Abstract
The curing kinetics of the hexahydro-4-methylphthalic anhydride (MHHPA)/diglycidyl 1,2-cyclohexane dicarboxylate (CY184) epoxy resin system and MHHPA/CY184 epoxy/episulfide resin system (containing 2 mass% DMP-30 as an accelerator) was comparatively investigated by non-isothermal differential scanning calorimetry with a model-fitting Málek method and a model-free advanced isoconversional method of Vyazovkin, and the curing behavior was discussed based on the proposed curing mechanism. The results indicated that both of the MHHPA/CY184 epoxy resin system and MHHPA/CY184 epoxy/episulfide resin system fitted Šesták–Berggren model. The activation energy of MHHPA/CY184 epoxy/episulfide resin system was lower than that of MHHPA/CY184 epoxy resin system, suggesting that the episulfide resin has higher reactivity and can accelerate the reaction. The value of m in the kinetic model equation in MHHPA/CY184 epoxy/episulfide resin system is much smaller than that in MHHPA/CY184 epoxy resin system, indicating, unlike the MHHPA/CY184 epoxy resin system, MHHPA/CY184 epoxy/episulfide resin system has much less autocatalytic effect.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The structures of episulfide compounds are similar to that of epoxy compounds with the thiirane groups replacing epoxide groups only. Compared to the corresponding epoxy compounds, episulfide compounds exhibit some unique characteristics, such as higher curing reactivity and faster curing rate especially at lower temperature, low shrinkage rate, lower water absorption, higher refractive index, and better adhesive strength on copper [1, 2]. Consequently, their broad range of applications can be prospected in the area of composite materials, coatings, and adhesives.
Until now, most of the studies focused on the synthesis and properties of episulfide resins synthesized by using bisphenol A epoxy resin (DGEBA) and bisphenol F epoxy resin (DGEBF) [1–3], and some researchers proposed the curing mechanism of epoxy/episulfide resin system using amine as a hardener. Ku and Bell [4] studied the thermo-mechanical properties and water absorption of the bisphenol A type epoxy/episulfide–polyamide curing systems and pointed out the system could be applied for fast curing at room temperature. Bell et al. [5] studied the curing behavior of synthesized bisphenol A type episulfide resins with amine as a curing agent and confirmed the thiirane group reacted with amine prior to the epoxide group at relatively lower temperature. Tsuchida and Bell [6, 7] studied the curing mechanism and the storage of epoxy/episulfide/dicyandiamide, suggesting that compared with standard epoxy/dicyandiamide systems, epoxy/episulfide/dicyandiamide systems could cure at lower temperature.
On the other hand, the episulfide resins synthesized by using DGEBA and DGEBF have high viscosity and poor fluidity at room or low temperature, which to some extent restrict the valuable applications of episulfide resins. Consequently, the preparation of epoxy/episulfide resin with low viscosity at room or low temperature is significative and interesting. CY184 epoxy resin has low viscosity, which contains six-membered ring and glycidyl ester bond in structure and presents high reactivity, high cohesive strength, excellent electrical insulating and ultralow temperature properties. The episulfide resin synthesized from CY184 epoxy resin has low viscosity (lower than 1.8 Pa s−1) and exhibits higher curing reactivity, faster curing rate and curing capacity at low temperature. Therefore, CY184 epoxy/episulfide resin is prospected to have potential application in the area of composite materials, coatings and adhesives.
And to the best of our knowledge, most researchers focused on the curing mechanism of epoxy/episulfide resin system using amine as a hardener. The curing mechanism and the curing kinetics of epoxy/episulfide resin system using anhydride as a hardener have not been reported. Anhydrides are important curing agents for epoxy resins and have the characteristics like high curing temperature and long curing time. Decreasing the curing temperature is an attractive and interesting issue in epoxy/anhydride systems. And currently, the common way used to reduce the curing temperature is to raise the content of accelerators [8], which inevitably leads to the intense exothermic reactions. In this study, the thiirane resin was partially used in epoxy resin (the CY184 epoxy/episulfide resin system) to decrease the curing temperature and increase the curing speed because of the high reactivity of thiirane groups, which is a novel way without reports found before. The curing behaviors of both MHHPA/CY184 epoxy/episulfide system and MHHPA/CY184 epoxy resin system were investigated by the curing kinetics using DSC. And, the curing temperature and the curing degree parameters obtained from DSC measurement at various heating rates showed the curing reactivity of both systems. And, activation energy and reaction order of both systems could be obtained to explain their curing behaviors. The curing kinetics was simulated by Málek method.
Experimental
Materials
CY184 epoxy resin (equivalent epoxy mass 172 g mol−1) was purchased from Shanghai Quan Xin import and export trading co., Ltd. MHHPA and DMP-30 were both purchased from Meryer (Shanghai) Chemical Technology Co., Ltd. KSCN and ethanol were purchased from Beijing Chemical Works Co., Ltd.
Synthesis of CY184 episulfide resin
The CY184 episulfide resin was prepared by the reaction between CY184 epoxy resin and KSCN under microwave heating. The synthesis route is shown in Fig. 1. 9.7 g KSCN was dissolved in 50 mL deionized water, and 14.1 g CY184 was dissolved in 100 mL ethanol, and the molar ratio of KSCN and CY184 was 2.4. Both solutions were stirred by EUROSTAR 20 digital stirrers to get a homogeneous mixture before added into a 250 mL three-neck flask. The three-neck flask with stirring was heated to 328 K by microwave (100 W) for 30 min to complete the reaction. The product was poured into separating funnel and washed by toluene and deionized water several times separately. The upper layer was separated and dried by anhydrous MgSO4 for 24 h, and then the solvent was removed by rotary evaporation to get light yellow liquid.
Characterization of CY184 episulfide resin
FTIR
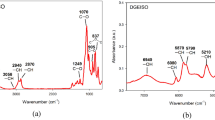
FTIR spectrum of CY184 episulfide resin was recorded by using a Nicole Nexus 670 FTIR spectrometer (USA) in the range of 4,000–400 cm−1 using KBr pellet. Figure 2 shows the result. It can be seen that the characteristic infrared absorption peak of epoxy group at around 916 cm−1 decreased remarkably, and the characteristic infrared absorption peak of episulfide group at around 617 cm−1 appeared showing the episulfide resin was synthesized.
ESI–MS
The CY184 episulfide resin was dissolved in methanol, and test was carried out on ESI–MS (Waters Corporation, USA). ESI–MS data: [M + 1]+= 285, 301, and 317, showing that the synthesized episulfide resin is the mixture of CY184 episulfide resin and CY184 epoxy resin and CY184 epoxy resin with one thiirane group.
Elemental analysis (EA)
The sample was investigated by the vario EL cube elemental analyzer under a test temperature of 1,223 K to determine the sulfur contents of the product. The elemental analysis revealed that the sulfur content was 10.3 mass%, and therefore the calculated sulfur conversion rate was 55.38 %.
DSC measurement
Epoxy and epoxy/episulfide were mixed with MHHPA in a certain ratio, respectively. The air bubbles of both systems were removed by using centrifuge. The non-isothermal reactions were investigated by using differential scanning calorimetry (DSC, Q2000, TA instruments). About 6 mg fresh resin mixtures were accurately measured, enclosed in an aluminum DSC crucible, and scanned from 298 to 573 K with an identical empty crucible as the reference. Dry nitrogen was used as protective gas, and the heating rates were controlled at 5, 10, 15, and 20 K min−1.
Curing kinetics
Curing kinetics is one important way to study the curing process of resins. DSC is a method to study thermal analysis kinetics. According to the DSC curves, the curing degree of resins and the relationships among reaction rate, time and temperature can be measured, and therefore the kinetic equations can be determined. The basic principle underlying this method is that the total area under the curve is proportional to the heat release of curing; the heat release rate is proportional to the rate of curing reaction, as Eq. (1):
The result of a DSC experiment is a curve of heat flux versus temperature. Heat release can be obtained by integrating the curve. The curing degree is calculated as Eq. (2):
where H t is the reaction heat released up to a time t, ΔH is the total reaction heat [9, 10]. According to the equation above, the degree of conversion at different times can be obtained by integrating the curve. By substituting them into the kinetic equation, kinetic parameters can be determined. Therefore, the basic equation describing curing process is obtained.
According to the different reaction mechanism functions, the kinetic models of resins are mainly divided into two types: nth order model and autocatalytic kinetic model [11–15], as following Eqs. (3) and (4):nth order model
Autocatalytic kinetic model
where m and n are reaction orders. If the parameters E a, A, m, and n are known, certain curing rate and curing degree will be achieved by changing curing temperature and curing time.
Results and discussion
DSC
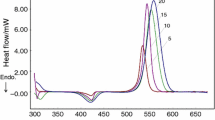
DSC curves at different heating rates are shown in Fig. 3. The initial reaction temperature (T i), peak reaction temperature (T p), and final reaction temperature (T f) can be obtained from Fig. 3, which are listed in Table 1. It can be found from Table 1 and Fig. 3 that the initial reaction temperature of epoxy/episulfide system is about 60 K lower than that of epoxy system, suggesting indeed the high curing reactivity of the episulfide resin. However, the peak reaction temperature and final reaction temperature of both neat epoxy system and epoxy/episulfide system are basically the same. Epoxy and episulfide can both react with MHHPA. And we consider carefully and assume that in epoxy/episulfide system, the reaction between episulfide and MHHPA is the main reaction compared to the reaction between epoxy and MHHPA at lower temperature, which is consistent with high reactivity of episulfide resin and explains the reason why initial temperature of epoxy/episulfide system is lower. At higher temperature, on the one hand, some of the episulfide resin is consumed, and on the other hand, the reaction rate between epoxy resin and MHHPA is increased, so the reaction between epoxy and MHHPA is the main reaction in epoxy/episulfide system. That is why the peak reaction temperature and final reaction temperature of epoxy system and epoxy/episulfide system are similar.
The exothermic enthalpy of MHHPA/CY184 epoxy resin system and MHHPA/CY184 epoxy/episulfide resin system is shown in Table 1. With the increase of the heating rate, the exothermic enthalpy of MHHPA/CY184 epoxy resin system decreases, while the exothermic enthalpy of epoxy/episulfide system increases. The trend of exothermic enthalpy of MHHPA/CY184 epoxy resin system is easy to understand. At higher heating rates, the reaction time decreases and therefore the curing conversion is influenced which contributes to the decrease of exothermic enthalpy of neat epoxy resin at higher heating rates. However, the MHHPA/CY184 epoxy/episulfide resin system has a totally different trend which is curious and cannot be explained by the curing conversion. To explain the phenomenon, we try to propose the curing mechanism of MHHPA/CY184 epoxy/episulfide resin system by combining the curing mechanism of epoxy/anhydride system [16] and unique elementary curing reactions of thiirane group with anhydride.
It is well known that the mechanism of epoxy resin system using anhydride as a hardener can be described by the reactions 1–3 when using strong Lewis base the tertiary amine. According to the mechanism above and the curing mechanism of epoxy/episulfide with polyamide as a hardener [6], we propose that besides the reactions (1)–(3), reactions (4)–(7) take place in the MHHPA/CY184 epoxy/episulfide resin system, which are shown in Fig. 4.
Reactions (4)–(6) are the elementary reactions of episulfide group with anhydride in the presence of DMP-30 corresponding to the epoxy group’s elementary reaction (1)–(3), respectively.
By comparing the bond dissociation energies of C–S, C–O, S–, O– [17], we can find out that the reaction heat of reactions (4)–(6) is less than the reactions (1)–(3), respectively. So, the overall exothermic enthalpy of MHHPA/CY184 epoxy/episulfide resin system should be less than that of MHHPA/CY184 epoxy resin system, which is consistent with the data collected in Table 1. Because of the high reactivity of episulfide group, episulfide resin has homopolymerization effect, as shown in reaction (7) whose reaction heat is less than reaction (1).
When the heating rate is low, the reaction of episulfide dominates because during the lower temperature range, the reactions (1)–(3) of epoxy resin in epoxy/episulfide system are relatively slow, and there is enough time for reactions (4)–(6) and the homopolymerization of episulfide reaction (7) to take place. So, the selectivity of reactions is significant. As a result, the main reactions of the system are reactions (4)–(7), and the exothermic enthalpy is lower.
However, at faster heating rate, there is not enough time for reactions (4)–(7) to complete. And during the higher temperature range, the selectivity of reactions loses its significance. The main reactions of the system are reactions (1)–(3), and the exothermic enthalpy increases as the increase of the heating rate.
Determination of curing mechanism functions and reaction kinetic parameters
In kinetic studies, Málek [18–22] proposed a relatively complete method of most probable mechanism functions. By analyzing the DSC curves at different heating rates, we can calculate kinetic parameters, determine curing kinetic model, as shown in the following steps: (1) using appropriate methods to obtain activation energy E a; (2) according to the shapes and characteristic values of two special functions y(α) and z(α) to determine mechanism function f(α); and (3) according to the mechanism function f(α), choosing proper methods to calculate kinetic parameters (including n, m, and pre-exponential factor A).
To achieve reliable E a of both systems, Flynn–Wall–Ozawa method (FWO) [23–25] was used to calculate the activation energy, as Eq. (5)
where β i represents heating rate; the subscript α represents the conversion rate; E a is activation energy; T α,i is obtained by conducting a series of thermal analysis at different heating rate β i. The value of activation energy E a is calculated by the fitting straight line of lnβ i versus 1/T α,i. Figure 5 shows the relationship between activation energy and the conversion. The overall E a for MHHPA/CY184 epoxy resin system is about 84.6 kJ mol−1 and for MHHPA/CY184, epoxy/episulfide resin system is about 75.7 kJ mol−1.
According to the data of DSC, in order to make further study on the curing behavior of two systems, we used Málek method to determine appropriate reaction mechanism functions, i.e., to determine f(α) through defined functions y(α) and z(α), which are shown in Eqs. (6) and (7).
where χ represents E a/RT, π(χ) represents the integral of temperature. The expression is the approximate temperature integral proposed by Senum and Yang [26], as following Eq. (8):
Reaction mechanism functions were estimated by the shapes of y(α) and z(α) and the characteristic values α M and \( \alpha_{\text{p}}^{\infty } \). If 0 < α M < 1 and \( \alpha_{\text{p}}^{\infty } \) ≠ 0.632, we would regard curing reaction as the autocatalytic kinetic model. α M was the curing degree corresponding to the maximum of y(α), and \( \alpha_{\text{p}}^{\infty } \) was the curing degree corresponding to the maximum of z(α). The curves y(α) – α and z(α) – α are shown in Fig. 6, and Table 2 shows the specific data.
In Table 2, it was observed that 0 < α M < 1 and \( \alpha_{\text{p}}^{\infty } \) ≠ 0.632. According to the criteria proposed by Málek, the kinetic mechanism functions fitted \( f\left( \alpha \right) = \alpha^{\text{m}} \left( {1 - \alpha } \right)^{\text{n}} \), i.e., SB (m, n) model, which is shown as Eq. (9):
where m and n are reaction orders.
Equation (11) can be obtained by transforming Eq. (10)
where, m/n equals to \( \varvec{ }\alpha_{\text{M}} /\left( {1 - \alpha_{\text{M}} } \right) \). So for, 0.2 ≤ α ≤ 0.95 [27], the curves of \( \ln \left[ {\left( {{\text{d}}\alpha /{\text{d}}t} \right)\exp \left( {E_{\text{a}} /{\text{RT}}} \right)} \right] \) versus \( \ln \left[ {\alpha^{{\text{m}}/{\text{n}}} \left( {1 - \alpha } \right)} \right] \) were linear, and the n was obtained from the slope, m was calculated according to the values of n and \( \varvec{ }\alpha_{\text{M}} /\left( {1 - \alpha_{\text{M}} } \right) \), and the value of lnA was obtained from the intercept of fitting straight line. Results are shown in Table 2. Figure 7 shows the dependence of \( \ln \left[ {\left( {{\text{d}}\alpha /{\text{d}}t} \right)\exp \left( {E/{\text{RT}}} \right)} \right] \)on \( \ln \left[ {\alpha^{{\text{m}}/{\text{n}}} \left( {1 - \alpha } \right)} \right] \) at the heating rate of 10 K min−1 in both systems in the conversion range from 0.2 to 0.95.
Substituting the results to Eq. (6), the curing kinetic fitting equations of MHHPA/CY184 epoxy resin system and MHHPA/CY184 epoxy/episulfide resin system were achieved, as following Eqs. (11) and (12):
MHHPA/CY184 epoxy resin system:
MHHPA/CY184 epoxy/episulfide resin system:
Compared with Eq. (11), the value of m of MHHPA/CY184 epoxy/episulfide resin system in Eq. (12) is far less than that of MHHPA/CY184 epoxy resin system in Eq. (11). Since α represents the conversion rate, the extremely small value of m shows that the reacted part has very little influence on the curing rate of MHHPA/CY184 epoxy/episulfide resin system compared with MHHPA/CY184 epoxy resin system [8]. That is to say, unlike the MHHPA/CY184 epoxy resin system which has significant autocatalytic effect, the consumed accelerator DMP-30 and resultant groups like O− and C(O)O−, which have an impact on the curing rate of MHHPA/CY184 epoxy resin system as shown in Eq. (11), have almost no influence on the curing rate of MHHPA/CY184 epoxy/episulfide resin system. We assume that because of the high reactivity and fast curing rate of episulfide groups and S−, the resultant S− can carry out reaction with thiirane groups and MHHPA immediately to form branch structures with slightly residual S− groups as autocatalytic effect. This is consistent with the DSC curves.
We compared the fitting curves with experimental curves, as shown in Fig. 8, and the initial conditions were set as α = 0 when T = 313 K for MHHPA/CY184 epoxy/episulfide resin system and α = 0 when T = 325 K for MHHPA/CY184 epoxy resin system. It can be observed that the fitting curves fit well, which indicates that using Eqs. (11) and (12) to describe the non-isothermal curing process of these two resin systems, respectively, is appropriate. From Fig. 8, Eqs. (11) and (12), it can be seen that MHHPA/CY184 epoxy resin/episulfide system can lower the initial curing temperature and have faster curing rate in the early stage. This model provides necessary theoretical basic for the selection of technological parameters for two resin systems.
Advanced isoconversional kinetic analysis
Advanced isoconversional method developed by Vyazovkin [28–30] can be used to determine the relationship between activation energy E a and conversion rate. Isoconversional kinetic methods follow isoconversional Flynn–Wall–Ozawa principle which is based on the assumption that the reaction rate at a given degree of conversion is only a function of the temperature. Without assuming a particular form of the reaction model, the method allows for evaluating effective activation energy as a function of the extent of reaction. Specific calculation process is given in references [31–33]. Linear heating non-isothermal curing process mainly used the following Eqs. (13) and (14).
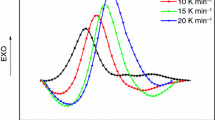
where β represents the heating rates; subscripts i and j represent the test results at different heating programs, i.e., the results at different heating rates of 5, 10, 15, and 20 K min−1 in this paper; n represents test number, i.e., four different heating processes; α represents conversion degree; and Δα represents the small increment of α. In order to reduce the error, Δα equals to 0.01. I represents temperature integral. Under different heating rates, the dependence of non-isothermal curing conversion degree α on the temperature is shown in Fig. 9. It can be seen that MHHPA/CY184 epoxy resin/episulfide system exhibits faster curing rate before the conversion rate α < 0.4.
After interpolating α–T curves by non-linear interpolation, temperature integral was approximately calculated by trapezoidal rule. Substituting the approximate temperature integral at [α–Δα, α] interval under different heating programs into Eq. (13). Keeping changing the value of E a, when the value of Eq. (13) was minimized, this E a was considered as the average value of this interval. Because Δα was relatively small, the calculation results maintain high accuracy.
From Fig. 10, it can be seen that the activation energy calculated by advanced isoconversional has the same trend with the activation energy calculated by FWO method.
The activation energy of the epoxy/episulfide system decreases with the increase of the conversion rate in the conversion range from 0.1 to 0.90 (average value is about 67.6 kJ mol−1). In the conversion range from 0.10 to 0.30, the decrease of activation energy is significant. In the conversion rate from 0.30 to 0.90, the activation energy slightly decreases (about 62.4 kJ mol−1). For the MHHPA/CY184 epoxy resin system, the conversion rate keeps basically the same in the conversion range from 0.10 to 0.40, and then slightly decreases in the conversion range from 0.4 to 0.90. The overall activation energy of epoxy system in the conversion range from 0.10 to 0.90 is about 86.3 kJ mol−1.
Several factors combined influence the activation energy of curing reaction process. Firstly, during the curing process, the molecular mass increases and therefore the branched and networked polymer chains were formed, contributing to the decrease in the mobility of reactive groups and the increase of flow activation energy. Secondly, the reaction centers, such as the sulfhydryl groups, alkoxide, and carboxylate anion, are formed by adding tertiary amine to epoxy system and epoxy/anhydride system both using anhydride as a hardener. As a result, the activation energy of reaction decreases during the curing process. Finally, during the non-isothermal curing reaction, the temperature increases and therefore the mobility of molecular chains and groups is improved. And the diffusion activation energy decreases [34]. The three factors mentioned above together result in the variation of activation of MHHPA/CY184 epoxy resin system and MHHPA/CY184 epoxy/episulfide resin system.
Compared with MHHPA/CY184 epoxy resin system, the relatively high viscosity of MHHPA/CY184 epoxy/episulfide resin system at lower temperature constricts the mobility of molecular chains and groups; so, the diffusion activation is high. The increase of temperature reduces the viscosity of MHHPA/CY184 epoxy/episulfide resin system and therefore the diffusion activation drops noticeably. With the increase of the conversion rate, not only the reaction centers like alkoxide and carboxylate anion which also exist in epoxy resin system, but also the highly active reaction centers, the sulfhydryl groups are formed. As a result, both the diffusion and reaction activation energy decrease, and the overall E a of MHHPA/CY184 epoxy/episulfide resin system decreases and is less than that of MHHPA/CY184 epoxy resin system.
For MHHPA/CY184 epoxy resin system, the slight decrease of activation energy at beginning is mainly due to the decrease of diffusion activation energy caused by the increase of temperature. And the balance of three factors contributes to the consistency of the activation energy in the conversion range from 0.10 to 0.40. And again, the decrease of diffusion activation energy largely causes the slight decrease of activation energy of MHHPA/CY184 epoxy resin system in the conversion range from 0.40 to 0.90.
Conclusions
CY184 epoxy resin was used to synthesize CY184 episulfide/epoxy resin system which was characterized by FTIR, ESI–MS, and EA. The episulfide conversion rate was 55.35 %. The non-isothermal curing kinetics of the MHHPA/CY184 epoxy resin system and MHHPA/CY184 epoxy/episulfide resin system has been studied comparatively. The curing mechanism of MHHPA/CY184 epoxy/episulfide resin system was proposed to explain the curing behavior. By using integral isoconversional methods of Vyazovkin and FWO method, the activation energy of MHHPA/CY184 epoxy resin system and MHHPA/CY184 epoxy/episulfide resin system was calculated. The curing kinetics has been simulated by Málek method. The results showed that both systems fitted SB model. The influence of episulfide resin in the MHHPA/CY184 epoxy/episulfide resin system has been discussed, and results show that episulfide resins have faster curing rate and could lower the initial reaction temperature and the exothermic enthalpy.
References
Bell J, Ku W. Epoxy/episulfide resins. Berlin: Walter de Gruyter; 1987.
Li Y, Cheng J, Zhang J. Study on the synthesis of thiirane. J Appl Polym Sci. 2006;101(6):4023–7.
Chino K, Suga K, Ikawa M, Satoh H. Novel rapid-cure adhesives for low temperature using thiirane compound. J Appl Polym Sci. 2001;82(12):2953–7.
Ku W, Bell JP. Fast curing epoxy–episulfide resin for uses at room temperature. Epoxy resin chemistry II(A 84-29376 12-27). Washington, DC: American Chemical Society; 1983. p. 153–69.
Bell J, Don TM, Voong S, Fernandez A, Ku W. Synthesis and properties of epoxy–episulfide resins. Die Angewandte Makromolekulare Chemie. 1996;240(1):67–81.
Tsuchida K, Bell JP. A new epoxy/episulfide resin system for coating applications: curing mechanism and properties. Int J Adhes Adhes. 2000;20(6):449–56.
Tsuchida K, Bell JP. A new epoxy/episulfide resin system for electronic applications—2: pot life and prepreg storage life evaluation. J Adhes Sci Technol. 2000;14(12):1515–26.
Yang T, Zhang C, Zhang J, Cheng J. The influence of tertiary amine accelerators on the curing behaviors of epoxy/anhydride systems. Thermochim Acta. 2014;577:11–6.
Yao L, Deng J, Qu B, Shi W. Cure kinetics of DGEBA with hyperbranched poly (3-hydroxyphenyl) phosphate as curing agent studied by non-isothermal DSC. Chem Res Chin Univ. 2006;22(1):118–22.
Chu F, McKenna T, Lu S. Curing kinetics of an acrylic resin/epoxy resin system using dynamic scanning calorimetry. Eur Polym J. 1997;33(6):837–40.
Šesták J, Berggren G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim Acta. 1971;3(1):1–12.
Ghaemy M, Rostami A, Omrani A. Isothermal cure kinetics and thermodynamics of an epoxy–nickel–diamine system. Polym Int. 2006;55(3):279–84.
Zhou T, Gu M, Jin Y, Wang J. Studying on the curing kinetics of a DGEBA/EMI-2, 4/nano-sized carborundum system with two curing kinetic methods. Polymer. 2005;46(16):6174–81.
Zhao L, Hu X. A variable reaction order model for prediction of curing kinetics of thermosetting polymers. Polymer. 2007;48(20):6125–33.
Yoo MJ, Kim SH, Park SD, Lee WS, Sun J, Choi J, Nahm S. Investigation of curing kinetics of various cycloaliphatic epoxy resins using dynamic thermal analysis. Eur Polym J. 2010;46(5):1158–62.
Rocks J, Rintoul L, Vohwinkel F, George G. The kinetics and mechanism of cure of an amino-glycidyl epoxy resin by a co-anhydride as studied by FT-Raman spectroscopy. Polymer. 2004;45(20):6799–811.
Luo Y-R. Handbook of bond dissociation energies in organic compounds. Boca Raton: CRC Press; 2002.
Hseih HK, Su CC, Woo EM. Cure kinetics and inter-domain etherification in an amine-cured phenoxy/epoxy system. Polymer. 1998;39(11):2175–83.
Málek J. A computer program for kinetic analysis of non-isothermal thermoanalytical data. Thermochim Acta. 1989;138(2):337–46.
Málek J. The kinetic analysis of non-isothermal data. Thermochim Acta. 1992;200:257–69.
Roşu D, Caşcaval C, Mustată F, Ciobanu C. Cure kinetics of epoxy resins studied by non-isothermal DSC data. Thermochim Acta. 2002;383(1):119–27.
Roşu D, Mustată F, Caşcaval C. Investigation of the curing reactions of some multifunctional epoxy resins using differential scanning calorimetry. Thermochim Acta. 2001;370(1):105–10.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38(11):1881–6.
Flynn JH, Wall LA. General treatment of the thermogravimetry of polymers. J Res Nat Bur Stand. 1966;70(6):487–523.
Liu J, Li J, Fan M, Zhang J, Cheng J. Comparative curing kinetics of 1, 4-bis (4-diaminobenzene-1-oxygen) n-butane and 4, 4′-bis-(diaminodiphenyl) methane with tetraglycidyl methylene dianiline systems. J Therm Anal Calorim. 2014;117(2):603–610.
Senum G, Yang R. Rational approximations of the integral of the Arrhenius function. J Therm Anal. 1977;11(3):445–7.
Koga N. Kinetic analysis of thermoanalytical data by extrapolating to infinite temperature. Thermochim Acta. 1995;258:145–59.
Sbirrazzuoli N, Vyazovkin S. Learning about epoxy cure mechanisms from isoconversional analysis of DSC data. Thermochim Acta. 2002;388(1):289–98.
Sbirrazzuoli N, Vyazovkin S, Mititelu A, Sladic C, Vincent L. A study of epoxy–amine cure kinetics by combining isoconversional analysis with temperature modulated DSC and dynamic rheometry. Macromol Chem Phys. 2003;204(15):1815–21.
Vyazovkin S, Sbirrazzuoli N. Mechanism and kinetics of epoxy–amine cure studied by differential scanning calorimetry. Macromolecules. 1996;29(6):1867–73.
Vyazovkin S. Advanced isoconversional method. J Therm Anal Calorim. 1997;49(3):1493–9.
Vyazovkin S. Modification of the integral isoconversional method to account for variation in the activation energy. J Comput Chem. 2001;22(2):178–83.
Vyazovkin S. Evaluation of activation energy of thermally stimulated solid-state reactions under arbitrary variation of temperature. J Comput Chem. 1997;18(3):393–402.
Vyazovkin S, Sbirrazzuoli N. Isoconversional kinetic analysis of thermally stimulated processes in polymers. Macromol Rapid Commun. 2006;27(18):1515–32.
Acknowledgements
The authors greatly appreciated the financial supports from National Natural Science Foundation of China (Project Nos. 21176017 and 21476013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, C., Liu, X., Cheng, J. et al. Study on curing kinetics of diglycidyl 1,2-cyclohexane dicarboxylate epoxy/episulfide resin system with hexahydro-4-methylphthalic anhydride as a curing agent. J Therm Anal Calorim 120, 1893–1903 (2015). https://doi.org/10.1007/s10973-015-4527-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4527-y