Abstract
The thermal behavior of coal gangue selected from Zhungeer, Inner Mongolia Autonomous Region of China, was investigated by X-ray diffraction (XRD), Fourier transform infrared (FT-IR) spectroscopy, thermogravimetry (TG), derivative thermogravimetry (DTG), and scanning electron microscope (SEM). The XRD data indicated that the mineral compositions of the coal gangue were kaolinite, boehmite, and quartz. The coal-gangue sample was considered as belonging to a typical mixture of kaolinite and boehmite. The XRD and FT-IR spectra clearly showed that the structural changes and dehydroxylation of coal gangue occurred with increased temperature from 100 to 900 °C. The reaction activity of coal gangue could be effectively improved by calcination. The calcined coal gangue contained considerable active amorphous Al2O3 and SiO2 and had significant loss on ignition. The optimum activation temperature range of coal gangue was from 600 to 700 °C. The dissolution contents of SiO2 and Al2O3 were 92.31 and 64.44 %, respectively, when the calcination temperature at 700 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coal gangue is a solid waste of coal produced in excavation and washing processes. Currently, coal gangue has been one of the largest industrial solid wastes in China. Coal gangue has caused serious damage to the environment, including taking up large land and farmland, and polluting the atmosphere and water quality [1–5]. In China, the total of accumulative stockpile of coal gangue reaches 4.5 billion metric tons. Kaolinite, a layered silicate mineral consisting of siloxane- and gibbsite-like layers, has a wide range of applications [6, 7]. Coal gangue with the high content of kaolinite can be modified by calcination and surface modification to be used as active polymer composite materials. The activation method of coal gangue includes thermal activation, mechanical activation, chemical activation, microwave radiation activation, and composite activation [8–11]. The thermal activation is an effective method to stimulate the activity of coal gangue due to that it could take advantage of the severe thermal motion produced by the microstructure of coal gangue particles at a high temperature, removed bound water in minerals, and reselected interstitial cations positions, such as calcium ion, magnesium ion, and iron ion. Coal gangue contains a large number of active alumina and silica after thermal activation [12, 13]. Studies have shown that the generated amorphous reactive SiO2 and Al2O3 after thermal activation improve the reactivity of coal gangue [14, 15]. Extensive studies have used specific types of coal gangue to find the optimal activation process to achieve high chemical reactivity of coal gangue. The mineral composition of Fujian coal gangue is studied, which mainly consists of illite, quartz, and calcite, and the optimal activation temperature is 750 °C. The dissolution of SiO2 and Al2O3 of coal gangue differs in different calcination temperatures [16]. The mineral composition of Zibo coal gangue in Shandong includes kaolinite, alpha quartz, gypsum, and calcite, and the optimum activation temperature is 700 °C. Thermal activation is necessary for activating coal gangue [17]. The physico-chemical transformations and dissolution of the active ingredients of Jiahe coal gangue calcined at different temperatures were investigated. The calcination of coal gangue could remove water and organic contents, form amorphous material and glass phase, and make the SiO2 and Al2O3 contents much more soluble [18]. In this study, the structure changes of coal gangue in Zhungeer in the thermal activation process were systematically analyzed by X-ray diffraction (XRD) and Fourier transform infrared (FT-IR) spectroscopy. Calcination is an effective means to stimulate the activity of coal gangue. The thermal activation process of coal gangue contains considerable active amorphous silicon dioxide and aluminum oxide [19–23].
Experimental
Material and method
The coal-gangue samples collected from Zhungeer, Inner Mongolia, China, were used in this study. All coal-gangue samples were ground and sieved to a particle size below 100 μm. The treated coal-gangue samples were heated at 100, 200, 300, 400, 500, 600, 700, 800, and 900 °C for 2 h at a rate of 10 °C min−1 in a muffle furnace under an air atmosphere. Activated coal gangue (10 g) and hydrochloric acid solution (60 g, 25 % mass) were mixed finely and stirred at 95 °C for 3 h in a three-neck flask, with a stirring speed of 200 r min−1. The products were filtered after the reaction. Filtrate A was obtained, and the filter residue was dried at 100 °C until constant mass. The filter residue and sodium hydroxide solution were then mixed and stirred at 95 °C for 3 h in a three-neck flask, with a stirring speed of 200 r min−1. Filtrate B was obtained, and the filter residue was dried.
Filtrate A was for determining the content of Al2O3 in activated coal gangue. The Al2O3 content was determined by the national standard GB15892-2009 aluminum polychloride [24]. The SiO2 content in the coal gangue was measured through the method of alkali-soluble carbon. The specific steps were as follows: Filtrate B was added to the three-neck flask equipped with a stirring system heated at 60 °C with a stirring speed of 200 r min−1, and then, CO2 gas was slowly passed into the flask with a uniform motion. The product was filtered after 0.5 h of reaction, and hydrochloric acid solution was continuously sprayed to the filter cake. The impurities in the filter cake were removed until the filter cake had no bubble. The filter cake was then rinsed with distilled water, weighed, and dried in a vacuum oven. The SiO2 content of activated coal gangue was extracted.
Characterization
The X-ray diffraction (XRD) patterns of the raw and the thermally treated samples were generated using a Rigaku D/MAX 2500PC powder X-ray diffractometer with Cu Kα radiation (λ = 1.54059 Å), with a scanning rate of 4° min−1 in the range of 2.6° to 60°, and operated at 40 kv and 40 mA.
Fourier transform infrared (FT-IR) spectra were analyzed by Nicolet 6700, Thermo Fisher. The samples were mixed with KBr and ground in an agate mortar for 5 min. The mixture was pressed into a pellet for transmittance infrared spectroscopic measurements. The FT-IR spectra of prepared samples between 400 and 4000 cm−1 were recorded.
A Switzerland Mettler Toledo TGA/DSC1/1600HT was used for thermogravimetric combustion experiments. The sample was heated from room temperature up to a maximum temperature of 1100 °C at a heating rate of 10 °C min−1.
The morphology of the raw coal gangue and the calcined coal gangue was observed by a cold field emission scanning electron microscope (SEM, S-4800, Hitachi). During the observation, an accelerating voltage of 15 kV was selected, and the resolution was of ±2 nm.
Results and discussion
XRD
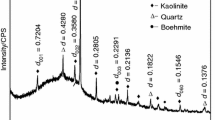
The XRD patterns of raw coal gangues are presented in Fig. 1. As illustrated in Fig. 1, the major mineral compositions of the original coal gangue are kaolinite (PDF card No. 14-0164), quartz (PDF card No. 65-0466), and boehmite (PDF card No. 21-1307). The strong diffraction peaks with the values of 0.72 and 0.359 nm at 2θ = 12°, 24° are attributed to the diffraction of (001) and (002) crystal planes, respectively. Three diffraction peaks with the values of 0.45, 0.439, and 0.420 nm at 2θ = 18°–24° are attributed to the diffraction of (020), (110), and (111) crystal surface reflections, respectively [25]. Six diffraction peaks at 2θ = 35°–40° have favorable separation condition and peak shape. Two diffraction peaks with d (001) value of 0.72 nm and d (002) value of 0.35 nm present six distinct peaks in the highly ordered kaolinite XRD patterns, and the relative intensity of the diffraction peaks can also be changed with the decreased number of the characteristic diffraction peaks [26]. The diffraction peaks by quartz at 2θ = 26.7° and 62.1° are weak, which display that the content of quartz is insignificant [27]. The characteristic diffractions with values of 0.617 and 0.317 nm at 2θ = 14.1°, 28° are attributed to boehmite [28, 29]. The characteristic peaks with the d (031) value of 0.234 nm and d (151) value of 0.166 nm are attributed to boehmite. A certain content of boehmite exists in the coal-gangue sample, which illustrates that the aluminum content in coal gangue is high, and the SiO2/Al2O3 molar ratio of the coal-gangue sample is less than the theoretical value. This result is consistent with the result of chemical analysis.
The XRD patterns of all thermally treated coal-gangue samples calcined at 100–900 °C are shown in Fig. 2. The diffraction peak shape of the calcined one at 100–400 °C is similar to that of the raw coal-gangue sample. The diffraction peak a value of 0.72 nm at 2θ = 12.6° gradually weakens as the temperature increases. At the calcination temperature of 600 °C, the diffraction peaks of kaolinite completely disappear through removing the inner hydroxyl structure, which may be due to the destruction of the crystal face (001) and the formation of an amorphous substance. The α-quartz diffraction peak with the value of 0.35 nm is intense. The result may be attributed to the further loss of water in kaolinite, boehmite, and free carbon, thereby generating amorphous Al2O3 and SiO2, which makes the content of quartz increase [30].
FT-IR analysis
The FT-IR spectra of raw coal gangue in Zhungeer are shown in Fig. 3. The infrared spectra of kaolinite mainly include the characteristic absorption bands of Si–O, –OH, and H–O–H. As illustrated in Fig. 2, in the high-frequency region (4000–3000 cm−1), two –OH absorption bands exist at 3694 and at 3619 cm−1, which are attributed to the surface and inner hydroxyl stretching vibration bands. The intensity of the absorption band at 3619 cm−1 is stronger than that of the absorption band at 3694 cm−1, and the band at 3411 cm−1 is associated with the OH stretching vibration mode of absorption water. However, the other two feature bands of kaolinite at 3668 and at 3652 cm−1 in the high-frequency region do not appear, thus showing that the bottom surface structure of coal kaolinite is imperfect. In the intermediate-frequency and low-frequency regions, the characteristic vibration bands at 2919 and 2850 cm−1 are assigned to aliphatic or naphthenic C–H. The band at 1598 cm−1 may be attributed to the vibration band of aromatic hydrocarbons or carboxylate salts. The band at 1384 cm−1 is attributed to the methyl or methylene vibration band. These bands indicate that coal-gangue samples contain carbonaceous components. The bands at 1094 and 1036 cm−1 are attributed to the symmetric stretching vibration of Si–O–Si. The bending vibration band differentiation of the inner surface hydroxyl groups is not obvious. The band approximately at 696 cm−1 is the stretching vibration mode of Si–O–Al, and the bands at 538, 472 cm−1 are the bending vibration mode of Si–O–Al.
The results of FT-IR under different calcined temperatures are presented in Figs. 4 and 5. The band at 3695 and 3650 cm−1 is formed by the vibration of the internal and external hydroxyl groups of kaolinite in coal gangue [31]. According to a study, the band at 3695 cm−1 is related to the stretching vibration of the hydroxyl groups of kaolinite in coal gangue [32]. The intensity of the two bands at 3694 and 3619 cm−1 gradually decreases and disappears at 600 °C, which indicates that the hydroxyl groups are removed in comparison with that of raw coal gangue. The intensity of the bands at 915 and 472 cm−1 gradually decreases and disappears at 900 °C. The result is attributed to the breakages of Al–OH and Si–O–Al as temperature increases. The intensity of the band at 1094 and 1036 cm−1 also gradually weakens as the temperature increases, and a wide band occurs when the temperature is 900 °C. The results are associated with the depolymerization and collapse of silica tetrahedrons structure [33, 34].
Thermal analysis
The TG-DTG curves of coal gangue samples in Zhungeer are presented in Fig. 6. An endothermic peak of raw coal gangue exists at approximately 100 °C, with mass loss of 10.23 %, which is attributed to the loss of the adsorbed water. The endothermic peak presented at 436 °C with mass loss of 29.4 % is associated with the dehydroxylation of kaolinite in coal gangue because of the dehydroxylation of the crystal lattice [35]. The theoretical loss of the structural water of kaolinite is 14.4 %. The mass loss of coal-gangue samples is higher than the theoretical value of the kaolinite structure water, which indicates that dehydroxylation reaction and carbon loss of kaolinite and boehmite occur [36].
The peak presented at 436 °C in the derivative TG curve is contributed by the dehydroxylation of coal gangue, which results in the metakaolin transformation of the thermodynamic metastable state [31], and indicates that the lattice is severely damaged and the structural water is driven off. This temperature is lower than the dehydroxylation reaction temperature for ordinary coal gangue. This phenomenon may be due to the poor crystallization degree of coal gangue in Zhungeer. Low crystallinity generally leads to the low temperature of dehydroxylation, and vice versa [37], which is consistent with the crystal-order analysis in the XRD analysis section.
The ignition loss of 32.34 % is associated with the considerable free carbon of coal gangue. The coal gangue in Zhungeer can be effectively utilized.
Thermal activation analysis
This section presents the thermal activation analysis of coal-gangue in Zhungeer. The dissolution contents of SiO2 and Al2O3 in different calcination temperatures are studied. The dissolution content of coal-gangue samples in Zhungeer is shown in Fig. 7. The dissolution content of Al2O3 and SiO2 is reviewed with increasing temperatures from 500 to 900 °C. The dissolution contents of Al2O3 and SiO2 increase as the temperature rises. At the calcination temperature of 700 °C, the dissolution contents of SiO2 and Al2O3 reach maximum at 92.3 and 64.44 %, respectively. When the calcination temperature continues to increase, the dissolution contents of SiO2 and Al2O3 gradually decrease. When the calcination temperature is 600 °C, the dissolution contents of SiO2 and Al2O3 are also high. Therefore, the optimal active region is 600–700 °C. The results are correlated with the results of TG–DTA and XRD analysis (Table 1).
The microstructure analysis
According to the results of the above analysis, the coal gangue calcined at 700 °C has the best activation effect. The SEM images of raw coal gangue and the coal gangue calcined at 700 °C are presented in Figs. 8 and 9. The microstructure of raw coal gangue presented clumps, schistose, and nonuniform size distribution. The chunks disappear and the fragments increase after coal gangue calcined at 700 °C. The overall particle distribution of calcined coal gangue is uniform and loose compared with that of raw coal gangue. Calcined coal gangue is basically loose because of the component volatilization and frame expansion [38]. Thermal activation could destroy the stable structure of coal gangue and improve the reaction activity.
Conclusions
The following conclusions can be drawn from this study:
-
1.
The mineral composition of coal gangue selected from Zhungeer was kaolinite, boehmite, and quartz.
-
2.
The free carbon and organic volatile matter in coal gangue could be removed by thermal activation and calcination. The long-chain polymeric structure that consisted of Si–O bond and Al–O bond was damaged. The reaction activity of coal gangue was improved, and activated amorphous Al2O3 and SiO2 were increased.
-
3.
Thermally activated analysis at different temperatures showed that the optimum activation temperature region of coal gangue in Zhungeer was 600–700 °C. The dissolution contents of Al2O3 and SiO2 reached 92.31 and 64.44 %, respectively.
References
Nichol D, Tovey NP. Remediation and monitoring of a burning coal refuse bank affecting the Southsea Looproad at Brymbo, North Wales. Eng Geol. 1998;50(3–4):309–18.
Zhang Y, Nakano J, Liu L, Wang X. Co-combustion and emission characteristics of coal gangue. J Therm Anal Calorim. 2015;120(3):1883–92.
Li Y, Yao Y, Liu X, Sun H. Improvement on pozzolanic reactivity of coal gangue by integrated thermal and chemical activation. Fuel. 2013;109(7):527–33.
Zhang Y, Li J, Cheng F, Guo Y. Study of the combustion behavior and kinetics of different types of coal gangue. Combust Explos Shock Waves. 2015;51(6):670–7.
Jaesuk R. Improvement on reactivity of cementitious waste materials by mechano-chemical activation. Mater Lett. 2003;58:903–6.
Cheng H, Zhang Z, Liu Q, Leung J. A new method for determining platy particle aspect ratio: a kaolinite case study. Appl Clay Sci. 2014;97–98(8):125–31.
Cheng H, Frostc RL, Yang J, Liu Q, He J. A new method for determining platy particle aspect ratio: a kaolinite case study. Spectrochim Acta Part A Mol Biomol Spectrosc. 2010;77(5):1014–20.
Cao Z, Cao Y, Dong H, Zhang J, Sun C. Effect of calcination condition on the microstructure and pozzolanic activity of calcined coal gangue. Int J Miner Process. 2016;146:23–8.
Mei Z, Huamin Z. Composite activation of gangue and preparation of high strength gepolyer material. Bull Chin Ceram Soc. 2014;33(8):1908–14.
Zhang C, Yang XY, Li YF. Mechanism and structural analysis of the thermal activation of coal-gangue. Adv Mater Res. 2011;356–360:1807–12.
Yuanyuan L, Qisheng W. Thermal activation of coal gangue. Environ Pollut Control. 2008;30(9):26–30.
Chakraborty AK. New data on thermal effects of kaolinite in the high temperature region. J Therm Anal Calorim. 2003;71(3):799–808.
Chen-chen G, Xu-yan S, Dong-xu L. Mechanism discussion on calcined activate coal gangue. J Mater Sci Eng. 2005;23(1):89–91.
Zhou C, Liu G, Yan Z, Fang T. Transformation behavior of mineral composition and trace elements during coal gangue combustion. Fuel. 2012;97(7):644–50.
Li Yu, Yao Y, Liu X, Sun H, Ni W. Improvement on pozzolanic reactivity of coal gangue by integrated thermal and chemical activation. Fuel. 2013;109:527–33.
Li D, Song X, Gong C, Pan Z. Research on cementitious behavior and mechanism of pozzolanic cement with coal gangue. Cem Concr Res. 2006;36(9):1752–9.
Zhang C. Study on the volcanic ash activity of low temperature burning coal 2003;369–373.
GuoWei LD, Jianhua C, Nanru Y. Research on cementing performance of actived coal gangue. Min Res Dev. 2007;27(3):35–42.
Yuanyuan L, Qisheng W. Thermal activation of coal gangue. Environ Pollut Control. 2008;30(9):26–30.
Koç S, Toplan N, Yildiz K. Effects of mechanical activaiton on the non-isothermal kinetics of mullite formation from kaolinite. J Therm Anal Calorim. 2003;71(3):799–808.
Chen-chen G, Xu-yan S, Dong-xu L, et al. Mechanism discussion on calcined activate coal gangue. J Mater Sci Eng. 2005;23(1):88–91.
Chakraborty AK. New data on thermal effects of kaolinite in the high temperature region. J Therm Anal Calorim. 2003;71(3):799–808.
Jieyu C, Chunjie Y, Weimin W, et al. Structure and thermal stability of Kaolinite/urea intercalated compound. J Chin Ceram Soc. 2010;38(9):1837–42.
GB15892-2009 determination of aluminium oxide in the national standard aluminium polychloride.
Cheng H, Kuo L, Liu Q, Zhang S, Li X, Frost RL. Insight into the thermal decomposition of kaolinite intercalated with potassium acetate: an evolved gas analysis. J Therm Anal Calorim. 2014;117(3):1231–9.
JunKai H, LuJun W, Qinfu L. A study on mineralogical properties of kaolinite in Huaibei coal bearing strata used as carrier for FCC catalyst. Acta Min. 2011;31(2):274–9.
Bayram H, Önal M, Yılmaz H, Sarıkaya Y. Thermal analysis of a white calcium bentonite. J Therm Anal Calorim. 2010;101:873–9.
Alex TC. An insight into the changes in the thermal analysis curves of boehmite with mechanical activation. J Therm Anal Calorim. 2014;117(1):163–71.
Guzmán-Castillo ML, Bokhimi X, Toledo-Antonio A. Effect of boehmite crystallite size and steaming on alumina properties. J Phys Chem B. 2001;105(11):2099–106.
Zhang C, Yang XY, Li YF. Mechanism and structural analysis of the thermal activation of coal-gangue. Adv Mater Res. 2012;356–360:1807–12.
Cheng H, Liu Q, Yang J, Frostc RL. Thermogravimetric analysis of selected coal-bearing strata kaolinite. Thermochim Acta. 2010;507–08(33):84–90.
Ming H. Modification of kaolinite by controlled hydrothermal deuteration–a DRIFT spectroscopic study. Clay Miner. 2004;39:349–62.
Cao Z, Cao Y, Dong H. Effect of calcination condition on the microstructure and pozzolanic activity of calcined coal gangue. Int J Miner Process. 2016;146:23–8.
Cao Z, Cao Y, Dong H, Zhang J, Sun C. Effect of calcination condition on the microstructure and pozzolanic activity of calcined coal gangue. Int J Miner Process. 2016;146:23–8.
Cheng H, Kuo L, Liu Q, Zhang S, Li X, Frost RL. Insight into the thermal decomposition of kaolinite intercalated with potassium acetate: an evolved gas analysis. J Therm Anal Calorim. 2014;117(3):1231–9.
Zhang Y, Xu L, Seetharaman S. Effects of chemistry and mineral on structural evolution and chemical reactivity of coal gangue during calcination: towards efficient utilization. Mater Struct. 2015;48:2779–93.
Yuanyuan L, Qisheng W. Thermal activation of coal gangue. Environ Pollut Control. 2008;30(9):26–30.
Zhang C, Yang XY, Li YF. Mechanism and structural analysis of the thermal activation of coal-gangue. Adv Mater Res. 2012;356–360:1807–12.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, L., Zhang, Y., Zhang, Y. et al. The thermal activation process of coal gangue selected from Zhungeer in China. J Therm Anal Calorim 126, 1559–1566 (2016). https://doi.org/10.1007/s10973-016-5711-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5711-4