Abstract
Ammonium polyphosphate (APP)-diatomaceous earth (DE) composite prepared by in situ polymerization was modified with silane coupling agent (KH550) and was applied as filler to prepare flame-retardant paper. Fourier transfer infrared, X-ray diffraction and thermogravimetry were used to characterize the structure and properties of the fillers, and limiting oxygen index (LOI), cone calorimeter and field-emission scanning electron microscope were used to investigate the flame-retardant properties of the filled paper. Results showed that chemical bonds occurred between APP-10 % DE filler and KH550, and APP-10 % DE filler had lower water solubility and better crystallization and thermostability after it was modified with KH550. Paper filled with modified APP-10 % DE filler had higher LOI value and lower heat release rate and mass loss at the same filler loading, and its charred residue collected after cone calorimeter test was more compact and strong. Surface modification of APP-10 % DE filler with KH550 improves its flame-retardant effect on paper.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well known that paper made from plant fibers is a kind of flammable material. With the development of society, an increasing number of application areas need to use flame-retardant paper base materials, such as automobile filter materials, building materials and decorative materials, so the improvement of flame-retardant property of paper has become a hotspot in the research of specialty paper [1–5]. The present ways to manufacture flame-retardant paper mainly include impregnation, surface coating of base paper with flame retardants and adding flame retardants to paper stock [6–9]. Paper absorbs a mass of flame retardant in the process of impregnation, which makes the flame-retardant paper weak in mechanical strength, dimensional stability and moisture resistance. Flame retardants are distributed on the surface of paper in the process of surface coating, and a large amount of organic adhesives are used, so the flame-retardant paper has no ideal flame-retardant effect. Adding inorganic flame retardants (such as aluminum hydroxide [Al(OH)3], magnesium hydroxide[Mg(OH)2]) to paper stock is a simple way to manufacture flame-retardant paper, but it has some major disadvantages, such as a large amount of inorganic flame-retardant requirement and an adverse effect on the physical properties of paper. Therefore, the development of an efficient flame-retardant filler is an important research aspect.
Ammonium polyphosphate (APP) is an efficient flame retardant containing phosphorus and nitrogen, which is widely used as the flame retardant in the fields of polymers, coatings, wood and other fibrous materials. However, paper sheet is a very thin material, papermaking is a wet process which is unlike the molding of polyethylene (PE), polypropylene (PP) and other polymers, in order to produce a uniform paper sheet, a large amount of water is applied to dilute and disperse fibers to a dilute suspension of fibers (typically 0.3–0.6 % consistency), and filler is also added to the dilute fiber suspension to obtain good dispersion. APP will dissolve easily in water and run away with drainage during papermaking process due to its moisture absorption and hydrophilicity [10, 11], so the use of APP alone as flame-retardant filler in paper cannot obtain good flame-retardant effect.
Diatomaceous earth (we shall hereby call DE because it is a lot easier to type) is a porous nonmetallic mineral with large specific surface area and strong adsorption and is usually used as papermaking filler for its rich resources, low price and high filler retention, but its flame-retardant effect is very poor.
Accordingly, APP-DE composite was prepared by in situ polymerization and modified with silane coupling agent (KH550) and was applied as filler to prepare flame-retardant paper. The effect of surface modification on structure, water solubility and thermal stability of the APP-10 % DE filler was studied by using FTIR, XRD and TG, and the flame-retardant effect of composite fillers on paper was investigated by using LOI, cone calorimeter and FESEM.
Experimental
Materials
Softwood pulp came from Chile, and hardwood pulp came from Brazil. Phosphoric acid (85 %), urea, absolute ethyl alcohol and glacial acetic acid were supplied by Shanghai Lingfeng Chemical Reagent Co., Ltd. Diatomaceous earth (DE) was obtained from Chinasun Specialty Products Co., Ltd; cationic polyacrylamide (CPAM) was obtained from NALCO (Shanghai) Trading Co., Ltd; silica sol was supplied by Suzhou Tianma Specialty Chemicals Co., Ltd; and (3-aminopropyl) triethoxysilane (KH550) was obtained from Zhejiang Feidian Chemical Co., Ltd.
Synthesis of APP-DE composite fillers
A certain amount of phosphoric acid was weighed and poured into a three-necked flask, a gas outlet was connected to the flask, the mixture was stirred and heated to 70 °C in an oil bath, and then a certain amount of urea was added in the flask. Heating the mixture was continued to 130 °C at the heating rate o f 2–3 °C/min, and then a certain amount of DE was added which is equivalent to 5, 10, 15, 20 and 25 % of the mass of the generated APP, respectively. The temperature was kept at 130 °C for 15–30 min, and then the product was poured into a tray and placed in an oven to solidify at the temperature of 210 °C for 2 h. Finally, the solidified materials were ground to obtain APP-DE composite fillers with different compositions, which were designated as APP-5 % DE, APP-10 % DE, APP-15 % DE, APP-20 % DE and APP-25 % DE, respectively.
Surface modification of APP-DE composite filler
Five grams of APP-DE composite filler was weighed and kept in a round-bottom flask, then 20 mL ethanol was added, and the mixture was dispersed by using ultrasonic dispersion for 20 min and stirred rapidly in a water bath at 70 °C. Two percent of KH550 (relative to the mass of composite filler) was taken, diluted with the same mass of absolute ethyl alcohol, adjusted to pH 3.5–5 with glacial acetic acid, and added to the above-mentioned round-bottom flask, and then the mixture was stirred at high speed for 1–2 h at the temperature of 70 °C. After suction filtration, the modified composite filler was placed in an oven at 105 °C, and it was dried to constant mass.
Preparation of flame-retardant paper
Softwood pulp was beaten to about 40° SR, and hardwood pulp was beaten to about 37° SR using a Valley beater. 25 mass % of softwood pulp and 75 mass % of hardwood pulp were mixed and disintegrated in a standard fiber disintegrator to form uniform fiber suspension, then 0.2 % of CPAM and 0.3 % of silica sol were added into the fiber suspension and stirred well, finally a certain amount of composite filler was added into the fiber suspension, and paper sheets of 100 g m−2 were formed on a standard sheet former (ZQJ1-200, Shannxi University of Science and Technology, China), pressed on a presser (400-1, LABTECH, Canada) at the pressure of 2.5 kgf cm−2 for 5 min and dried by a drum dryer (E-100, AMC, USA).
Measurement and characterization
Water solubility was measured as follows [12]: 4.00 g APP-DE composite filler was weighed and dispersed well in 100 mL distilled water at room temperature, the mixture was oscillated in an oscillator (150 r min−1) for 40 min, and the supernatant was transferred into a centrifugal tube. After 30-min centrifugal separation (2000 r min−1), 20 mL supernatant was taken into a beaker and weighed with an analytical balance (m 1), then the beaker was placed in an oven (110 °C), and the supernatant was dried to a constant mass (m 2). The water solubility (S) of APP-DE composite filler can be obtained according to the following equation:
The FTIR spectra were recorded with a FTIR-8400S spectrometer (Shimadzu, Japan) in the range of 400–4000 cm−1, and the samples were prepared with KBr pellets.
The X-ray diffraction (XRD) patterns were recorded at room temperature on a Bruker D8 ADVANCE X-ray diffractometer (Bruker, Germany) using Cu Ka radiation (λ Cu Ka = 1.5418 Å, 40 kV, 40 mA) and Ni filter in the 2θ range of 5°–60°. The scanning step was 0.02°, and the scanning rate was 0.1 s per step.
Thermogravimetry (TG) was performed on a STA 449 F3 DSC/DTA–TG simultaneous thermal analyzer (Netzsch, Germany) at a heating rate of 10 °C min−1 under nitrogen atmosphere (at a flow rate of 50 mL min−1), and the sample mass used in thermal analysis is 10 mg.
LOI tests were performed with a JF-3 digital display limiting oxygen index tester (Nanjing ShineRay Instrument Co., Ltd, China) according to ASTM D2863-97, and the samples used for the test were 100 mm × 10 mm (length × width).
The cone calorimeter tests were carried out with a FTT2000 cone calorimeter (FTT, England) in accordance with the procedures in ASTM international standard method E1354 [13]. The samples were 100 mm × 100 mm × 0.20 mm (length × width × thickness), and the tests were conducted using the standard optional retainer frame and grid. Each specimen was wrapped in an aluminum foil and exposed horizontally to a heat flux of 30 kW m−2, and the data scans were taken every second. The distance between the bottom surface of the cone heater and the top of the specimen was adjusted to 25 mm as specified in the standard.
Digital camera was used to take pictures of the charred residues after cone calorimeter test, and a field-emission scanning electron microscope (FESEM, JSM-7001F, JEOL Ltd.) was used to obtain the images of the charred residues.
Results and discussion
Composition optimization of APP-DE composite flame-retardant filler
Composite fillers with different compositions were added to paper stock to prepare flame-retardant paper, and LOI was used to evaluate the flame-retardant property of paper. The optimal filler composition was determined according to the LOI value of filled paper, and the results are shown in Fig. 1.
Figure 1 shows that the LOI value of paper with no filler is about 19 %. Paper with no filler is very flammable because it is made from flammable wood fibrous raw materials. For paper with same filler composition, as filler loading increases, the flame-retardant property of paper is improved, as reflected by the increased LOI value of paper. When same filler loading is used in paper, the LOI value of paper loading APP-10 % DE filler is significantly higher than that of other paper loading composite fillers, reaches 25.2 %, and meets the basic requirement of fire resistance, when the loading of APP-10 % DE filler is 20 mass %.
The results of water solubility measurement show that APP-10 % DE filler has the lowest water solubility than other fillers, as shown in Table 1, and this explains the reason why APP-10 % DE filler has the best flame-retardant effect on paper. APP-DE filler with lower water solubility has higher filler retention in paper, resulting in better flame-retardant effect on paper.
Effect of surface modification on structure and properties of APP-10 % DE filler
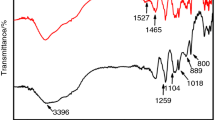
Coupling agent containing silica itself has certain water resistance and fire resistance and is usually used as surface modifier for APP and inorganic particles [14–16]. Figure 2 shows the FTIR spectra of APP-10 % DE filler before and after modification with KH550. The absorption bands at 2972 and 2928 cm−1 in Fig. 2a have been assigned to the stretching vibration of C–H group in KH550. In Fig. 2b, c, the absorption bands at 760, 680 and 600 cm−1 have been assigned to the stretching vibration of O=P–O, –OH and O–P–O groups, respectively, and the absorption bands at 1068 and 496 cm−1 have been assigned to the antisymmetric stretching vibration and bending vibration of Si–O–Si group. Comparing Fig. 2b with c, it can be known that the infrared spectrogram of APP-10 % DE has obviously changed after surface modification with KH550, and new absorption bands have occurred at 2920 and 2853 cm−1, which are corresponding to the asymmetric and symmetrical stretching vibration of C–H group, respectively. In addition, the absorption Si–O–Si group at 1068 cm−1 has enhanced. These changes demonstrate that chemical reaction has occurred between KH550 and APP-10 % DE and new Si–O–Si bond has been successfully formed. The formation mechanism of Si–O–Si bond is shown in Fig. 3. The silanol group [–Si(OH)3] produced by the hydrolysis of silane coupling agent can react with the silicon hydroxyl on the surface of DE to form Si–O–Si covalent bond by condensation. At the same time, silanol group can also associate each other to form network film and cover the surface of DE, which makes the surface organic and improves its hydrophobicity [17].
Figure 4 presents the XRD patterns of APP, DE, APP-10 % DE and APP-10 % DE-KH550. The diffraction peak appearing at the 2θ of 14.82 ° in Fig. 4a is the characteristic diffraction peak of APP-I, and the diffraction peak appearing at the 2θ of 21.88 ° in Fig. 4b is the characteristic diffraction peak of DE. The maximum intensity diffraction peak position of APP-10 % DE, in Fig. 4c, is consistent with that of APP, except for a small peak occurring at near the 2θ of 21.88 °, indicating that in situ polymerization of APP and DE has barely changed the crystal form of APP. The diffraction peak position of APP-10 % DE-KH550 is coincident with that of APP-10 % DE, but surface modification with KH550 causes a significant increase in XRD diffraction peak intensity at the 2θ of 14.82 °, as shown in Fig. 4d. KH550 functions as a molecular bridge between APP and DE, improves the crystallization of APP-10 % DE filler, and thus improves its stability. The functional mechanism of KH550 is shown in Fig. 5. The Si–O– group on the surface of silane coupling agent reacts with the silicon hydroxyl on the surface of DE to form Si–O–Si bond, and the –NH– group on the surface of silane coupling agent forms intermolecular hydrogen bond with the hydroxyl group on the surface of APP. These reactions can reduce water solubility of APP-10 % DE filler and make it have good hydrophobicity and flame-retardant property [18].
Water solubility measurement shows that water solubility of APP-10 % DE reduces from 1.15 g/100 mL H2O to 0.97 g/100 mL H2O after modification; that is to say, surface modification with KH550 can improve the stability of APP-10 % DE filler in water and decrease its sewer loss during papermaking process.
The TG, DTG and DSC curves of APP-10 % DE and APP-10 % DE-KH550 are shown in Fig. 6, and the relevant characteristic parameters are listed in Table 2. TG curves show that both APP-10 % DE and APP-10 % DE-KH550 have two main mass loss phases when being heated from 20 to 800 °C. The first phase appears at the temperature range of 280–550 °C, and the mass loss is mostly due to the release of NH3 and H2O and the crosslinking of P-OH to form polyphosphoric acid [19]. The second phase occurs at the temperature range of 550–700 °C, which can be attributed to the release of phosphoric acid, polyphosphoric acid and meta-phosphoric acid [20]. The initial decomposition temperature of APP-10 % DE- KH550 is the same as that of APP-10 % DE; however, the mass loss of APP-10 % DE-KH550 at 550 °C is 5 % higher than that of APP-10 % DE in the first phase; this is due to the combustion of KH550 and the pyrolysis of impurities brought in the process of modification [21]. Two peaks of mass loss rate are observed before 550 °C (at 343 and 383 °C) in the DTG curve of APP-10 % DE-KH550: one attributes to the release of NH3 and H2O, and the other one attributes to the combustion of KH550. DSC curves show that APP-10 % DE-KH550 has lower endothermic peak before 550 °C than APP-10 % DE (the heat enthalpy of APP-10 % DE-KH550 is 111.5 J g−1, while that of APP-10 % DE is 161.6 J g−1), and this phenomenon is due to the combustion heat release of KH550, which further confirms the presence of KH550 combustion and gives the reason why APP-10 % DE-KH550 has higher mass loss in this phase. The final residue of APP-10 % DE-KH550 is slightly higher than that of APP-10 % DE because the peak mass loss rate of APP-10 % DE-KH550 (4.95 % min−1) is significantly lower than that of APP-10 % DE (6.64 % min−1) in the second mass loss phase. Therefore, surface modification of APP-10 % DE with KH550 slows its mass loss at high temperature and improves its thermal stability, which is favorable for its flame-retardant effect.
Effect of APP-DE fillers on the flame-retardant properties of paper
The LOI values of paper loading 20 mass % APP-10 % DE and APP-10 % DE-KH550 fillers are shown in Fig. 7. It can be seen that the LOI values of paper loading APP-10 % DE-KH550 are 2 % higher than those of paper loading APP-10 % DE.
Cone calorimeter tests were carried out for paper with no filler, with 20 mass % APP-10 % DE and APP-10 % DE-KH550 fillers at a heat flux of 30 kW m−2, and the heat release rate (HRR, kW m−2) is shown in Fig. 8; the time to ignition (TTI, s), peak heat release rate (PHRR, kW m−2), time to PHRR (TPHRR), total heat released (THR, MJ m2), mass loss (ML, %)) and mean mass loss rate (mean MLR, g s−1) are shown in Table 3.
It can be found that all three samples burn out within 100 s after ignition. The TTI of paper with no filler is 18 s, and the PHRR occurs at 28 s with a PHRR of 73.7 kW m−2 and a THR of 2.05 MJ m−2. However, the paper loading APP-10 % DE and APP-10 % DE-KH550 show dramatic drop of the PHRR and delay of the TTI and the tPHRR. The PHRR and THR for paper loading APP-10 % DE-KH550 is 33.9 kW m−2 and 1.07 MJ m−2, which are lower than 41.0 kW m−2 and 1.22 MJ m−2 for paper loading APP-10 % DE. And also the TTI and tPHRR for paper loading APP-10 % DE-KH550 prolong to 24 s and 33 s, longer than 23 s and 31 s for paper loading APP-10 % DE. The PHRR is a very important parameter used to express the intensity of fire [22], and the ratio of TTI to PHRR is often used to predict whether a material can easily develop drastic combustion after ignition, which is designated as fire performance index (FPI) [23]. The greater the FPI value, the better is the fire resistance [24]. It can be known from Table 3 that paper loading APP-10 % DE-KH550 has the greatest value of FPI (0.708) and paper without it has the lowest value of FPI (0.244), and the PFI value (0.561) of paper loading APP-10 % DE is in between.
Mass loss rate is another important parameter to express the flammability of a material. Table 3 also shows that the ML and mean MLR of paper with no filler are 99.6 % and 0.0053 g s−1, much higher than those of paper loading APP-10 % DE and APP-10 % DE-KH550. The ML and mean MLR of paper loading APP-10 % DE-KH550 are 72 % and 0.0038 g s−1, which are lower than those of paper loading APP-10 % DE.
The data above indicate that loading APP-10 % DE composite filler significantly reduces the THR, ML and the MLR of paper and improves its flame-retardant properties. After modification with KH550, APP-10 % DE filler has better flame-retardant effect on paper.
APP-10 % DE composite filler in paper displays condensed phase flame retardation, and phosphoric acid dehydrating agent generated by APP decomposition under high temperature promotes the dehydration and charring of paper [25]. The compact char layer can efficiently isolate heat and oxygen, cut off further spread of temperature and flame and prevent paper from being further burned [26]. The photographs and FESEM images of the charred residues collected after the cone calorimeter tests are shown in Figs. 9 and 10, respectively. It can be observed that paper with no filler only leaves a small amount of residue (Fig. 9a), whereas the residue is gray and looses char layer with some blisters and cracks for paper loading APP-10 % DE (Fig. 9b) and black and compact char layer for paper loading APP-10 % DE-KH550 (Fig. 9c). Figure 10 shows that the charred residue of paper with no filler is very loose and porous, the charred residue of paper with APP-10 % DE-KH550 is very smooth, dense and strong, while the charred residue of paper with APP-10 % DE is in between. Therefore, surface modification of APP-10 % DE filler with KH550 is helpful to improve its flame-retardant effect on paper.
Conclusions
Addition of APP-DE composite fillers prepared by in situ polymerization improves the flame-retardant properties of paper. The optimal flame-retardant effect is achieved when the ratio of APP/DE in composite fillers is 100/10, and the LOI value of paper reaches 25.2 % when the loading of APP-10 % DE filler is 20 mass %.
Si–O–Si bonds occur between APP-10 % DE filler and KH550, and surface modification of APP-10 % DE filler with KH550 decreases its water solubility and improves its crystallization and thermostability. The LOI value of paper loading modified APP-10 % DE filler is 2 % higher than that of paper loading unmodified APP-10 % DE filler, and paper loading modified APP-10 % DE filler has lower PHRR, THR and mean MLR and higher residual char rate than paper loading unmodified APP-10 % DE filler. The addition of modified APP-10 % DE filler promotes paper to form strong and compact char layer during combustion, thereby isolates heat and oxygen and improves the flame-retardant properties of paper. Surface modification of APP-10 % DE filler with KH550 improves its flame-retardant effect on paper.
References
Hong L, Hu J, Xu GL, Liang Y. Preparation of flame-retardant air filter paper with ammonium polyphosphate. China Pulp Pap Ind. 2011;32:49–52.
Chang CP, Hung SC. Manufacture of flame retardant foaming board from waste papers reinforced with phenol-formaldehyde resin. Bioresour Technol. 2003;86:201–2.
Rie DH, Moon SW, Lim KB. Combustion and thermal properties of paper honeycomb. J Therm Anal Calorim. 2012;107:535–9.
Yang HS, Kim DJ, Kim HJ. Combustion and mechanical properties of fire retardant treated waste paper board for interior finishing material. J Fire Sci. 2002;20:505–17.
Simkovic I, White RH, Fuller AM. Flammability studies of impregnated paper sheets. J Therm Anal Calorim. 2012;107:519–26.
Shang CX, Yang S. Flame retardant effect of water-soluble ammonium polyphosphate on paper. Hangzhou Chem Ind. 2010;40:26–8.
Cui JF, Guo JH, Yang BP, Zhou YP. Investigation and preparation of sizing agent with waterborne epoxy bromine carbon resin for flame retardant paper. J Lanzhou Univ Technol. 2010;36:62–5.
Zhou H, Liu Z, Wei YJ. Preparation of fire-retardant paper using magnesium hydroxide as flame retardant. China Pulp Pap. 2009;28:13–6.
Wang SL, Huang JL, Chen FS. Study on Mg–Al hydrotalcites in flame-retardant paper preparation. Bioresource. 2012;7:997–1007.
Lewin M. Unsolved problems and unanswered questions in flame retardance of polymers. Polym Degrad Stab. 2005;88:13–9.
Zhang ZM, Wang SF. Water solubility and hydrolytic behavior of ammonium polyphosphate. Fire Sci Technol. 2001;4:42–3.
Li YT, Wang HX, Wang ZC, He JS. Study on synthetic technology of high efficiency flame retardant ammonium polyphosphat. Inorg Chem Ind. 2012;44:26–9.
ASTM international. Standard test method for heat and visible smoke release rates for materials and products using an oxygen consumption calorimeter. West Conshohocken: ASTM E1354-04a; 2004.
Xi Q, Chang L, Kuang SL. Study on ammonium polyphosphate surface modification with silane coupling agent. Adhes China. 2005;26(19–20):23.
Tan XM, Feng AS, Zhao HQ. Graft modification of SiO2 nano-particles with silane coupling agent. China Powder Sci Technol. 2011;17:14–7.
Xu H, Sun T. Surface modification of nanosized TiO2 with silane coupling agent. Paint Coat Ind. 2008;38:1–17.
Du GX, Zuo RF, Mei LF, Liao JH, Guo WJ. Surface modification of diatomite by silane coupling agent and its effects on the reinforcing efficiency of NR/SBR blend. Rare Metal Mater Eng. 2013;42(Suppl. 1):412–7.
Hao JW, Liu GS, Du JX, Yang RJ, Qiu XY, Zhu F. A study on the surface treatment of ammonium polyphosphate and its application in flame retardant polypropylene. Trans Beijing Inst Technol. 2009;29:556–9.
Camino G, Costa L, Trossarelli L. Study of the mechanism of intumescence in fire retardant polymers: part V—Mechanism of formation of gaseous products in the thermal degradation of ammonium polyphosphate. Polym Degrad Stab. 1985;12:203–11.
Gu JW, Zhang GC, Dong SL, Zhang QY, Kong J. Study on preparation and fire-retardant mechanism analysis of intumescent flame-retardant coatings. Surf Coat Tech. 2007;201:7835–41.
Shan FR, Yu ZM, Luo LS, Zhang Y. Study on surface modification of nano-alumina by silane coupling agent KH550. New Chem Mater. 2013;41(169–170):185.
Hirschler MM. Heat release from plastic materials. In: Babrauskas V, Grayson SJ, editors. Heat release in fires, chapter 12a. London: Elsevier; 1992. p. 375–422.
Ou YX, Chen Y, Wang XM. Flame retarded polymeric materials. Beijing: National Defence Industry Press; 2001.
Ye L, Qu BJ. Flammability characteristics and flame retardant mechanism of phosphate-intercalated hydrotalcite in halogen-free flame retardant EVA blends. Polym Degrad Stab. 2008;93:918–24.
Pan LL, Li GY, Su YC, Lian JS. Fire retardant mechanism analysis between ammonium polyphosphate and triphenyl phosphate in unsaturated polyester resin. Polym Degrad Stab. 2012;97:1801–6.
Qu BJ, Xie RC. Intumescent char structures and flame-retardant mechanism of expandable graphite-based halogen-free flame-retardant linear low density polyethylene blends. Polym Int. 2003;52:1415–22.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sha, LZ., Chen, KF. Surface modification of ammonium polyphosphate-diatomaceous earth composite filler and its application in flame-retardant paper. J Therm Anal Calorim 123, 339–347 (2016). https://doi.org/10.1007/s10973-015-4941-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4941-1