Abstract
The aim of this study was to evaluate the physical quality of Tabebuia caraiba (Mart.) Bur. powder, separately in three batches of different particle sizes, through the adapted Ozawa method. The Ozawa model was used for thermogravimetry (TG) data analysis in dynamic synthetic air and nitrogen atmospheres in different heating rates (5, 10, 20 and 40 °C min−1). The software TA-50 was used to data treatment. Kinetic data showed different values of activation energy and same reaction order according to the particle powder sample size in a specific model (derivative or tangent) of data treatment. The difference factor (F 1) was used to compare the parallelism line of the samples constructed by Ozawa graphs. Different kinetic results discriminate the particles size of the samples. TG technique allowed discriminating different particle sizes of herbal medicine powder.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tabebuia (Bignoniaceae) plants are native of Central and South America and are widely used in folk medicine. Their extracts are employed for the treatment of ulcers and other gastrointestinal disorders, syphilis, candidiasis, prostatitis, allergies and among others. The major constituents include quinones, furanonaftoquinonas, benzoic acid and flavonoids [1].
Tabebuia caraiba (Mart.) Bur., known as craibeira, caraíba, paratudo-do-campo, carobeira and craíba, is a regular-sized tree that can reach up to 20 m when in good soil and moisture conditions, naturally occurring in the scrub, savanna and wetland [2]. It is a native semiarid plant used in folk medicine often reported in the literature, mainly for its expectorant and antipyretic action. However, studies investigating the nature of these activities are scarce [3].
The commercial value of the plant derivatives is growing, making the safety, efficacy and quality of products become constant concerns for regulation authorities [4].
The main desirable characteristics of medicinal plants are effectiveness, low-risk use, as well as reproducibility and consistency of its quality [5]. The technical information about the most medicinal plants, regarding the characteristics mentioned above, is still insufficient [6].
The dry plant milling process is a critical step in the production of phytotherapic drugs intermediaries, due to its role in particle size control [7]. The particle size distribution of the powders should be considered for physical, chemical and biological characterization, aiming its standardization and to increase quality of popular use of herbal drugs and their derivatives, suggesting that it is necessary to develop methodologies to discriminate particle powder size.
Analytical methods have been used to characterize compounds from herbal medicine, its products and extracts. Thermal analysis such as thermogravimetry (TG) is a technique of high sensitivity, reproducibility and rapid response to variations in mass, obtaining results related to the composition and thermal stability of the sample, being important to the characterization of raw plant [8, 9].
The kinetic of Ozawa obtained from the dynamic TG data has been employed to evaluate the kinetic parameters: activation energy (E a), frequency factor (A) and reaction order (n), using the decomposed fraction (alpha) between the start and end of the step of decomposition [10].
Ozawa kinetic model was already used to differentiate pharmaceutical raw material physical quality. Recent research showed that Ozawa method through kinetic parameters (reaction order) differentiated three simvastatin raw material batches [11].
The aim of this work was to evaluate the physical quality of herbal medicine (Tabebuia caraiba) samples with different particle size powders, using the adapted Ozawa method through different data treatment models.
Materials and methods
Raw material
The raw material used was harvested in the municipality of Conchichola, PB, with the support of the Empresa de Assistência Técnica e Extensão Rural da Paraíba (EMATER-PB).
The plant was identified as Tabebuia caraiba by comparison with voucher number EAN 566, 1318, 12,278 and 8306 stored in the Prof. Jayme Coelho de Moraes herbarium, by the botanic Prof. Dr. Leonardo Félix.
Powder preparation
The leaves were dehydrated in a stove at 40 °C. The dried leaves were milled using a knife mill, with four fixed and four rotating knives (Willey, Marconi), coupled to a 10-mesh sieve.
The different particle sizes were segregated using a sieve set with distinct mesh: 48, 100 and 200 µm. Each granulometric fraction was stored in a frosted white polyethylene container, properly sealed and protected from light humidity at room temperature, averaging 25 °C.
Particle size determination
SEM images for particles of different granulometries were taken. The model of microscope used was TM-1000, from Hitachi.
Thermogravimetric characterization
The dynamic thermogravimetric curves of the different particle sizes of herbal medicine, namely CAR 01, CAR 02 and CAR 03, were obtained in a thermobalance (Shimadzu, Model TGA-50) using an alumina crucible. The instrument was calibrated with calcium oxalate monohydrate. The temperature range used was 25–900 °C in the heating ratios of 5, 10, 20 and 40 °C min−1 under synthetic air and nitrogen flow of 20–50 mL min−1, respectively. The sample mass was 5.0 ± 0.1 mg. Data were initially analyzed using the software Tasys, Shimadzu.
Kinetic parameters determination
The reaction order (n) and activation energy (E a) were determined using the Ozawa model with the data of TG dynamic atmospheres of synthetic air and nitrogen, using the software TA-50 (TGA Kinetic Analysis Program).
The determination of the temperature range (start and end) to obtain the mass loss percentual of the determining step (to apply the model of Ozawa in different heating rates) was obtained by two different data collection types:
-
1.
Tangent
-
2.
First derivative.
Thermal decomposition kinetic interpretation
The Ozawa graph, log β versus 1/T, the temperature (T) in Kelvin, was obtained using the TA-50 software. For parallelism, evaluation was determined by the difference factor (F 1), using the mathematical equation below:
Results and discussion
The powders particle size distribution should be considered for physical, chemical and biological characterization of raw materials, its standardization increases the safety/quality popular use of herbal drugs and their derivatives.
Herbal medicine powder, its products and extracts have been characterized by analytical methods. Scanning electron microscopy (SEM) is used to characterize the morphology and particle size of herbal medicine powder [12, 13]; thermal analysis such as thermogravimetry (TG) and differential thermal analysis (DTA) together with pyrolysis coupled to gas chromatography/mass spectrometry is employed to evaluate the quality of pharmaceuticals [14–16], as well as atomized extracts of herbal medicine like E. mulungu [17] and distinct brands and batches of Cymbopogon citratus teas [18].
Granulometry
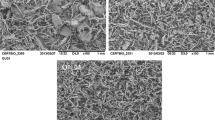
Figure 1 shows the micrographs from samples CAR 01, 02 and 03. The diversity of particles morphology confirms the process limitations to produce a uniform plant powder.
Assessing SEM images, it was possible to verify the heterogeneity in particle sizes into the same batch (same mesh range) and its morphological diversity of the herbal medicine powder, which demonstrates limitations in the plants processes to obtain a uniform powder.
The determination of particle size by SEM obtained by sieving process allowed the perception of different particle sizes of the powders for CAR samples. It is evident the difference between the samples CAR 01 to CAR 03, for example. The characterization by SEM could detect particle size and morphology anomalies. Search for herbal medicine technology powder characterization has importance because influences the derivatives and plant products preparations for therapeutic use of the population.
Use the process of sieving to minimize the granulometric differences into a raw material obtained after milling is a way to try to standardize the range mesh of the particle, despite the full uniformity is not achieved. It is still necessary to use techniques like SEM and others to its characterization.
The particle size determination by granulometry sieves cannot ensure that the particles sizes are smaller than the stated mesh. SEM enabled us to detect the existence of divergences from the expected results, allowing a better quality evaluation of the herbal medicine powder production technology [19].
Thermogravimetric characterization of herbal medicine
Recent study showed through TG and others techniques that herbal medicine particle size powder samples demonstrate differences in its analytical signal, showing that samples in different granulometries could have different physical and chemical qualities [19].
Evaluating the thermogravimetric profile of the herbal medicine Caraibeira (CAR 01, CAR 02 and CAR 03) in a nitrogen atmosphere and using the derivative model, the following thermal behaviors were observed according to the different heating rates (which allow the applicability of Ozawa’s method). In a heating rate of 5 °C min−1, five decomposition steps were observed; the main one occurred in the temperature range of 169.8–341.6 °C, with higher mass loss corresponding to 42.7 % of the total mass for the samples CAR 01 and CAR 02. At a heating rate of 10 °C min−1, there were four decomposition steps for CAR 01 and CAR 02 and five for CAR 03. The temperature range was from 191.8 to 350.5 °C, with a maximum mass loss of 41.7 %, corresponding to the sample CAR 01. The results to the heating rate of 20 °C min−1 to the same samples showed the presence of three decomposition steps for all studied samples. The major step occurred in the range of 199.7–370.1 °C, with a maximum mass loss of 45.6 %, attributed to the sample CAR 01. The samples submitted to a heating rate of 40 °C min−1 from demonstrated three decomposition steps; the main step occurred in the range of 212.6–383.4 °C with maximum mass loss of 47.8 %, related to CAR 02.
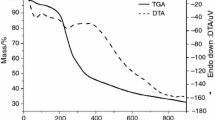
Figure 2 illustrates thermogravimetric curves for herbal medicine Caraibeira powder samples, in different particle sizes (CAR 01, CAR 02 and CAR 03), submitted to different heating rates (5, 10, 20 and 40 °C min−1) and different atmospheres: nitrogen and synthetic air.
The results obtained in the oxidative atmosphere for the samples evaluated (CAR 01, CAR 02 and CAR 03) showed different thermogravimetric profiles according to its different heating rates. In the heating rate of 5 and 40 °C min−1, the curves showed five decomposition steps. To 5 °C min−1, the main step for the three samples occurred in the range of 160.6–329.9 °C and the maximum mass loss corresponded to 39.8 % of the total mass. In the heating rate of 10 °C min−1, the temperature range to the main step was of 202.8–344.9 °C, with a maximum mass loss to the sample CAR 02, corresponding to 41.1 % of the total mass. In the heating rate of 20 °C min−1, the major step occurred in the range of 197.3–358.5 °C, with a maximum mass loss of 41.0 %, corresponding to the sample CAR 02. In the ratio of 40 °C min−1, the range of the main step was 207.6–371.3 °C with maximum loss for CAR 03 sample, corresponding to 44.3 % of the initial mass.
The first decomposition step refers to the volatiles, mainly water, to all samples in all conditions. The second step represents the macro- and micro-compounds degradation. The last loss mass step correlates with the no degradable residues, inorganic constituents.
Fewer overlapping mass loss events were observed to the samples submitted to lower heating rates, distinguishing more decomposition steps, which begin at lower temperatures than the others at higher heating rates. The crescent heating rates increase the initial temperature of the main steps.
The two atmosphere conditions used showed differences in the thermal profile of the samples. Like the derivative model of data treatment, through the tangent model of calculation, the variations of temperature range (main step) between the different heating rates, sample particle sizes and mass losses were observed. These data obtained were used to determine the kinetic parameters by Ozawa method.
Determination of the reaction order and activation energy
The reaction order of the samples CAR 01, CAR 02 and CAR 03 showed the same values using models of data treatment by tangent and first-derivative curve methods, analyzed in different atmospheres conditions. The reaction order determined was zero order for all the samples.
The activation energy showed different values using tangent and first-derivative curve, in an inert and oxidative atmosphere. Comparing the two models of treatment into the air synthetic atmosphere condition, the obtained activation energy values (kJ mol−1) were of the following order: CAR 02 > CAR 01 > CAR 03. The values obtained had a variation of 130.19–195.33 and 136.54–200.45 kJ mol−1, corresponding to tangent and derivative, respectively. Analyzing the same samples in an inert atmosphere of nitrogen, the obtained values of activation energy according to the different granulometries were not of the same order. Tangent treatment results followed the order: CAR 03 > CAR 02 > CAR 01. The order followed by derivative model was CAR 02 > CAR 03 > CAR 01. Through this condition, bigger particle size sample powder showed lower activation energy. These values vary from 213.3 to 186.6 kJ mol−1 (tangent) and 178.9 to 169.18 kJ mol−1. Through this condition, smaller particle size sample powder showed lower activation energy.
Tables 1 and 2 show the activation energy for each sample by Ozawa method into inert and oxidative atmospheres.
Comparing the activation energy between the two data treatment models in an inert condition, differences in result were observed. The tangent model showed bigger values than derivative. The values obtained to oxidative condition were similar when compared the models.
Activation energy showed different values between sample particle size, model of treatment and atmosphere condition. The samples in inert conditions required more energy to react and to decompose.
Activation energy as a function of different particle sizes for Caraibeira increases only in a nitrogen atmosphere for the tangent method. While in synthetic air atmosphere, a decrease in the energy was observed. Figures 3 and 4 show a non-uniformity and variation in the particle decomposition process of Caraibeira.
The results showed that the thermal decomposition was not similar to the herbal medicine studied in different granulometries. The activation energy values of the samples (CAR 01, CAR 02 and CAR 03) varied when submitted to the same conditions and data treatment.
Beyond differentiate the samples of different particle sizes according to TG data and its derived kinetic results, it was applied F 1 model to the parallel lines obtained from Ozawa’s method to each sample. It was done a correlation between the two heating rates results (5–10; 10–20; 20–40), the ratio between them was of two times. Differences between the samples were verified.
According to the comparative parallel line results, it was possible to discriminate the different particle size samples. In all conditions studied and ratios relations used, F 1 values were not similar between the samples.
The values of F 1, generated from the Ozawa graph data, log β versus 1/T, calculated by the derivative method, in an atmosphere of nitrogen and synthetic air are represented by Figs. 5 and 6. The data analysis shows that there is no similarity between different particle size F 1 values.
Through the model used, different particle sizes of powder can be distinguished by thermogravimetry, what is interesting considering that previous works [6, 7] demonstrated the influence of herbal medicine particle size powder in its physical and chemical properties, pharmacological and toxicological effects.
The presented model is still being developed with the goal of being used to sort through thermogravimetry the different particle sizes, which will be interesting, considering that previous studies [19] demonstrated the influence of the herbal medicine powder particle size in the physical, chemical, pharmacological and toxicological effects.
The development of analytical methodologies that help in the characterization of herbal drugs is an alternative to the standardization of powdered herbal drugs. Differences in particle size of the material may result in products with different compositions.
The particle size distribution influences significantly at various stages of production; thus, its determination is a critical step in all processes that somehow involve materials as powders.
Conclusions
TG allows the discrimination of different particle size samples of herbal medicine. Modified Ozawa method could be used as an auxiliary tool to differentiate different granulometry powders through the interpretation of the kinetic data. It was concluded that thermal analysis using adapted Ozawa method constitutes an analytical method to evaluate physical quality of herbal medicine.
References
Lemos OA, Sanches JCM, Silva IEF, Silva MLA, Vinhólis AHC, Felix MAP, Santos RA, Cecchi AO. Genotoxic effects of Tabebuia impetiginosa (Mart. Ex DC.) Standl. (Lamiales, Bignoniaceae) extract in Wistar rats. Genet Mol Biol. 2012;35:498–502.
Lorenzi H, Matos FJA. Plantas Medicinais no Brasil: Nativas e Exóticas Cultivadas. Vol II. Nova Odessa, SP: Instituto Plantarum, 2002.
Coelho AAM, Paula JE, Espíndola LS. Efeito de extratos de plantas do Cerrado em Dipetalogaster máxima. Rev Bras Entomol. 2009;3:444–51.
Carvalho ACB, Santos LA, Silveira D. La regulación de los medicamentos herbarios en Brasil. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas. Bol Latinoam Caribe Plant Med Arom. 2008;8:7–11.
Nakazawa TA. Particularidades de formulações para fitoterápicos. Rev Racine. 1999;53:38–41.
Calixto JB. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents). Braz J Med Biol Res. 2000;2:179–89.
Júnior Silva. JOC. Brazil: Universidade de São Paulo; 2006. p. 120.
Skoog DA, Holler FJ, Nienman TA. Principles of instrumental analysis. 5th ed. USA: Harcourt Brace & Company Philadelphia; 1998.
Silva EC, Paola MVRV, Matos JR. Análise Térmica Aplicada à Cosmetologia. Rev Bras Ciên Farm. 2007;47:347–56.
Boer TM, Procópio JVV, Nascimento TG, Macêdo RO. Correlation of thermal analysis and pyrolysis coupled to GC–MS in the characterization of tacrolimus. J Pharm Biomed Anal. 2013;73:18–23.
Santos AFO, Procópio JVV, Moura EA, Correia LP, Pinto MF, Macêdo RO. Ozawa kinetic model application for physical quality differentiation of simvastatin raw material. Lat Am J Pharm. 2013;32:927–30.
Wang C, Wang Z, Pan Y, Sun XZ. Application of pyrolysis-gas chromatography and hierarchical cluster analysis to the discrimination of the Chinese traditional medicine Dendrobium candidum Wall. ex Lindl. J Anal Appl Pyrol. 2011;90:13–7.
Yuan M, Zhang MG, Yuan P, Zeng Z, Yan CL. Pyrolysis-gas chromatography fingerprint with fuzzy cluster analysis for Curcuma aromatica Salisb., Chinese. J Chromatogr. 2003;21:469–71.
Procópio JVV, Souza VG, Costa RA, Correia LP, Souza FS, Macêdo RO. Application of thermal analysis and pyrolysis coupled to GC/MS in the qualification of simvastatin pharmaceutical raw material. J Therm Anal Calorim. 2011. doi:10.1007/s10973-010-1274-y.
Moura EA, Correia LP, Pinto MF, Procópio JVV, Souza FS, Macêdo RO. Thermal characterization of the solid state and raw material fluconazole by thermal analysis and pyrolysis coupled to GC/MS. J Therm Anal Calorim. 2010;100:289–93.
Fernandes FHA, Santana CP, Santos RL, Correia LP, Conceição MM, Macêdo RO, Medeiros ACD. Thermal characterization of dried extract of medicinal plant by DSC and analytical techniques. J Therm Anal Calorim. 2013;113–2:443–7.
Macêdo RO, Tecnologia analítica baseada na pirólise acoplada à cromatografia gasosa/espectrometria de massa para caracterização e obtenção de compostos químicos a partir de extratos de Erythrina mulungu Linné secos por nebulização, PI0800530-3 A2, 2010.
Oliveira EJ, Alvarez EDA, Lima NGPB, Macêdo RO. Usefulness of pyrolysis coupled to gas chromatography/mass spectrometry for evaluating the reproducibility of commercial samples of Cymbopogon citratus Stapf. Poaceae. Braz J Pharmacogn. 2010;20:93–9.
Correia LP, Procopio JVV, Santana CP, Santos AFO, Cavalcante HMM, Macedo RO. Characterization of herbal medicine with different particle sizes using pyrolysis GC/MS, SEM and thermal techniques. J Therm Anal Calorim. 2013;111:1691–8.
Acknowledgements
To CNPq, for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Correia, L.P., Procópio, J.V.V., Santana, C.P. et al. Herbal medicine physical quality evaluation by thermal analysis using adapted Ozawa method. J Therm Anal Calorim 122, 207–214 (2015). https://doi.org/10.1007/s10973-015-4638-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4638-5