Abstract
Estradiol (E2) is the main drug used in menopause therapy. This study aimed to evaluate the drug-excipient compatibility of binary mixtures (BMs) (1:1 BMs, w/w), initially by differential scanning calorimetry (DSC), and subsequently, by complementary techniques such as X-ray powder diffraction (XRPD) and high performance liquid chromatography (HPLC) if there was any evidence of interaction. The samples were stored under accelerated stability conditions (40 °C at 75 % relative humidity). The DSC curves of estradiol and the BMs with excipients (corn starch, lactose, xanthan gum, microcrystalline cellulose, magnesium stearate, dibasic calcium phosphate, and talc) were obtained. The results show that estradiol was compatible with all the selected excipients. XRPD and HPLC analysis were instrumental in interpreting the DSC results and excluding relevant pharmaceutical incompatibilities in all cases. Overall, the compatibility of the selected excipients with estradiol was successfully evaluated using a combination of thermal and spectroscopic methods, and the formulations developed using the compatible excipients were found to be stable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
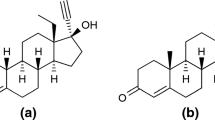
Estradiol (E2) is a natural sex steroid hormone that is used to improve the symptoms of menopause, reduce the risk of bone fractures occurring after menopause, and to prevent and reverse female ovarian dysfunction. Estradiol can promote the cell synthesis of DNA and RNA and regulate the female sex organs and secondary sex characteristics. Estradiol is the primary estrogenic hormone elaborated by the human ovary, and although it is available in pure form, it has not been used extensively in oral menopausal therapy because it is considered ineffectual due to poor gastrointestinal absorption. A new dosage form of estradiol, i.e., a micronized estradiol and dydrogesterone double-layer tablet with better absorption, is not only well-tolerated but also clinically effective as shown in a preliminary study of menopause therapy. This new dosage form may actually help to prevent the increase in body fat mass and fat redistribution. Informing women about the effects of menopause on body weight/fat distribution and the potential beneficial effects of some HRT regimens helps to improve HRT compliance. The chemical structure of estradiol corresponds to 3,17-β-dihydroxy-1,3,5(10)-estratriene and is given in Fig. 1.
Study of drug-excipient compatibility is an important stage in pre-formulation studies during the development of pharmaceuticals. Indeed, potential physical and chemical interactions between drug and excipients can affect the chemical nature, stability, and bioavailability of a drug and thus its effectiveness [1–6]. In the literature, two types of chemical incompatibilities have been described: (i) excipient-promoted intrinsic degradation of the drug, such as hydrolysis or oxidation and/or (ii) a covalent reaction between the drug and the excipient [5, 7].
In recent years, a number of techniques have been introduced for the evaluation of drug-excipient compatibility. Differential scanning calorimetry (DSC) is a well-established technique for the detection of drug-excipient incompatibility. DSC has become the first choice in the pharmaceutical industry for compatibility studies. The main benefit of DSC is its ability to quickly screen potential excipients for incompatibilities derived from the appearance, shift, disappearance of peaks or variations in the corresponding ΔH (enthalpy of transition) [8]. In this method, the sample is exposed to high temperatures, the conclusion drawn with which may not always be valid under ambient conditions. Thus, conclusions based on DSC results alone can often be misleading or inconclusive. Moreover, the presence of a solid–solid interaction does not necessarily indicate pharmaceutical incompatibility, as it might instead be advantageous, e.g., as a more desirable form of the drug delivery system [9–13]. Therefore, the use of other analytical techniques, such as Fourier transform infrared spectroscopy, X-ray powder diffractometry, and high performance liquid chromatography (HPLC) as complementary tools to assist in the interpretation of DSC findings is greatly advisable [14–17].
Thus, this study aimed to evaluate the thermal stability of estradiol and the impact of excipients used in the development of solid dosage forms when combined in binary mixtures (BMs) 1:1 (w/w).
Experimental
Materials and samples
Estradiol and the following excipients were purchased from commercial sources: estradiol (Zhejiang Xianju Pharmaceutical Co., Ltd, China), lactose (Foremost, USA), magnesium stearate (Mg stearate, Peter Greven, Netherlands), corn starch (Beijing Fengli Jingqiu Commerce and Trade Co., Ltd., China), microcrystalline cellulose (MCC) (Avicel PH-301, FMC, USC), xanthan gum (Shanghai Huayi Biotechnology Co., Ltd., China), dibasic calcium phosphate (Lai Fengyu Talcum Powder Co., Ltd., China), HPLC grade acetonitrile (Tedia, USA) were used to prepare the mobile phase for HPLC analysis. Water was used throughout the HPLC analysis and was prepared using a water purifier (ATC-2001-P, Aquapro, China).
Physical mixtures of estradiol with each selected excipient were prepared in a 1:1 (w/w) ratio by simple mixture of the components in an agate mortar and pestle for approximately 5 min. For the stability study, samples were submitted to physical homogenization using a vortexer for 3 min. After preparation, samples were analyzed immediately. The same samples were also stored for 1 month in a stability chamber at 40 ± 0.5 °C using a saturated NaCl solution (average of 75 ± 1 % RH) monitored by a temperature/humidity data logger. The mixtures were divided into two groups: dried heat using sealed vials of penicillin and moist heat using open vials of penicillin, with both submitted to the same chamber.
Differential scanning calorimetry
A differential scanning calorimeter (DSC 821e, Mettler Toledo, Switzerland) was used for the thermal analysis of the drug and mixtures of the drug and the excipients in a 1:1 w/w ratio. Individual samples of the drug and the selected excipients (all passed through a 100-mesh sieve) were weighed to 5~10 mg in a DSC aluminum pan and scanned in the temperature range of 40–400 °C under an atmosphere of nitrogen. The heating rate was 10 °C min−1, and the obtained curves were observed for any type of interaction. Enthalpy calculations were completed using STARe software (version 102).
X-ray powder diffraction (XRPD)
The diffractogram of the drug was obtained using a Rigaku diffractometer (X-ray diffractometer, TTR III), equipped with a copper anode. The samples were analyzed at angle intervals of 2θ in the range of 2–45° at a digitalization speed of 0.02° 2θ s−1. The samples were prepared on glass slides with a thin layer of powder without solvent.
High performance liquid chromatography (HPLC)
The Shimadzu HPLC system consisted of an LC-20 AD pump, a DGU-20A5R on-line degasser, a SIL-20 a autoinjector, a CTO-20 AD column oven, and an SPD-M20A UV–VIS detector. Shimadzu CLASS-VP software (Version 5.03) was used for data acquisition and mathematical calculations. Chromatographic separation of estradiol was performed on a C18 Spherisorb column (4.6 mm × 250 mm; 5 µm). The mobile phase used was acetonitrile-deionized water in a ratio of 55:45 v/v at a flow rate of 1 mL min−1. The temperature of the column oven was maintained at 40 °C. All the samples (20 µL) were injected and analyzed at 205 nm using a UV detector. For peak purity testing, a PDA detector in the range of 190–800 nm was used, and the results analyzed by HPLC are presented in Table 1.
Formulation of tablets
The detail of the formulation development of the core tablets of E2 immediate release tablets is presented in Table 2. In brief, micronized estradiol immediate release tablets were prepared by wet granulation using single stroke tablet punching machine (XYP-5B, Leimai, China) fitted with 7 mm standard concave punches. E2 and all the excipients were mixed and passed through 100-mesh sieve, and they were mixed for 20 min in a polyethylene bag. Granules were prepared by kneading with 1 % sodium dodecyl sulfate and kept in oven at 50 °C for 1 h. The dried granules were passed through sieve 32-mesh sieve to get uniform granules. These sized granules were then blended with magnesium stearate (80-mesh passed) and compressed into tablets having an average mass of 100 mg.
Results and discussion
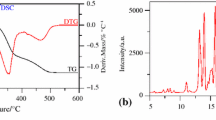
Selected DSC scans of the drug and drug-excipient mixtures are shown in Figs. 2a, 3a, 4a, 5a, 6a, 7a. The thermal behavior of the pure drug, the respective excipient, and the combination of the drug and excipient was compared in the DSC thermograms. The peak temperature (T peak), onset transition temperature (T onset), and heat of fusion or enthalpy (ΔH f) of estradiol in various excipient mixtures are summarized in Table 3.
The DSC trace of estradiol showed a sharp endothermic peak at 177.35 °C, and the heat of transition (ΔH f) was 59.85 J g−1 K−1. In the majority of cases, the melting endotherm of the drug was well preserved with slight changes in terms of broadening or shifting toward a lower temperature. It has been reported that the quantity of material used, especially in drug-excipient mixtures, affects the peak shape and enthalpy [18, 19]. Thus, these minor changes in the melting endotherm of the drug could be due to the mixing of the drug and the excipient, which lowers the purity of each component in the mixture but may not necessarily indicate potential incompatibility.
In the DSC curve of corn starch, a broad endothermic peak was seen at 74.12 °C due to the evaporation of adsorbed moisture (Fig. 2a). The melting endotherm of the drug was found to be 177.65 °C with a small change in the ΔH f value (58.85 J g−1 K−1) in the DSC trace of the estradiol–corn starch mixture, which ruled out any incidence of incompatibility.
The DSC scan of lactose was found at 148.43 °C (corresponding to the dehydration of bound water), and small peaks at 174.20 °C (crystalline transition) [20] and 218.19 °C (melting point), and a broad peak at 240.30 °C were also found [21]. The DSC curve of estradiol in the presence of lactose is shown in Fig. 3a. The DSC curve of the estradiol and lactose mixture showed features of both estradiol and lactose with the drug melting endotherm present at 177.49 °C; the accompanying ΔH f value was 59.68 J g−1 K−1.
The DSC trace of xanthan gum showed a broad endothermic peak at 88.36 °C, due to the evaporation of adsorbed moisture (Fig. 4a). The melting endotherm of the drug was present at 177.92 °C, with an accompanying ΔH f value of 64.39 J g−1 K−1 in the DSC trace of the estradiol–xanthan gum mixture, which ruled out any incompatibility.
The DSC scan of MCC showed a broad endotherm at 66.06 °C (starting from 30.31 °C and ending at 105.37 °C), which may be attributed to the loss of adsorbed water [22, 23]. The curve of the estradiol–MCC mixture showed an endothermic peak of the drug at 177.70 °C with a small change in the ΔH f value (65.47 J g−1 K−1), indicating that there was no interaction (Fig. 5a). The melting endotherm of estradiol was well retained in the DSC trace of the estradiol–MCC mixture (208.58 °C).
In the DSC trace of magnesium stearate, two endothermic peaks were observed at 95.20 and 116.35 °C (Fig. 6a). A small peak was also present at 218.35 °C, which might be due to palmitate impurities [24, 25]. The curve of the estradiol and magnesium stearate mixture showed a shift in the peak of estradiol to a temperature 178.11 °C; the accompanying ΔH f value was 61.94 J g−1 K−1. This phenomenon may be due to the purity of each component. This result suggests that there was no interaction between magnesium stearate and estradiol.
In the DSC scans of dibasic calcium phosphate, no peaks were observed in the range of 40–400 °C (Fig. 7a). However, the endothermic peak of estradiol was well preserved at 178.95 °C with a small change in the ΔH f value (55.42 J g−1 K−1) in the DSC traces of the estradiol–dibasic calcium phosphate mixtures, respectively.
X-ray powder diffraction has been used for qualitative and quantitative identification of crystallinity to investigate possible interactions with estradiol in BM [4, 26–30]. The X-ray diffraction patterns of the estradiol BM mixtures are shown in Figs. 2b, 3b, 4b, 5b, 6b, 7b.
The additional prominent DSC peaks in the mixtures of the drugs and excipients are a positive indication of a chemical interaction between the drug and the excipient. Such an interaction should result in the partial or complete disappearance of the reactant phases and the appearance of new phases, which can be inferred from X-ray diffraction patterns. The X-ray diffraction pattern of a mixture, prepared at room temperature, when compared with those of its individual components, may show the appearance of new lines and the disappearance of some of the lines present for the individual components.
The X-ray patterns of the E2-lactose mixture prepared at room temperature showed an increased number of lines in addition to those present in the patterns of the individual components (Fig. 3b; Table 4). The same table indicates the enhancement of the diffraction lines of higher or moderate intensity in the mixture. The significant difference in the X-ray patterns of the drug-excipient mixtures compared to those of the individual drug and excipient indicates compatibility of the drug with the excipient at room temperature.
The diffractogram of the E2-magnesium stearate mixtures shows the appearance of some new lines. The number of new lines is shown in (Fig. 6b; Table 5). The same diffractogram and table indicate that the number of lines present in the XRPD patterns of the individual components was found in the similar pattern recorded for the mixture. At the same time, the intensities of the majority peaks were appreciably reduced. The number of lines that vanished was little reduced, indicates compatibility between the estradiol and magnesium stearate at room temperature.
The results of the HPLC analysis of these samples are shown in Table 1, in which changes in the drug content could reveal drug-excipient compatibility. There were no significant interactions between estradiol and the individual excipients. Moreover, the mixtures of estradiol and the individual excipients were compared with pure estradiol during accelerated conditions by HPLC. It was found that the retention times remained unchanged. This indicates that estradiol was not degraded in the drug–excipient mixtures in the accelerated samples. Therefore, the accelerated results further verify that all the selected excipients are compatible with estradiol.
Conclusions
The results confirm that DSC can be used as a rapid method to evaluate the compatibility between a drug and an excipient. However, after storage of a mixture of estradiol and individual excipients under stressed conditions, the HPLC technique should be adopted in conjunction with DSC and XRPD studies in order to reach a definite conclusion. In this study, DSC analysis along with XRPD and HPLC analysis (for accelerated studies) were successfully employed to assess the compatibility of estradiol with selected excipients used in the development of a sustained release tablet formulation, i.e., novel micronized estradiol and dydrogesterone double-layer tablets.
References
Oliveira PR, Stulzer HK, Bernardi LS, Borgmann SHM, Cardoso SG, Silva MAS. Sibutramine hydrochloride monohydrate thermal behavior, decomposition kinetics and compatibility studies. J Therm Anal Calorim. 2010;100:277–82.
Soares MFR, Soares-Sobrinho JL, Silva KER, Alves LDS, Lopes PQ, Correia LP, Souza FS, Macedo RO, Rolim-Neto PJ. Thermal characterization of antimicrobial drug ornidazole and its compatibility in a solid pharmaceutical product. J Therm Anal Calorim. 2011;104:307–13.
Soares-Sobrinho JL, Soares MFR, Lopes PQ, Correia LP, Souza FS, Macedo RO, Rolim-Neto PJA. Preformulation study of a new medicine for chagas treatment: physico-chemical characterization, thermal stability and compatibility of benznidazole. AAPS PharmSciTech. 2010;11(3):1391–6.
Tita B, Fulias A, Bandur G, Marian E, Tita D. Compatibility study between ketoprofen and pharmaceutical excipients used in solid dosage forms. J Pharm Biomed Anal. 2011;56:221–7.
Verma RK, Garg S. Selection of excipients for extended release formulations of glipizide through drug-excipient compatibility testing. J Pharm Biomed Anal. 2005;38:633–44.
Viana OS, Arauijo AAS, Simoes RA, Matos CRS, Grangeiro-Júnior S, Lima CM, Rolim-Neto PJ. Kinetic analysis of the thermal decomposition of efavirenz and compatibility studies with selected excipients. Lat Am J Pharm. 2008;27(2):211–6.
Cunha-Filho MSS, Martínez-Pacheco R, Landín M. Compatibility of the antitumoral β-lapachone with different solid dosage from excipients. J Pharm Biomed Anal. 2007;45:590–8.
Gao R, Sun BW, Lin J, Gao XL. Compatibility of medroxyprogesterone acetate and pharmaceutical excipients through thermal and spectroscopy techniques. J Therm Anal Calorim. 2014;117:731–9.
Oliveira PR, Bernardi LS, Murakami FS, Mendes C, Silva MAS. Thermal characterization and compatibility studies of norfloxacin for development of extended release tablets. J Therm Anal Calorim. 2009;97:741–5.
Lira AM, Araujo AAS, Baslio IDJ, Santos BLL, Santana DP, Macedo RO. Compatibility studies of lapachol with pharmaceutical excipients for the development of topical formulations. Thermochim Acta. 2007;457:1–6.
Tomassetti M, Catalani A, Rossi V, Vecchio S. Thermal analysis study of the interactions between acetaminophen and excipients in solid dosage forms and in some binary mixtures. J Pharm Biomed Anal. 2005;37:949–55.
Barboza F, Vecchia DD, Tagliari MP, Silva MAS, Stulzer HK. Differential scanning calorimetry as a screening technique incompatibility studies of acyclovir extended release formulations. Pharm Chem J. 2009;43:363–8.
Moyano MA, Broussalis AM, Segall AI. Thermal analysis of lipoic acid and evaluation of the compatibility with excipients. J Therm Anal Calorim. 2010;99:631–7.
Tita B, Stefanescu M, Tita D. Complex of anti-inflammatory non-steroidal drugs from carboxylic acids family. 1 Synthesis and characterization of Zn(II) complex with ibuprofen. Rev Chim (Buchar). 2011;62:1060–4.
Tita B, Stefanescu M, Tita D. Complex of anti-inflammatory non-steroidal drugs from oxicam family. 1 Synthesis and characterization of Zn(II) complex with piroxicam. Rev Chim (Buchar). 2011;62:1002–7.
Bannach G, Cervini P, Gomes Cavalheiro T, Ionashiro M. Using thermal and spectroscopic data to investigate the thermal behavior of epinephrine. Thermochim Acta. 2010;499:123–7.
El-Gamel AEN, Hawash FM, Fahmey AM. Structure characterization and spectroscopic investigation of ciprofloxacin drug. J Therm Anal Calorim. 2012;108:253–62.
Kandarapu R, Grover V, Chawla HPS. Evaluation of the compatibility of ketorolac tromethamine with selected polymers and common tablet excipients by thermal and isothermal stress testing. STP Pharm Sci. 2011;11:449–57.
Mura P, Manderioli A, Bramanti G, Furlanetto S, Pinzauti S. Utilization of differential scanning calorimetry as a screening technique of determine the compatibility of ketoprofen with excipients. Int J Pharm. 1995;11:971–9.
Luo YH, Wu GG, Sun BW. Antisolvent crystallization of Biapenem: estimation of growth and nucleation kinetics. J Chem Eng Data. 2013;58:588–97.
Araujo AAS, Storpirtis S, Mercuri LP, Carvalho FMS, Filho MS, Matos JR. Thermal analysis of the antiritroviral zidovudine (A2T) and evaluation of the compatibility with the excipients used in solid dosage forms. Int J Pharm. 2003;260:303–14.
Mura P, Manderioli A, Bramanti G, Furlanetto S, Pinzauti S. Utilization of differential scanning calorimetry as a screening technique to determine the compatibility of ketoprofen with excipients. Int J Pharm. 1995;119:71–9.
Durig T, Fassihi AR. Thermal analysis study of captopril coated tablets by thermogravimetry (TG) and differential scanning calorimetry (DSC). Int J Pharm. 1993;117:161–70.
Serajuddin AT, Thakur AB, Ghoshal RN, Fakes MG, Ranadive SA, Morris KR, Varia SA. Selection of solid dosage form composition through drug-excipient compatibility testing. J Pharm Sci. 1999;88:696–704.
Gu L, Strickley RG, Chi L, Chowhan ZT. Drug-excipient incompatibility studies of the dipeptide angiotensin-converting enzyme inhibitor, moexipril hydrochloride: dry powder vs wet granulation. Pharm Res. 1990;7:379–83.
Tita B, Fulias A, Szabadai Z, Rusu G, Bandur G, Tita D. Compatibility study between ibuprofen and excipients in their physical mixtures. J Therm Anal Calorim. 2011;105:517–27.
Gombas A, Szabo-Revesz P, Kata M, Regdon G Jr, Eros I. Quantitative determination of crystallinity of α-lactose monohydrate by DSC. J Therm Anal Calorim. 2002;68:503–10.
Desai SR, Shaikh MM, Dharwadkar SR. Preformulation compatibility studies of etamsylate and fluconazole drugs with lactoseby DSC. J Therm Anal Calorim. 2003;71:651–8.
Marini A, Berbenni V, Pegoretti M, Bruni G, Cofrancesco P, Sinistri C, Villa M. Drug-excipient compatibility studies by physico-chemical techniques. The case of atenolol. J Therm Anal Calorim. 2003;73:547–61.
Stulzer HK, Rodrigues PO, Cardoso TM, Matos JSR, Silva MAS. Compatibility studies between captopril and pharmaceutical excipients used in tablets formulations. J Therm Anal Calorim. 2008;91:323–8.
Acknowledgements
This work has been supported by the Program for Changjiang Scholars and Innovative Research Team in University (IRT13095), and prospective joint research project of Jiangsu province (BY2012193).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Rui Gao and Yi Jin have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Gao, R., Jin, Y., Yang, QY. et al. Study of stability and drug-excipient compatibility of estradiol and pharmaceutical excipients. J Therm Anal Calorim 120, 839–845 (2015). https://doi.org/10.1007/s10973-014-4234-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4234-0