Abstract
The thermal decomposition behaviors of 4,10-dinitro-2,6,8,12-tetraoxa-4,10-diazaisowutrzitane (TEX) were studied by using a C-500 type Calvet microcalorimeter at five heating rates. The kinetic and thermodynamic parameters of exothermic decomposition reaction were obtained. The specific heat capacity of TEX was determined with a Micro-DSC III calorimeter, the specific heat capacity equation and the molar specific heat capacity were also obtained. The self-accelerating decomposition temperature, adiabatic decomposition temperature rise, and critical temperature of thermal explosion are 512.69, 7115.59, and 527.42 K, respectively. The adiabatic time-to-explosion is calculated to be a certain value between 53.69 and 54.05 s.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
To meet on the demands of the various weapon and equipment development, new materials have focused not only on high energy but also on low sensitivity to external stimuli [1–4]. 4,10-dinitro-2,6,8,12-tetraoxa-4,10-diazaisowutrzitane(TEX) is a new highly energetic and insensitive explosive with a cage structure. TEX has a high density (ρ = 1.99 g cm−3), high thermal stability (decomposition sets on above 240 °C), and low sensitivity to external stimuli (impact sensitivity H 50 = 23 J, friction sensitivity >360 N, sensitivity to electric spark 6.7 J). In addition, it exhibits desirable explosion parameters such as a high detonation rate (8170 ms−1) and a high detonation pressure (p cj = 31.4 GPa) [5–8]. Many papers have been concerned with the synthesis and properties, and applications in explosives [9–13], but the studies of thermal behavior and safety have not been reported by using microcalorimetry method.
In the present paper, the thermal decomposition behaviors of TEX were investigated by a C-500 type Calvet microcalorimeter at five heating rates. The self-accelerating decomposition temperature, the adiabatic decomposition temperature rise, the critical temperature of thermal explosion, and the adiabatic time-to-explosion are also obtained at the same time. The results can provide helpful information for its applications in explosive and propellant in the future.
Experimental
Sample
The sample(TEX) used in the investigation was prepared by Beijing Institute of Technology and Xi’an Modern Chemistry Research Institute and kept under vacuum before use. Its purity was higher than 99.5 %.
Equipment and conditions
The specific heat capacity of TEX was determined with a Micro-DSC III calorimeter (SETARAM, France). The thermal decomposition processes were measured by using a C-500 type Calvet microcalorimeter (SETARAM, France), which has a high sensitivity and was equipped with two 10 mL-vessels. The precision of enthalpy measurement was better than 0.5 % after calibration with the standard materials of In and Sn.
Results and discussion
Thermal decomposition behavior
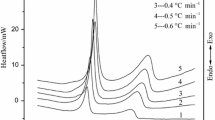
The heat flow curves of TEX at different heating rates are shown in Fig. 1, which shows only one exothermic peak. The characteristic temperatures obtained by heat flow curves at different heating rates are listed in Table 1.
In order to obtain the apparent activation energy (E) and the pre-exponential constant (A) of the thermal decomposition for TEX, The multiple heating methods (Kissinger method and Ozawa method) [14–16] were employed. Kissinger equation and Ozawa equation are as follow [17, 18]
where β i is the linear heating rate(K min−1), T pi represents the peak temperature of the thermal decomposition process (K), R is the gas constant (J mol−1 K−1).
From the original data in Table 1, the values of E and A obtained by Kissinger method (with a subscript of k) and Ozawa method (with a subscript of o) are listed in Table 2.
From Table 2, one can see that the apparent activation energy obtained by Kissinger method is in good agreement with that obtained by Ozawa method, and the linear correlation coefficients are all close to one, which means that the results is reliable.
The specific heat capacity of TEX
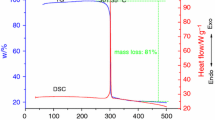
Figure 2 shows the determination results of specific heat capacity of TEX using continuous specific heat capacity mode of a Micro-DSC III apparatus. The sample mass was 413.71 mg and the heating rate was 0.2 °C min−1 from 10 to 80 °C. In determined temperature range, specific heat capacity of TEX presents a good linear relationship with temperature and the linear correlation coefficient is 0.9993. The equation [19, 20] describing the relationship can be written as
The molar specific heat capacity is 0.916 J g−1 K−1 at 298.15 K.
Self-accelerating decomposition temperature (T SADT)
The value T e0 and T p0 of T e and T p corresponding toβ →0 can be obtained by substituting the T ei and T pi from Table 1 into Eq. 4 and the self-accelerating decomposition temperature (T SADT) [21, 22] can be also obtained by Eq. 5.
where b and c are coefficients.
The values of T e0 (T SADT) and T p0 are 512.69 and 526.89 K, respectively.
Thermodynamic parameters of activation reaction
With the help of the data in Table 2, the entropy of activation (ΔS ≠), the enthalpy of activation (ΔH ≠), and the Gibbs free energy of activation (ΔG ≠) [23] are obtained by Eqs. 5–7 taken from as 40.19 Jmol−1 K−1, 152.27 kJ mol−1, and 131.09 kJ mol−1 corresponding to T = T po = 526.89 K, E = E k = 156650 Jmol−1, A = A k = 1015.14 s−1. The value of ΔG ≠ is positive, showing that the thermal decomposition reactions of TEX will not take place until the proper temperature is reached.
where k B is the Boltzmann constant and h is the Planck constant.
Adiabatic decomposition temperature rise (ΔT ad)
The adiabatic decomposition temperature rise is defined as ΔT ad. It can be obtained by Eq. 9 [19].
where H d is the quantity of heat released from the thermal decomposition, and C p is the specific heat capacity of TEX.
The specific heat capacity of TEX was C p = 0.916 J g−1 K−1 at 298.15 K, H d was obtained from the average of ΔH at different heating rates, and the value is −6517.88 J g−1.
Critical temperature of thermal explosion (T b)
The critical temperature of Thermal explosion (T b) is an important parameter of evaluating the safety and elucidating transition tendency from thermal decomposition to thermal explosion for small-scale EMS.
For TEX, the value of T b obtained from Eq. 10 [19, 20] using the value of T e0, and the value T b is 527.42 K.
Adiabatic time-to-explosion
Energetic materials need a time from the beginning thermal decomposition to thermal explosion in the adiabatic conditions. The time is named the adiabatic time-to-explosion. The adiabatic time-to-explosion is an important parameter for assessing their thermal stability and the safety. It can be calculated by the following Eqs. 11–14 [21, 23–25].
where t is the adiabatic time-to-explosion(s), C p is the specific heat capacity (J g−1 K−1), A is the pre-exponential constant (s−1), Q is the exothermic value (J g−1), E is the apparent activation energy (J mol−1), R is the gas constant (J mol−1 K−1), α is the fraction of conversion, f(α) is the decomposition reaction mechanism function and the limit of temperature integral is from T e0(K) to T b(K).
The combination of Eqs. 11–14 can give the following equation:
For TEX, C p (J g−1 K−1) = 0.196 + 2.414 × 10−3 T, Q = 6511.88 J g−1, A = 1011.54 s−1, E = 156650 J mol−1, T e0 = 512.69 K, T b = 527.42 K, then t = 53.69 s (n = 0), t = 53.88 s (n = 1), and t = 54.05 s (n = 2). We can see that the adiabatic time-to-explosion is little affected by the reaction order, so the adiabatic time-to-explosion of TEX is a certain value between 53.69 and 54.05 s.
Conclusions
-
1.
The thermal decomposition behaviors of TEX were studied by a C-500 type Calvet microcalorimeter at five heating rates and the values of enthalpy were calculated accurately. The apparent activation energy (E) and the pre-exponential constant (A) of the thermal decomposition for TEX were determined by using Kissinger method and Ozawa method.
-
2.
The standard molar specific heat capacity of TEX is 0.916 J g−1 K−1 at 298.15 K, and the equation describing values of the specific heat capacity versus temperatures is C p (Jg−1 K−1) = 0.196 + 2.414 × 10−3 T (283.15 K < T < 353.15 K).
-
3.
The values of the thermodynamic parameters of activation reaction ΔS ≠, ΔH ≠, ΔG ≠ are 40.19 J mol−1 K−1, 152.27 kJ mol−1, and 131.096 kJ mol−1, respectively.
-
4.
The self-accelerating decomposition temperature, the adiabatic decomposition temperature rise, and the critical temperature of thermal explosion of exothermic decomposition reaction are 512.69, 7115.59, and 527.42 K, respectively. The adiabatic time-to-explosion of exothermic decomposition reaction is a certain value between 53.69 and 54.05 s.
References
Dong HS. The development and countermeasure of high energy density materials. Chin J Energ Mater. 2004(Supplement):1–11.
Larry CW. Conducting polymers for improved impact and friction sensitivities of composite propellants for guided tactical missiles. US 6521063, 2003.
Nakashita G, Kubota N. Energetics of nitro/azide propellants. Propellants Explos Pyrotech. 1991;16:177–81.
Li YB, Huang H, Li JS, Li HB. A new HMX-based low-sensitive high energy PBX explosive containing LLM-105. Chin J Explos Propellants. 2008;31:1–4.
Ramekrishman VT, Vedachalam M, Boyer JH. 4,10-dinitro-2,6,8,12-tetraoxa-4,10-diazatetracyclo [5.5.0.05,903,11] dodecane. Heterocycles. 1990;31:478–80.
Lei YP, Xu SL, Yang SQ, Zhang T. Progress in high energetic explosive: TEX. Chin J Energ Mater. 2006;6:467–70.
Vagenknecht J. TEX—a lova explosive. Chin J Energ Mater. 2008;8:56–9.
Vagenknecht J, Marecek P, Trzcinski W. Sensitivity and performance properties of TEX explosives. J Energy Mater. 2002;20:245–53.
Maksimowski P, Golofit T. 4,10-dinitro-2,6,8,12-tetraoxa-4,10-diazatetracyclo[5.5.0.05,903,11] dodecane synthesis. J Energy Mater. 2013;31:224–37.
Xu R, Zhou XQ, Ceng GY, Liu C. Study on the synthesis of TEX. Chin J Explos Propellants. 2006;29:26–8.
Karaghiosoff K, Klapotke TM, Michailovski A, Holl G. 4,10-dinitro-2,6,8,12-tetraoxa-4,10- diazaisowutrzitane(TEX): a nitramine with an exceptionally high density. Acta Crystallogr C. 2002;58:580–1.
Lund GK, Highsmith TK, Braithwaite PC, Wardle RB. Insentive high performance explosive compositions. US 5529649, 1996.
Vagenknecht J, Marecek P, Trzcinski WA. Sensitivity and performance properties of TEX explosives. J Energ Mater. 2002;3:245–53.
Koga N. Ozawa’s kinetic method for analyzing thermoanalytical curves. J Therm Anal Calorim. 2013;113:1527–41.
Rajeshwari P, Dey TK. Structural and thermal properties of HDPE/n-AlN polymer nanocomposites. J Therm Anal Calorim. 2014;118:1513–30.
Zhang ZY, Chen J, Liu HJ, Xiao CF. Applicability of Kissinger model in nonisothermal crystallization assessed using a computer simulation method. J Therm Anal Calorim. 2014;117:783–7.
Kissinger HE. Reaction kinetics in differential thermalanalysis. Anal Chem. 1957;29:1702–6.
Ozawa T. A new method of analyzing thermogravinetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Xu KZ, Zuo XG, Zhang H, Yan B, Huang J, Ma HX, Wang BZ, Zhao FQ. Synthesis and thermal behavior of a new high-energy organic potassium salt K(AHDNE). J Therm Anal Calorim. 2012;110:585–91.
Zhang Y, Wu H, Xu KZ, Qiu QQ, An T, Song JR, Zhao FQ. Nonisothermal decomposition kinetics, specific heat capacity, and adiabatic time-to-explosion of Zn(NH3)(FOX-7)2. J Therm Anal Calorim. 2014;116:817–23.
Xue L, Zhao FQ, Xing XL, Zhou ZM, Wang K, Gao HX, Yi JH, Xu SY, Hu RZ. Thermal behavior of 1,2,3-triazole nitrate. J Therm Anal Calorim. 2011;104:999–1004.
Yan B, Ma HX, Zhao NN, Mai T, Song JR, Zhao FQ, Hu RZ. Thermal behavior, non-isothermal decomposition reaction kinetics and thermal-safety evaluation on N-2,4-dinitrophenyl-3,3-dinitroazetidine under two different pressures. J Therm Anal Calorim. 2012;110:1253–7.
Hu RZ, Gao SL, Zhao FQ, Shi QZ, Zhang TL, Zhang JJ. Thermal analysis kinetics. 2nd ed. Beijing: Science Press; 2008.
Xu KZ, Chen YS, Wang M, Luo JA, Song JS, Zhao FQ, Hu RZ. Synthesis and thermal behavior of 4,5-dihydroxyl-2-(dinitromethylene)-imidazolidine(DDNI). J Therm Anal Calorim. 2011;105:293.
Liu Y, Jiang YT, Zhang TL, Feng CG, Yang L. Thermal kinetic performance and storage life analysis of a series of high-energy and green energetic materials. J Therm Anal Calorim. 2015;119:659–70.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21473131) and the Science and Technology Foundation of Grant (No. 9140C350301120C35131).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiao, LB., Zhao, FQ., Luo, Y. et al. Thermal behavior and safety of 4,10-dinitro-2,6,8,12-tetraoxa-4,10-diazaisowutrzitane. J Therm Anal Calorim 121, 839–842 (2015). https://doi.org/10.1007/s10973-015-4577-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4577-1