Abstract
Amorphous boron is usually employed as the most important fuel of boron-based fuel-rich propellants, and NH4ClO4 (AP), cyclotetramethylenetetranitramine (HMX), KClO4 and KNO3 are the solid oxidizers of most used in solid propellants. The mixtures of boron and different solid oxidizers with mass ratio 1:1 were prepared in this paper, and the effect of these oxidizers on the thermal oxidation and combustion performance of amorphous boron was studied by simultaneous thermogravimetry–differential scanning calorimeter–Fourier transform infrared spectroscopy and CO2 laser ignition experiments. The experimental results show that the main reactions during the heating process of B/AP and B/HMX samples are the decomposition of oxidizers, and the decomposition process of oxidizers rather than the decomposition temperature is affected by amorphous boron; boron could react with KClO4 and KNO3 violently with the release of large amounts of heat, and then both of the oxidizers, especially KClO4, have positive effect on the oxidation and combustion performance of amorphous boron.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The combustion heat of boron is 58.83 kJ g−1 which is much higher than that of magnesium and aluminum (24.79 and 31.09 kJ g−1, respectively); thus, boron could be employed as a metal additive of solid propellants with high energy density [1, 2]. Considering the combustion of boron needs more oxygen, boron is generally regarded as the promising fuel of ramjet [3–5]. The thrust of normal solid rocket is generated by the combustion of fuels and oxidizers in the propellant which result in the low specific impulse (I s); however, ramjet employs air (or part of air) as oxidizer, and then, the specific impulse of ramjet is much higher than that of normal solid rocket. Ducted rocket is a kind of ramjet with solid fuel-rich propellants as its fuel, and boron-based fuel-rich propellants are considered to be one of the best energy sources because of the high energy.
Elemental boron could be divided into crystalline boron and amorphous boron, and amorphous boron is the more preferable fuel because of the high reaction activity and low price [6, 7]. Amorphous boron is the most important fuel in boron-based fuel-rich propellants due to the high content, and there are also the other fuels in such kind of propellants, such as binder, magnesium and aluminum. However, the combustion performance of boron is important for the heat release of the propellants because boron is difficult to be ignited and usually has low combustion efficiency [8–11].
The combustion of propellants for ducted rocket could be divided into primary combustion process and secondary combustion process, and the oxygen needed during the primary one all comes from the solid oxidizer. Therefore, oxidizer plays an important role for the combustion performance of boron and thus the propellants [12]. AP is the most widely used oxidizer in solid composite propellants mainly because of high content of oxygen, high specific volume of gases products and low price. HMX is another commonly used oxidizer for composite propellants and modified double-base propellants because of the high formation of heat, high density and high specific volume [13]. In addition, there is no chlorine in HMX; thus, HMX is also an important oxidizer for smokeless propellants [14]. Potash including KClO4 and KNO3 is also common oxidizer of solid propellants, and the mixture of boron and KNO3 is even a common kind of pyrotechnic [15].
In this paper, mixtures of amorphous boron and different oxidizers were prepared, and the effect of oxidizers on the oxidation performance of amorphous boron was investigated by TG–DSC–FTIR and XRD experiments. In addition, the effect of oxidizers on the combustion performance of amorphous boron was explored by CO2 laser ignition experiments.
Experimental
Sample preparation
Amorphous boron (95 % in purity, 1 μm) was purchased from Dandong Chemical Engineering Institute Co., Ltd. Analytical-grade ammonium perchlorate (AP) and KClO4 were obtained from Dalian North Potassium Chlorate CO., Ltd, analytical-grade cyclotetramethylenetetranitramine (HMX) was provided by Gansu Baiyin silver chemical materials plant, and KNO3 was purchased from Xuzhou Jingke Reagent Instrument Co., Ltd. All the oxidizers were analytical reagents with particle size of 50 μm. Mixtures of amorphous boron and different oxidizers with mass ratio 1:1 were prepared with a mortar and pestle for about 30 min.
The morphology of the samples was investigated with a scanning electron microscope (TESCAN VEGA 3 LMH), and the results are shown in Fig. 1.
Figure 1 indicates that the amorphous boron particles are much irregular and porous, and the particle size is much smaller than that of oxidizers. There are oxidizer particles of a few microns dispersed in the samples, and the interfacial areas of boron and oxidizers for different samples are similar because of the similar particle size of oxidizers after mixing process.
TG–DSC–FTIR and XRD experiments
Simultaneous TG–DSC (METTLER TOLEDO TG/DSC 1) and FTIR (Bruker VERTEX 70) were used in the TG–DSC–FTIR experiments. Samples weighing about 3 mg were heated from 50 to 700 °C with a heating rate of 10 °C min−1 under argon atmosphere (the flow rate of the sweeping gas is 30 mL min−1). The component of evolved gases during the heating process was analyzed by FTIR with wavelength of 400–4000 cm−1.
Samples weighing about 1 g were heated from room temperature to 700 °C in muffle furnace under argon atmosphere. In order to avoid the flying of the solid products of small particle size, samples were wrapped by graphite paper before the heating. Samples were cooled to room temperature when the temperature reached 700 °C, and then the cooled solid residues were analyzed by X-ray diffractometer (Bruker D8ADVANCE) using Cu Kα radiation (λ = 1.5406 Å) with scattering angles (2θ) of 10°–60°.
CO2 laser ignition experiments
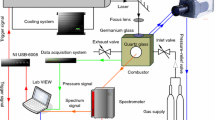
Samples weighing 10 mg were put into the aluminum oxide crucible and ignited in a pressured and windowed combustor under atmospheric argon pressure. CO2 laser with maximum output of 150 W was used to ignite the samples, and the spectrometer (AvaSpec-2048) was employed to record the ignition and combustion process. In order to get more time-resolved spectrum, the integration time of the spectrum was set as 1.05 ms.
Results and discussion
Effect of oxidizers on the thermal oxidation characteristics of amorphous boron
TG–DSC results of the samples are shown in Figs. 2 and 3.
Figures 2 and 3 show that TG and DSC curves of B/AP and B/HMX mixtures are similar to that of pure AP and HMX decomposition, and the main differences are the value of mass loss and heat release [16]. Figure 2 also suggests that the maximum mass loss of B/AP and B/HMX mixtures is 45.3 and 48.1 %, respectively, which is close to the content of oxidizer in the mixtures. AP and HMX can decompose to gases completely, and then, TG and DSC results preliminarily indicate that the main reactions during the heating process of the mixtures are the thermal decomposition of the oxidizers, and there may be only small amount of boron oxidized by the thermal decomposition products of oxidizers.
It also can be seen from Figs. 2 and 3 that the mass loss and heat release of B/KClO4 and B/KNO3 mixtures occur at a similar temperature, but the maximum mass loss is higher and heat release is lower for the sample of B/KNO3. The gaseous products percentage is 53.42 and 46.19 % after the decomposition of KNO3 and KClO4, while the maximum mass loss of B/KNO3 and B/KClO4 is 11.2 and 5.9 %, respectively, which indicates that most of these two oxidizers react with amorphous boron and generate less gaseous products. However, the reaction mechanisms could not be concluded by the TG results because there are some solid products escaped from the crucible. In addition, a large amount of heat was produced during the heating process of B/KNO3 and B/KClO4 mixtures which suggests that these two oxidizers, especially KClO4, have a more positive effect on the oxidation of amorphous boron.
Reaction mechanisms of amorphous boron with different oxidizers
The evolved gases during the TG–DSC experiments were analyzed by FTIR. The experimental results show that the shape of FTIR curves is similar at different stages of heating process, and the main difference of these curves only lies in the signal strength (depending on the amount of gaseous products). FTIR curves with the highest signal strength correspond to the time that heat flow of DSC curves reaches maximum, and are shown in Fig. 4.
It can be seen from Fig. 4 that there are many absorption peaks in the wavelength range of 3500–3900 and 1300–2000 cm−1 which correspond to the water vapor, while the absorption peaks at 2361 and 2341 cm−1 correspond to CO2. There are always these two kinds of gases in the FTIR curves for the evolved gases of different samples at different time which results largely from the impurity of the sweep gas.
Figure 4 also indicates that there are only absorption peaks corresponding to water vapor and CO2 during the heating process of B/AP, B/KClO4 and B/KNO3 samples. Although the decomposition of AP will produce water vapor, the absorption peaks are mainly due to the impurity of sweep gas because they exist in all FTIR curves. In addition, Fig. 4b suggests that there are absorption peaks at 2206 and 2237 cm−1 which are assigned to CO. As a small amount of boron was oxidized during the heating process of B/HMX, CO should be the decomposition products of HMX.
It should be noted that there may be some infrared inactive gases such as N2, H2 and O2 which exist in the evolved gases, so not all the gases products could be determined by FTIR. However, many possible gases products could be excluded through the experiments. For example, AP starts to decompose at about 260 °C with the main gases products of N2O, NO2, HCl, Cl2, O2, H2O, etc. [17], but there is only water vapor in the gases products from Fig. 4a which indicate the decomposition of AP may be affected by amorphous boron.
XRD patterns of solid residues after the heating process are represented in Figs. 5–8.
TG and DSC results show that only a small amount of boron was oxidized during the heating process of B/AP mixture, and Fig. 5 suggests that NH4B5O8·4H2O and H3BO3 were also produced during the process. Oxidizing gases (O2,HClO4, HClO3…) and water were produced during the decomposition of AP, and then, boron oxide was produced firstly by the reactions of these oxidizing gases and boron. Boron oxide reacted with water to produce H3BO3. Ammonia was another important decomposition product of AP, and NH4B5O8·4H2O may result from the reaction among ammonia, boron oxide and water.
N2O, NO2 and HCl are all important gases products during the decomposition of AP, but there are no absorption peaks of these products in FTIR results which indicates the decomposition process is affected by the presence of amorphous boron. A large amount of researches using TG and DSC as experimental facility were conducted to thermal decomposition mechanism of AP, and a process of proton transfers regarded to be most consistent with experimental results [18]. AP decomposes to NH3 and HClO4 firstly, and NH3 is then oxidized by HClO4 to produce the other gases products. The surface of amorphous boron is irregular which results in the high specific area, so the produced NH3 by the decomposition of AP is easy to be adsorbed onto the surface of boron. Amorphous boron particles are coated with acidic boron oxide film, and NH3 could react with boron oxide which makes the decomposition process of AP changes.
Figure 6 suggests that there are BN, H3BO3 and elemental boron in the solid products of B/HMX sample, and most of the boron was not oxidized during the heating process from the results of TG and DSC results. Some oxidizing gases were also produced during the decomposition of HMX which results in the production of boron oxide, and H3BO3 could be produced by the reaction of boron oxide and water. There are nitrogen in HMX, and boron nitride may be produced by boron and nitride decomposed by HMX. The decomposition products of HMX are mainly N2O, CO and H2O [17], but there are only absorption peaks of CO and water vapor in the FTIR curve. Therefore, the decomposition process of HMX is also affected by the presence of amorphous boron.
Figure 7 shows that KCl and KB5O6(OH)4·2H2O were produced through the reaction between KClO4 and amorphous boron. Potassium borate could be produced when KClO4 reacts with boron, and KB5O6(OH)4·2H2O is produced ultimately by the moisture absorption of potassium borate. TG and DSC results also show that amorphous boron reacts with KClO4 very violently, and then, a large proportion of boron should take part in the reactions. The decomposition of KClO4 starts at about 670 °C with KCl and O2 as the main products [19], but the mass loss starts at about 450 °C and speeds up at about 450 °C when the B/KClO4 sample was heated. Therefore, the presence of amorphous boron improves the decomposition of KClO4. It is worthwhile to note that the melting point of boron oxide is also 450 °C, so boron could contact with KClO4 directly when boron oxide coated on the surface of boron particles exists in liquid state which benefits the reactions between amorphous boron and KClO4.
There are no obvious absorption peaks in the FTIR results of B/KClO4 sample, and it is consistent with the decomposition process of B/KClO4. Then, the possible reaction mechanisms between boron and KClO4 could be illustrated as follows [20]
Meanwhile, the decomposition of KClO4 is carried out [21]
Boron could react with oxygen to produce boron oxide, and the other kinds of potassium borate could be produced by the reaction of boron oxide and KBO2.
Figure 8 shows that the mainly solid product after the reactions between amorphous boron and KNO3 is KB5O8·4H2O which also results from the moisture absorption of potassium borate produced by the reactions. Similar to the TG results of B/KClO4 sample, the mass loss also started at about 450 °C and speeded up at about 500 °C. However, the decomposition of KNO3 also starts at 450 °C and decomposes completely at 500 °C. Therefore, the presence of amorphous boron may inhibit the decomposition of KNO3.
Boron oxide coated on the surface of boron oxide exists in liquid state, and KNO3 started to decompose when the temperature reaches 450 °C, and then, boron could react with KNO3 to produce potassium borate. The possible reaction mechanism is [20]
The produced oxygen also could react with boron to produce boron oxide because the reactions between boron and KNO3 could release huge amount of heat which benefits for the oxidation of boron, and the other kinds of potassium borate also could be produced by the reactions between boron oxide and KBO2.
To sum up, the main reactions are the decomposition of oxidizer during the heating process of B/AP and B/HMX samples, and the presence of amorphous boron could affect the decomposition process of the oxidizers with the decomposition temperature not affected. Boron could react with KClO4 and KNO3 violently to produce potassium borate, so these two oxidizers have positive effect on the oxidation process of amorphous boron.
Effect of oxidizers on the combustion characteristics of amorphous boron
The spectra shape at different time is almost the same except the signal strength during the combustion process of the mixtures, and the spectra with the highest signal strength are shown in Fig. 9.
The highest peak at 588 nm in Fig. 9 is the interference of sodium, and the five peaks located from 470 to 580 nm are all assigned to the emission of BO2 which agrees well with the Refs. [22, 23]. In addition, the strong peaks with the wavelength from 740 to 810 nm are the results of potassium oxides. Figure 9b also shows that the combustion spectrum of B/KClO4 sample has the highest intensity because of the much violent combustion, and the combustion spectra in Fig. 9 represent the similar results with the ones of TG and DSC, and B/KClO4 sample has the highest combustion violence while the lowest combustion violence for B/HMX sample.
In summary, TG, DSC and CO2 ignition experiments all indicate that KClO4 and KNO3, especially KClO4, could improve the oxidation and combustion performance of amorphous boron. The content of oxygen in KClO4 is lower than that in KNO3, so KClO4 should be the preferable solid oxidizer of boron-based fuel-rich propellants considering the combustion performance of amorphous boron. However, KClO4 produces less gaseous products than AP and HMX because of potassium; thus, it should be added into the propellants in moderation.
Conclusions
Only small amount of amorphous boron is oxidized when AP and HMX are employed as the oxidizer, but the decomposition process of both the oxidizers changes at the presence of amorphous boron. KNO3 and KClO4, especially KClO4, could improve the thermal oxidation and combustion performance of amorphous boron through the violent reactions of the production of potassium borate. KClO4 may be the preferable solid oxidizer of boron-based fuel-rich propellants considering the combustion performance of amorphous boron, but it should be added into the propellants in moderation due to the low content of oxygen.
References
Kuo KK, Summerfield M. Fundamentals of solid-propellant combustion. New York: American Institute of Aeronautics and Astronautics; 1984.
Gany A, Timnat YM. Advantages and drawbacks of boron-fueled propulsion. Acta Astronaut. 1993;29(3):181–7. doi:10.1016/0094-5765(93)90047-Z.
Besser HL, Strecker R. Overview of boron ducted rocket development. Int J Energ Mater Chem Propuls. 1993;2(1–6):133–78. doi:10.1615/IntJEnergeticMaterialsChemProp.v2.i1-6.60.
Buchner E, Langel G. Elementary boron as a propellant component in ducted rockets: thermodynamic study. Z Flugwissensch. 1976;24(5):275–8.
Fry RS. A century of ramjet propulsion technology evolution. J Propuls Power. 2004;20(1):27–58. doi:10.2514/1.9178.
Pang WQ, Fan XZ, Zhang W, Xu HX, Li JZ, Li YH, et al. Application of amorphous boron granulated with hydroxyl-terminated polybutadiene in fuel-rich solid propellant. Propellant Explos Pyrotech. 2011;36(4):360–6. doi:10.1002/prep.200900112.
Liu L, He G, Wang Y. Thermal reaction characteristics of the boron used in the fuel-rich propellant. J Therm Anal Calorim. 2013;114(3):1057–68.
Hussmann B, Pfitzner M. Extended combustion model for single boron particles—part I: theory. Combust Flame. 2010;157(4):803–21. doi:10.1016/j.combustflame.2009.12.010.
Hussmann B, Pfitzner M. Extended combustion model for single boron particles—part II: validation. Combust Flame. 2010;157(4):822–33. doi:10.1016/j.combustflame.2009.12.009.
Foelsche RO, Burton RL, Krier H. Boron particle ignition and combustion at 30-150 ATM. Combust Flame. 1999;117(1–2):32–58. doi:10.1016/S0010-2180(98)00080-7.
Yeh CL, Kuo KK. Ignition and combustion of boron particles. Prog Energy Combust. 1996;22(6):511–41. doi:10.1016/S0360-1285(96)00012-3.
Liu L, He G, Wang Y. Effect of oxidizer on the combustion performance of boron-based fuel-rich propellant. J Propuls Power. 2014;30(2):285–9.
Kubota N. Energetics of HMX-based composite modified double-base propellant combustion. J Propuls Power. 1999;15(6):759–62.
Muthiah R, Varghese T, Rao SS, Ninan K, Krishnamurthy V. Realisation of an eco-friendly solid propellant based on HTPB-HMX-AP system for launch vehicle applications. Int J Energ Mater Chem Propul. 1997;4(1–6):134–9.
Sivan J, Haas Y. Spectroscopic characterization of B/KNO3 diode-laser induced combustion. J Phys Chem A. 2013;117(46):11808–14.
Liu L, He G, Wang Y, Liu P. Effect of catocene on the thermal decomposition of ammonium perchlorate and octogen. J Therm Anal Calorim. 2014;117(2):621–8.
Boldyrev V. Thermal decomposition of ammonium perchlorate. Thermochim Acta. 2006;443(1):1–36.
Jacobs P, Pearson G. Mechanism of the decomposition of ammonium perchlorate. Combust Flame. 1969;13(4):419–30.
Lee J-S, Hsu C-K, Jaw K-S. The thermal properties of KClO 4 with different particle size. Thermochim Acta. 2001;367:381–5.
Miyata K, editor. Combustion of boron-pyrotechnics. AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, 37th, Salt Lake City, UT; 2001.
El-Awad A. Catalytic effect of some chromites on the thermal decomposition of KClO4. Mechanistic and non-isothermal kinetic studies. J Therm Anal Calorim. 2000;61(1):197–208.
Spalding MJ, Krier H, Burton R. Boron suboxides measured during ignition and combustion of boron in shocked Ar/F/O 2 and Ar/N 2/O 2 mixtures. Combust Flame. 2000;120(1):200–10.
Ao W, Yang W, Wang Y, Zhou J, Liu J, Cen K. Ignition and combustion of boron particles at one to ten standard atmosphere. J Propuls Power. 2014;30(3):760–4.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Pj., Liu, Ll. & He, Gq. Effect of solid oxidizers on the thermal oxidation and combustion performance of amorphous boron. J Therm Anal Calorim 124, 1587–1593 (2016). https://doi.org/10.1007/s10973-016-5252-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5252-x