Abstract
Host–guest complexes of an amphiphilic water-soluble p-tert-butylcalix[5]arene bearing 4-sulphonatobutoxy groups at the narrow rim with trace amines (2-phenylethylamine and tyramine) and a neurotransmitter (dopamine), originally investigated via 1H NMR, have been re-examined via ITC in order to double check the reliability of the values obtained through a van’t Hoff analysis of the NMR data. The calorimetrically determined data confirm the existence of a 1:1 host–guest species; however, there are inconsistencies between the van’t Hoff derived values and the values determined via direct calorimetry. These discrepancies do not result from proton displacement due to inclusion, but rather result from the temperature dependence of the van’t Hoff enthalpies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Monoamine neurotransmitters (such as dopamine) and trace amines (such as 2-phenylethylamine and tyramine) are known to play a key role in mammalian central nervous systems by regulating the physiological processes of signaling transmission [1, 2]. Abnormal levels of these biogenic amines are responsible for several psychiatric disorders (e.g., schizophrenia) and neurodegenerative pathologies (e.g., Parkinson’s disease) [3] and, because of this, are commonly monitored in biological fluids (cerebrospinal fluids, plasma and urine) not only to evaluate physiological functions like the activity of the nervous system [4], but also to detect pathologies such as hypotension, hypertension, neuroblastoma and pheochromocytoma [5–7]. The mechanism of neurotransmitter release and subsequent binding to the G protein-coupled receptors in the synaptic cleft is a field of multidisciplinary interest, currently attracting considerable attention [8–10].
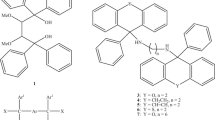
Several artificial receptors [11, 12] have been synthesized over the past few decades with the aim of gaining a deeper understanding of the binding pocket of G protein-coupled receptors and, further down the line, developing and testing new drugs for pharmaceutical purposes and/or new sensing agents for diagnostic clinical chemistry. Within this research context, inspired by the known affinity of the calix[5]arene framework [13] for biologically relevant substrates containing an arylethylammonium moiety, we have recently synthesized an amphiphilic water-soluble p-tert-butylcalix[5]arene (1) bearing 4-sulphonatobutoxy groups at the narrow rim (Fig. 1) and studied its aggregation and host–guest properties [14–16].
In particular, we have found that calixarene 1, below its critical micelle concentration (cmc) [14] (i.e., in its monomeric form), is able to act as a selective artificial receptor toward a series of hydrochloride salts of monoamine neurotransmitters and trace amines [15], while the micelles formed above the cmc efficiently encapsulate a number of these guests in different regions of the micellar environment (core, palisade, Stern layer) [16]. These findings make the water-soluble calixarene 1 an excellent candidate for the development of new systems for the detection, solubilization and separation of biological substrates.
Host–guest complexes of receptor 1 with trace amines 2 and 3 and neurotransmitter 4 (Fig. 1) were originally investigated by 1H NMR spectroscopy to assess their mode of binding in water. NMR data were then used not only to confirm that, in analogy to previous observations in organic media [13], arylethylammonium substrates are included endo-cavity with the ammonium moiety pointing inside the host cavity, but also to provide the 1:1 host–guest association constants and, by way of a van’t Hoff analysis [15], the enthalpy change values of the binding process. In the present work, the free energy of binding determined by 1H NMR experiments was double checked by isothermal titration calorimetry (ITC). The latter was also employed to determine the energetics of the inclusion process in aqueous solution by direct measurements of the reaction heats in order to have a more accurate thermodynamic characterization of these biologically active ammonium-calixarene complex systems.
Experimental
Materials
Calixarene 1 was synthesized as previously reported [14], while the amine hydrochloride salts (Pea·HCl, 2; Tyr·HCl, 3; and Dopa·HCl, 4) as well as deuterium oxide (99.9 % isotopic purity) and maleic acid (qNMR standard grade) were purchased from Sigma-Aldrich and used as received. An accurate determination of the thermodynamic parameters implies a precise knowledge of the reactant concentrations; consequently, the purity of both host 1 and guests 2–4 was examined thermogravimetrically by heating the sample from 50 to 800 °C (at 10 °C min−1 in air) with a PerkinElmer TGS-2 instrument. High purity water (Millipore, Milli-Q Element A 10 ultrapure water) and A grade glassware were employed throughout.
NMR measurements
Spectra were recorded at 25 °C in D2O or H2O on a Varian 500 MHz instrument, using solvent suppression pulse sequences (either PRESAT or WET). The determination of the effective concentration of calixarene 1 present as a monomer in aqueous solution (i.e., in the non-aggregated form) was carried out according to the qNMR protocol reported by Henderson [17]. Typically, a 0.4 mM solution of 1 in pure water was left to equilibrate at room temperature for about 2 h and then a 530 μL sample was transferred into a 5-mm Wilmad NMR tube containing a Wilmad coaxial insert (WGS-5BL, stem length 50 mm) loaded with 60 μL of a 20 mM D2O solution of maleic acid used as an internal reference. Accurate integration of the CH resonance of the maleic acid (δ = 6.28 ppm) versus the signal of the aromatic hydrogen atoms of 1 showed that the effective concentration of the monomeric form of the host has a constant value (about 0.2 mM) over a period of 1 week; thus, the concentration of host monomer determined according to the above-mentioned procedure may be used for the analysis of the calorimetric data.
ITC titrations
ITC titrations were carried out at 25 °C in water by using a Nano-ITC2G calorimeter (TA Instruments) with an active cell volume of 0.988 mL and a 250- or 100-μL injection syringe. Measurements were run in the overfilled mode which does not require any correction for liquid evaporation and/or for the presence of the vapor phase [18]. The reaction mixture in the sample cell was stirred at 250 rpm during the titration. The power curve was integrated by using the NanoAnalyze software (TA Instruments) to obtain the gross heat evolved/absorbed in the reaction. The instrument was initially calibrated chemically by titrating an HCl solution (1 mM) into buffered TRIS (30 mM containing 10 mM HCl, [TRIS]/[TRISH+] = 2:1) according to the procedure previously described [19]. The calorimeter was also checked through an electrical calibration. ITC measurements were carried out by titrating aqueous solutions of Pea·HCl (0.01257–0.01450 M), Tyr·HCl (0.01190–0.01550 M) or Dopa·HCl (0.01150–0.01290 M) into a host 1 solution (the concentration of the monomer, determined via NMR, ranged between 1.88 and 2.30 × 10−4 M). Before running each titration, all solutions were degassed under vacuum for about 15 min. Typically, 4–5 independent titrations were run for each host–guest system with a final guest/host ratio of up to 18–20 in order to meet the saturation fraction criteria proposed for the formation of 1:1 complexes [20]. The heats of dilution were determined in separate blank experiments by titrating solutions of guests 2, 3 or 4 into plain water.
Data fitting
The net heats of reaction, obtained by subtracting the heat evolved/absorbed in the blank experiments, were analyzed by HypΔH [21]. This software calculates equilibrium constants and/or formation enthalpies of complexes in solution by nonlinear least-squares minimization of the function
Q obs is the observed heat for a given reaction step, corrected for the dilution (blank) effects, while Q calc is calculated as
where δn is the change in the number of moles of a reaction product (calculated in terms of a set of equilibrium constants) and ΔH° is the molar formation enthalpy of the reaction product. The summation is carried out over all the reaction steps in the system. The squared residuals (Q obs − Q calc)2 are summed over all the titration points. For each host–guest system, log K values and thermodynamic parameters were obtained by simultaneously fitting calorimetric data obtained from different titrations.
Results and discussion
When studying the thermodynamics of a complex binding event, such as the one described here, one generally seeks to split the free energy term into the ∆H° and ∆S° components to reveal specific features and/or differences that are not expressed in the ∆G° term. The enthalpy change of a reaction, ∆H°, a key quantity describing the amount of heat released or absorbed in the course of the reaction [22, 23], is usually determined directly by using calorimetry or indirectly from the temperature dependence of the binding constant (i.e., the van’t Hoff method). Of these two methods, only the calorimetric approach provides direct estimates of enthalpy changes for the binding process. The validity of van’t Hoff enthalpies derived from the temperature variation of equilibrium constants has been extensively debated over the past two decades [24–30].
Usually, enthalpy values are calculated by dividing Eq. (3) by T
and taking the derivative with respect to 1/T
In the above equations, R is the gas constant, K obs is the equilibrium constant observed at a given temperature, T is the absolute temperature, and ∆H vH denotes the van’t Hoff enthalpy.
A plot of lnK obs versus 1/T should be linear with a slope equal to −∆H vH/R, provided enthalpy does not change with temperature. If, as it is often the case [24–30], (∂∆H vH /∂T) p is different from zero, i.e., if ∆C p ≠ 0, then Eq. (4) becomes
where T Ref is the reference temperature and ∆C p,vH is the van’t Hoff derived heat capacity change [25]. A nonzero ∆C p,vH leads to a curvature in the van’t Hoff plots [24, 25, 29]. Even when taking into account curvatures [25], data analysis may be non-trivial [26]; it has also been pointed out that (1) estimates of ∆H and ∆C p are highly correlated and neither parameter can be reliably estimated unless one can be constrained and (2) small heat capacity changes can bias the slope of van’t Hoff plots without producing curvature that is visible within the noise level of typical data [24]. This and other problems have induced some authors to question the validity of the van’t Hoff enthalpy [24, 28–30]. In any case, the precision of the ∆H vH estimates is considerably lower than that for ∆H values obtained directly by calorimetry [24, 25]. Regardless of the reasons leading to discrepancies, these remain uncomfortably large [31]. Based on the above considerations, we have reinvestigated the inclusion process of the biologically active amines 2–4 inside the calixarene receptor 1 in water [15] and determined the ∆H° values by nano-ITC.
Since the accurate determination of the thermodynamic parameters requires concentrations of the reactants to be precisely known, the purity of both host 1 and guests 2–4 was preliminary checked through a thermogravimetric analysis. Heating the guest samples at 10 °C min−1 in air showed that the water present as absorption solvent amounts to <1 % by mass; all the guests decompose completely in the range 300–500 °C, thus indicating that they are free from inorganic impurities. The first drop in the TG curve of host 1 is observed around 150 °C (Fig. 2) and accounts for the adsorbed residual solvent that amounts to 5–7 % of the total mass. The final mass % at 800 °C (~20 % of the total) is entirely consistent with the expected value based on the inorganic components of the penta-sulphonato calix[5]arene 1.
Preliminarily, potentiometric measurements [32] were carried out on the host–guest systems under the same conditions employed for the ITC experiments to assess the influence of protons on the molecular recognition process. The pH value of a solution of host 1 was initially 6.5, and the change observed upon addition of up to 9–12 equivalents of either Tyr·HCl or Dopa·HCl solution was always within the experimental error of the instrument. This result allowed us to rule out any contribution resulting from the interaction of either the host or the guests with protons; consequently, ITC titrations were carried out in plain water rather than an aqueous buffered solution [26].
We have previously shown that the amphiphilic anionic calixarene 1 aggregates in water [14–16] even well below the critical micelle concentration. Specifically, according to dynamic light scattering measurements, a 0.1 mM aqueous solution of 1 was found to contain calixarene aggregates with a hydrodynamic radius of about 100 nm. Given that species of such a huge size fall outside the detection range of 1H NMR spectroscopy, prior to each batch of ITC titrations, it was necessary to assess the effective concentration of the host present in solution, in the monomeric form, by a quantitative NMR protocol [17] based on the use of a stem coaxial NMR tube loaded with an internal reference (a maleic acid D2O solution having an accurately known concentration). The addition of a volume of D2O equal to that employed for the ITC titrations did not cause any detectable change in the concentration of the monomer, which rules out any sizable dissociation of the micellar aggregate upon dilution.
The calorimetric measurements were carried out in water at 25 °C by usually titrating a 12–15 mM guest solution into a calixarene solution having a 0.2 mM monomer concentration. Typical experiments for the complexation of guests 2–4 with receptor 1 are shown in Figs. 3, 4 and 5.
The thermodynamic parameters for the interaction of the different guests with calixarene 1, determined by using a software that refines data obtained from multiple titrations, are reported in Table 1.
The species distribution diagrams indicate that, under the experimental conditions employed, the saturation criteria (“measurements should be made over roughly 75 % of the saturation curve”) [20] is satisfied for all inclusion complexes. The reliability of the results obtained is further strengthened by the precision and accuracy of the calorimetric apparatus which was thoroughly tested by using a chemical calibration procedure [19] rather than the simple electrical calibration recommended by the ITC manufactures [33, 34].
These data nicely reproduce the speciation and the trend of the binding constants formerly obtained via 1H NMR for the same host–guest systems [15], provided due allowance is made for the different experimental procedures and conditions employed (affinity constants were determined by direct integration of suitable 1H NMR signals of free and complexed species of host and guest). Both techniques indicate that the sulphonato calix[5]arene 1 is able to form stable 1:1 complexes in aqueous solution [35] with the biologically active amines investigated here. Different species and combinations thereof were examined, but the data analysis consistently converged toward the species and values reported in Table 1.
As previously reported [15], a structural comparison between Tyr·HCl and Dopa·HCl indicates that the presence of an additional hydroxyl group on the aromatic ring of the latter guest enhances the hydrophilic nature of the molecule, thus reducing its affinity for the lipophilic environment provided by the calixarene cavity, and may also cause steric hindrance upon complexation.
A clear-cut picture of the enthalpic and entropic contributions to the free energy term is shown in Fig. 6.
Among several weak non-covalent interactions occurring between a host and a guest, Coulombic interactions, hydrogen bonds, CH-π, π-stacking and van der Waals interactions mainly contribute to the enthalpy changes [36, 37], whereas conformational/structural rearrangements and desolvation events contribute to the entropy changes [38, 39]. As shown both in Table 1 and Fig. 6, the complexation of guests 2–4 by 1, that yields the 1:1 species, is always both enthalpically favored and driven regardless of the size/shape of the amine molecule. Concerted hydrogen bonds (+NH···O), CH-π and π–π interactions between the guest and the aromatic calixarene cavity drive the host–guest complex formation. Such enthalpically favorable contributions prevail over the cost in energy needed for the desolvation of the reactant molecules. The unfavorable entropic contribution (ΔS° < 0) to ΔG° accounts for the loss of degrees of freedom and consequent “structural freezing” of the system due to complex formation which is not paid off by the release of water molecules by each component upon inclusion [40, 41]. Similar observations have been previously reported for other calixarene receptors upon endo-cavity inclusion of (either positively or negatively) charged guests in water [42–44].
Interesting insights, which are not expressed in the log K value, are unveiled by the above thermodynamic parameters. For instance, the complexation of Pea·HCl by calixarene 1, which has a comparable affinity for Dopa·HCl and a slightly larger one for Tyr·HCl, occurs with the largest enthalpy gain as a consequence of the enthalpically favorable π–π interactions between the lipophilic moiety of 2, which lacks polar OH groups, and the electron-rich cavity of the host. Such strong interactions, along with the smaller- and less-hindered size of this guest, result in a more stiffened and conformationally less flexible host–guest complex as indicated by the ΔS° value which is more unfavorable than those found for the inclusion of the other guests.
Conclusions
The aromatic cavity of the water-soluble calix[5]arene 1 is able to mimic the hydrophobic binding pockets of G protein-coupled receptors of neurotransmitters and trace amines such as 2-phenylethylamine, tyramine and dopamine in aqueous solution. All guests are efficiently complexed by the host with a 1:1 stoichiometry and, in all cases, the inclusion process is enthalpically favored and driven. Concerted hydrogen bonds, CH-π and π–π interactions drive the insertion of the positively charged arylethylammonium guests inside the host cavity. The direct measurement of the heat of reaction by nano-ITC provided reliable and accurate ΔH° values which, along with the entropy change, revealed features/aspects that are not expressed in the free energy term of a chemical reaction. Though the thermodynamic data determined by direct calorimetry reproduce the trend reported previously, there is a discrepancy between the van’t Hoff estimates of ∆H° and the calorimetrically determined values obtained using ITC. This further stresses the need to determine enthalpy values directly since the van’t Hoff values are temperature dependent and ∆Cp are, in most cases, different from zero.
The above findings make lower-rim sulphonato calix[5]arenes promising candidates for the development of successful sensing devices or recognition agents for monitoring the level of biologically relevant amines in natural fluids.
References
Gringrich JA, Caron MG. Recent advances in the molecular biology of dopamine receptors. Annu Rev Neurosci. 1993;16:299–321.
Sotnikova TD, Caron MG, Gainetdinov RR. Trace amine-associated receptors as emerging therapeutic targets. Mol Pharmacol. 2009;76:229–35.
Kurian MA, Gissen P, Smith M, Heales SJR, Clayton PT. The monoamine neurotransmitter disorders: an expanding range of neurological syndromes. Lancet Neurol. 2011;10:721–33.
Marc DT, Ailts JW, Campeau DC, Bull MJ, Olson KL. Neurotransmitters excreted in the urine as biomarkers of nervous system activity: validity and clinical applicability. Neurosci Biobehav Rev. 2011;35:635–64.
Hyland K. Clinical utility of monoamine neurotransmitter metabolite analysis in cerebrospinal fluid. Clin Chem. 2008;54:633–41.
Monsaingeon M, Perel Y, Simonnet G, Corcuff JB. Comparative values of catecholamines and metabolites for the diagnosis of neuroblastoma. Eur J Pediatr. 2003;162:397–402.
Reisch N, Peczkowska M, Januszewicz A, Neumann HP. Pheochromocytoma: presentation, diagnosis and treatment. J Hypertens. 2006;24:2331–485.
Südhof TC. The molecular machinery of neurotransmitter release. Angew Chem Int Ed. 2014;53:12696–717.
Lefkowitz RJ. A brief history of G-protein coupled receptors. Angew Chem Int Ed. 2013;52:6367–78.
Kobilka B. The structural basis of G-protein coupled receptor signaling. Angew Chem Int Ed. 2013;52:6380–8.
Chui JKW, Fyles TM. Artificial catecholamine receptors in aqueous media. Supramol Chem. 2008;20:397–405.
Späth A, König B. Molecular recognition of organic ammonium ions in solution using synthetic receptors. Beilstein J Org Chem. 2010;6:1–111.
Gargiulli C, Gattuso G, Liotta C, Notti A, Parisi MF, Pisagatti I, Pappalardo S. Calix[5]arene-based heteroditopic receptor for 2-phenylethylamine hydrochloride. J Org Chem. 2009;74:4350–3.
Gattuso G, Notti A, Pappalardo A, Pappalardo S, Parisi MF, Puntoriero F. A supramolecular amphiphile from a new water-soluble calix[5]arene and n-dodecylammonium chloride. Tetrahedron Lett. 2013;54:188–91.
Gattuso G, Notti A, Pappalardo S, Parisi MF, Pisagatti I. Recognition in water of bioactive substrates by a sulphonato p-tert-butylcalix[5]arene. Supramol Chem. 2014;26:597–600.
Gattuso G, Notti A, Pappalardo S, Parisi MF, Pisagatti I, Patanè S. Encapsulation of monoamine neurotransmitters and trace amines by amphiphilic anionic calix[5]arene micelles. New J Chem. 2014;38:5983–90.
Henderson TJ. Quantitative NMR spectroscopy using coaxial inserts containing a reference standard: purity determinations for military nerve agents. Anal Chem. 2002;74:191–8.
Hansen LD, Fellingham GW, Russell DJ. Simultaneous determination of equilibrium constants and enthalpy changes by titration calorimetry: methods, instruments, and uncertainties. Anal Biochem. 2011;409:220–9.
Sgarlata C, Zito V, Arena G. Conditions for calibration of an isothermal titration calorimeter using chemical reactions. Anal Bioanal Chem. 2013;405:1085–94.
Derenleau DA. Theory of the measurement of weak molecular complexes. I. General considerations. J Am Chem Soc. 1969;91:4044–9.
Gans P, Sabatini A, Vacca A. Simultaneous calculation of equilibrium constants and standard formation enthalpies from calorimetric data for systems with multiple equilibria in solution. J Solution Chem. 2008;37:467–76.
Sgarlata C, Mugridge JS, Pluth MD, Tiedemann BEF, Zito V, Arena G, Raymond KN. External and internal guest binding of a highly charged supramolecular host in water: deconvoluting the very different thermodynamics. J Am Chem Soc. 2010;132:1005–9.
Bonaccorso C, Brancatelli G, Forte G, Arena G, Geremia S, Sciotto D, Sgarlata C. Factors driving the self-assembly of water-soluble calix[4]arene and gemini guests: a combined solution, computational and solid-state study. RSC Adv. 2014;4:53575–87.
Chaires JB. Possible origin of differences between van’t Hoff and calorimetric enthalpy estimates. Biophys Chem. 1997;64:15–23.
Horn JR, Russell D, Lewis EA, Murphy KP. van’t Hoff and calorimetric enthalpies from isothermal titration calorimetry: Are there significant discrepancies? Biochemistry. 2001;40:1774–8.
Horn JR, Brandts JF, Murphy KP. van’t Hoff and calorimetric enthalpies II: effects of linked equilibria. Biochemistry. 2002;41:7501–7.
Weber G. van’t Hoff revisited: enthalpy of association of protein subunits. J Phys Chem. 1995;99:1052–9.
Holtzer A. Comment on “van’t Hoff revisited: enthalpy of association of protein subunits”. J Phys Chem. 1995;99:13048–9.
Naghibi H, Tamura A, Sturtevant JM. Significant discrepancies between van’t Hoff and calorimetric enthalpies. Proc Natl Acad Sci USA. 1995;92:5597–9.
Liu Y, Sturtevant JM. Significant discrepancies between van’t Hoff and calorimetric enthalpies II. Protein Sci. 1995;4:2559–61.
Mizoue LS, Tellinghuisen J. Calorimetric versus van’t Hoff binding enthalpies from isothermal titration calorimetry: Ba2+-crown ether complexation. Biophys Chem. 2004;110:15–24.
Sgarlata C, Bonaccorso C, Gulino FG, Zito V, Arena G, Sciotto D. Stereochemistry and thermodynamics of the inclusion of aliphatic and aromatic anionic guests in a tetracationic calix[4]arene in acidic and neutral aqueous solutions. New J Chem. 2009;33:991–7.
Demarse NA, Quinn CF, Eggett DL, Russell DJ, Hansen LD. Calibration of nanowatt isothermal titration calorimeters with overflow reaction vessels. Anal Biochem. 2011;417:247–55.
Wadso I, Wadso L. Systematic errors in isothermal micro and nanocalorimetry. J Therm Anal Calorim. 2005;82:553–8.
Arena G, Gentile S, Gulino FG, Sciotto D, Sgarlata C. Water soluble pentasulfonatocalix[5]arene: selective recognition of ditopic trimethylammonium cations by a triple non-covalent interaction. Tetrahedron Lett. 2004;45:7091–4.
Meyer A, Castellano RK, Diederich F. Interactions with aromatic rings in chemical and biological recognition. Angew Chem Int Ed. 2003;42:1210–50.
Nishio M. CH/π hydrogen bonds ion organic reactions. Tetrahedron. 2005;61:6923–50.
Rekharsky MV, Inoue Y. Complexation thermodynamics of cyclodextrins. Chem Rev. 1998;98:1875–917.
Biedermann F, Nau WM, Schneider HJ. The hydrophobic effect revisited. Studies with supramolecular complexes imply high-energy water as a noncovalent driving force. Angew Chem Int Ed. 2014;53:11158–71.
Li Q, Guo DS, Qian H, Liu Y. Complexation of p-sulfonatocalixarenes with local anaesthetics guests: binding structures, stabilities, and thermodynamic origins. Eur: J Org Chem; 2012. p. 3962–71.
Liu Y, Ma YH, Chen Y, Guo DS, Li Q. Molecular recognition thermodynamics of pyridine derivatives by sulfonatocalixarenes at different pH values. J Org Chem. 2006;71:6468–73.
Arena G, Casnati A, Contino A, Lombardo GG, Sciotto D, Ungaro R. Water-soluble calixarene hosts that specifically recognize the trimethylammonium group or the benzene ring of aromatic ammonium cations: a combined 1H NMR, calorimetric and molecular mechanics investigation. Chem Eur J. 1999;5:738–44.
Bonaccorso C, Sgarlata C, Grasso G, Zito V, Sciotto D, Arena G. A gemini guest triggers the self-assembly of a calixarene capsule in water at neutral pH. Chem Commun. 2011;47:6117–9.
Wintgensa V, Biczókb L, Miskolczy Z. Thermodynamics of host-guest complexation between p-sulfonatocalixarenes and 1-alkyl-3-methylimidazolium type ionic liquids. Thermochim Acta. 2011;523:227–31.
Acknowledgements
MIUR (FIRB MERIT RBNE08HWLZ) and Universities of Catania and Messina are gratefully acknowledged for partial financial support. We are grateful to Dr. Giuseppe Mineo for TG measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arena, G., Pappalardo, A., Pappalardo, S. et al. Complexation of biologically active amines by a water-soluble calix[5]arene. J Therm Anal Calorim 121, 1073–1079 (2015). https://doi.org/10.1007/s10973-015-4522-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4522-3