated polylactide biodegradable composite films. Adv Mater Res. 2012;488–489:671–5.
Tabi T, Sajo IE, Szabo F, Luyt AS, Kovacs JG. Crystalline structure of annealed polylactic acid and its relation to processing. Express Polym Lett. 2010;4:659–68.
Lee JH, Jeong YG. Preparation and crystallization behavior of polylactide nanocomposites reinforced with poss-modified montmorillonite. Fiber Polym. 2011;12:180–9.
Gedde UFLW. Polymer physics. 1st ed. London: Chapman & Hall; 1995.
Hwang JJ, Huang SM, Liu HJ, Chu HC, Lin LH, Chung CS. Crystallization kinetics of poly(L-lactic acid)/montmorillonite nanocomposites under isothermal crystallization condition. J Appl Polym Sci. 2012;124:2216–26.
Xiao HW, Li P, Ren X, Jiang T, Yeh JT. Isothermal crystallization kinetics and crystal structure of poly(lactic acid): effect of triphenyl phosphate and talc. J Appl Polym Sci. 2010;118:3558–69.
Acknowledgements
The authors acknowledged the financial support from Ratchadapiseksomphot Endowment Fund, Chulalongkorn University (cu-58-034-AM) and The 90th Anniversary of Chulalongkorn University Fund. Additionally, this research was partially supported by Ratchadapiseksomphot Endowment under Outstanding Research Performance Program (GF_58_08_23_01). W. Phetwarotai gratefully thanks the Development and Promotion of Science and technology Talents project (DPST).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Phetwarotai, W., Aht-Ong, D. Nucleated polylactide blend films with nanoprecipitated calcium carbonate and talc. J Therm Anal Calorim 127, 2367–2381 (2017). https://doi.org/10.1007/s10973-016-5802-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5802-2